Abstract
The expression of the Hox gene Distal-less (Dll) directs the development of appendages in a wide variety of animals. In Drosophila, its expression is subjected to a complex developmental control. In the present work we have studied a 17 kb genomic region in the Dll locus which lies downstream of the coding sequence and found control elements for the expression of Dll in the leg and in other tissues. Of particular interest is a control element, which we have called LP, which drives expression of Dll in the leg primordium from early embryonic development, and whose deletion causes severe truncation and malformation of the adult leg. This is the first Distal-less enhancer for which, in addition of the ability to drive expression of a reporter, a role can be demonstrated in the expression of the endogenous Dll gene and in the development of the leg. In addition, our results suggest that some enhancers, contrary to the widely accepted notion, may require a specific 5′ or 3′ position with respect to the transcribed region.
Keywords: Distal-less, appendage, gene expression, reporter
INTRODUCTION
An important anatomical feature in many animal phyla is the presence of appendages which are used mainly for locomotion, but also for a variety of other roles. The embryonic origin and mode of development of such appendages are different between phyla, yet they show remarkable similarities in the choice of genes controlling these processes (Pueyo and Couso, 2005). One of the most conserved genes in appendage development is the family of HOX-encoding transcription factors similar to Drosophila Distal-less (Dll) (Cohen et al, 1989; reviewed in Panganiban, 2002).
The Dll gene is of crucial importance in the development of all the ventral appendages in Drosophila, including leg, clypeolabrum, maxillary and labial palps, antennae, legs and analia (Cohen and Jurgens, 1989a; Cohen and Jurgens, 1989b; Gorfinkiel et al., 1997; Gorfinkiel et al., 1999; Sunkel and Whittle, 1987). In addition to this role on appendage development, Dll is also required for the development of components of the peripheral nervous system, such as larval Keilin organs, antennal, maxillary, labial and labral sensory organs; and bristles in the adult leg and wing margin (Campbell and Tomlinson, 1998; Cohen and Jurgens, 1989a; Gorfinkiel et al., 1997; Sunkel and Whittle, 1987) and is expressed in the central nervous system (Kaphingst and Kunes, 1994). Most of these roles seem to be conserved throughout the animal kingdom, as seen by the universal expression of Dll in the central and peripheral nervous systems and, interestingly, several kinds of appendages or body outgrowths in general (Panganiban and Rubenstein, 2002; Pueyo and Couso, 2005). Remarkably, studies in mouse have shown that the vertebrate orthologue of Dll, Dlx, is required for the patterning of the distal part of the limb (Robledo et al., 2002).
The expression of Dll in such a wide variety of organs and tissues, and in a temporally dynamic fashion, requires a complex regulation. It is not then surprising then that the coding region of the gene is surrounded by several kilobases of non-coding DNA (Cohen et al., 1989) which are thus prime candidates to contain multiple cis regulatory elements. Among the multiple territories where Dll is expressed, the developing leg involves the most extensive and best studied Dll-dependent gene network. Expression of all the genes required for distal leg development, such as rotund, bric-a-brac, spineless, tarsal-less, Bar, vein, aristaless, etc., is dependent on Dll expression and function (Campbell and Tomlinson, 1998; Duncan et al., 1998; Galindo et al., 2005; Galindo et al., 2007; Kojima et al., 2000; St Pierre et al., 2002). Therefore, considerable effort has been dedicated to the study of the expression of Dll during leg development, and to the identification of the enhancers controlling this expression. The identification of enhancer elements and their functional characterisation is helping to understand the complex regulation of Dll and how the developmental switches on regulation are achieved.
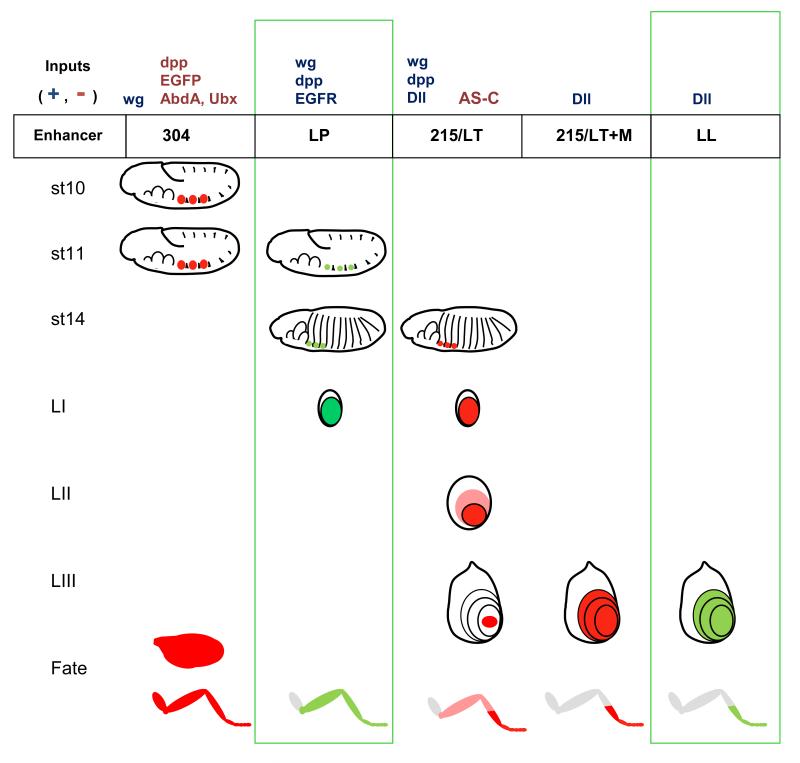
In Figure 1 we illustrate the dynamics of the expression of Dll throughout the development of Drosophila, and the related enhancers that have been found (including novel ones described in this work) . Dll is expressed in the three pairs of thoracic primordia from stage 10 of the embryonic development (Cohen et al., 1990, although it is not required for their determination (Cohen et al., 1993; Estella et al., 2003). Each one of these primordia will give rise to a Keilin organ, a sensory organ that is considered to be a rudiment of the larval leg, a ventral imaginal disc, precursor of the adult leg, and a dorsal imaginal disc, humeral, wing or haltere depending on the particular segment (Cohen et al., 1993; Goto and Hayashi, 1997). This early expression is induced by the Wnt homolog Wingless (Wg), expressed in the parasegmental boundaries (Cohen et al., 1993; Cohen, 1990); and repressed dorsally by the BMP homologue Decapentaplegic (Dpp), and ventrally by EGFR signalling (Goto and Hayashi, 1997). wg is expressed in all the segments, but Dll is repressed in the abdominal segments by the posterior homeotic genes Abd-A and Ubx (Castelli-Gair and Akam, 1995; Vachon et al., 1992) . In the 5′ non-coding region of the Dll genomic locus, a 1 kb fragment was identified 12 kb upstream of the start of transcription that can drive a lacZ reporter in this early pattern of Dll (Fig. 1 and Fig. 2A). This element, termed fragment 304, is activated by Wg, and can be repressed by ectopic expression of Ubx (Vachon et al., 1992).
Figure 1. Current view of Dll regulation in leg development.
The pattern of expression imposed on the Dll gene by the different enhancers during the leg development is depicted. The developmental stages are embryonic stages 10, 11 and 14; and second instar (LII) and late third instar (LIII) leg imaginal dics. In the lower row we illustrate the adult fates derived from these patterns of expression. Previously known enhancers are colored in red, and the novel enhancers described in the present work, in green. In the top row we indicate the known positive and negative regulatory inputs for each enhancer. of the Dll gene is depicted in red during different developmental periods: stage 10 and stage 14 embryos, 2nd and 3rd larval instar leg imaginal discs. The region depicted in pink in the 3rd instar leg disc represents the cells that used to express Dll, and is bound by the remaining central domain and the new ring of expression corresponding to the presumptive trochanter. The adult leg regions derived from these regions are indicated in the bottom panel. To the right of the images, the enhancers known to be active at each developmental stage and their main regulatory inputs are indicated. Leg segments are labeled: coxa (co), trochanter (tr), femur (fe), tibia (ti) and tarsal segments 1 to5 (ta).
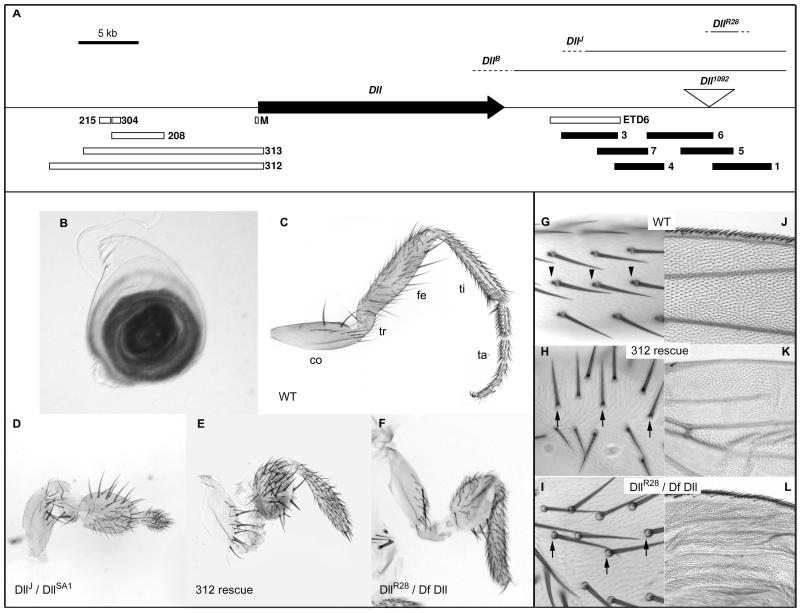
Figure 2. Genetics of the Dll downstream region.
A. Map of the Dll locus indicating the Dll transcription unit (black arrow), the positions of the fragments already known to contain enhancers (open bars), the new ones explored in this study (black bars), the Dll1092 transposable element insertion (inverted triangle) and the relevant mutations (continuous line for the region covered and a broken line for the region of indetermination). B. Leg disc of a Dll1092 late third instar larva stained with X-gal showing the pattern of expression of the β-galactosidase reporter. C. Wild type adult leg, segments are labeled as follows: coxa (co), trochanter (tr), femur (fe), tibia (ti) and tarsal segments 1 to5 (ta). D. DllJ / DllSA1 leg showing a deformed femur and a truncation of all the tissues from tibia. E. Similar phenotype in a DllSA1 / Df Dll; Dll312 rescued leg. F. Mutant leg of a DllR28 / Df Dll also showing femoral deformation and truncation from distal tibia.. G. Detail of a wild type wing margin. H. Wing margin of a DllSA1 / Df Dll; Dll312 rescue in which the mechanosensory bristles of the wing margin are missing. I. In a DllR28 / Df Dll wing, despite the crumpled morphology of the artificially inflated wing, the presence of the wing margin bristles can be appreciated. J. Wild type femur bristles showing bracts at their bases (arrowheads). K. In a DllSA1 / Df Dll; Dll312 rescue, bristles are misaligned due to the deformation of the femur, and bracts are missing (arrows). L. In a DllR28 / Df Dll femur the bristles are less severely misaligned, but bracts are also missing (arrows).
This induction phase driven by 304 is transient, and after a few hours the 304 enhancer is switched off (Cohen et al., 1993), and Dll expression must become dependent on other enhancers. A good candidate for the continuation of Dll expression was a second enhancer contained in fragment 215 (Fig. 1 and 2A), which becomes fully active by stage 14 (Estella et al., 2008; Vachon et al., 1992). The domain of expression of 215 (referred to as LT in Estella and Mann, 2008; Estella et al., 2008, see below) is more restricted than the one of 304 (Estella et al., 2008). 215 drives Dll expression only in the cells that will give rise to medial and distal leg (telopodite), but Dll expression is lost from proximal leg (coxopodite) and non-leg imaginal derivatives (Cohen et al., 1993; Goto and Hayashi, 1997). The cells that retain Dll will give rise to the following leg segments: trochanter, femur, tibia, tarsal segments and pretarsus (Fig. 2C). 215 is also repressed in the Keilin organ precursor cells by proneural genes, and Dll expression in these is driven by another element termed DKO (Estella et al., 2008). 215 expression is activated by Wg and Dpp signalling (Estella et al., 2008), which is surprising considering that Dpp was a repressor input for early Dll expression (Goto and Hayashi, 1997). 215 also requires an autoactivatory input from Dll (Castelli-Gair et al., 1994; Estella et al., 2008).
The cells of the adult appendage primordia, including the leg ones, delaminate from the embryonic epidermis and form structures called imaginal discs (reviewed in Cohen, 1993). During the three larval instars the leg imaginal disc grows and new proximal-distal (PD) fates are generated as concentric domains in the imaginal discs (reviewed in Couso and Bishop, 1998; Kojima, 2004). When the discs evert during pupal metamorphosis, the cells at the centre of the disc will become distal structures and cells at the periphery, proximal ones (see figure 1). During the first larval instar Dll is still present in the medial and distal parts of the leg imaginal disc. During the transition from second to third instar, Dll expression is lost from the presumptive medial leg, and these cells now express the dachshund gene (Abu-Shaar and Mann, 1998; Campbell and Tomlinson, 1998; Mardon et al. 1994). At this stage new Dll expression can still be experimentally activated by ectopic Wg signalling from the ventral cells and dpp signalling from the dorsal ones (Diaz-Benjumea et al., 1994; Lecuit and Cohen, 1997). At the end of larval development in late third instar, Dll is restricted to the future distal tibia, tarsal segments and pretarsus, and in a ring of cells corresponding to the trochanter that appears de novo. At this stage the central domain of Dll expression is independent of Wg and Dpp and dependent on self-activation (Campbell, 2002; Galindo et al., 2002). The requirements for Dll function in imaginal discs mirror this dynamic developmental expression: deprivation of Dll function from early larval stages in strong hypomorphic combinations or in early-induced somatic clones can affect medial and distal leg segments; and weak hypomorphs or late clones affect just the segments distal to the tibia (Campbell and Tomlinson, 1998; Cohen et al., 1993; Cohen and Jurgens, 1989a; Cohen and Jurgens, 1989b; Sunkel and Whittle, 1987).
The imaginal leg enhancers have been more elusive than the embryonic ones. Recently, candidates for both the Wg and Dpp responsive element in early larval stages and the self-maintenance in late larva have been proposed (Estella et al., 2008). The first one (leg trigger or LT) maps within fragment 215, which also contains the embryonic late enhancer. Expression driven by 215/LT requires Wg and Dpp. Another element (M) overlaps the Dll promoter and start of transcription (Fig. 2A) and can only drive weak expression in isolation, but is much more efficient when fused to 215/LT. This fusion of LT/M can drive expression during the whole of larval development in a pattern identical to native Dll, with exception of the trochanteral ring. However, M can also work when in cis to fragments other than LT, and LT itself also requires Dll function in addition to Wg and Dpp signals (Estella and Mann, 2008; Estella et al., 2008).
It had been known that ‘promoter bashing’ studies using reporter gene expression had a tendency to show a picture more complex than anticipated a priori, often revealing multiple enhancers with apparently redundant activities (Bachmann and Knust, 1998; Kassis, 1990; Werner et al., 2007). Increasingly, functional appraisal of such enhancers is revealing that in fact they are not wholly redundant, but follow a functional hierarchy consisting of primary (i.e. most strongly required) and secondary or ‘shadow’ (i.e. required minimally or only under environmental or genetic stress) enhancers (Camprodón and Castelli-Gair, 1994; Frankel et al., 2010; Hong et al., 2008). In the present work we analyse functionally the control of Dll expression using mutant alleles and reporter constructs, with an emphasis on leg development. Despite the recent characterisation of sequences driving leg expression in the 5′ region of Dll described above, here we define several 3′ enhancers controlling both reporter-mediated and endogenous Dll gene expression and function in the leg and in other organs. Furthermore, we also show that these regulatory elements are absolutely required for the corresponding specific functions of Dll in these tissues, and propose the idea that some of these enhancers must be located downstream of the transcript to achieve full functionality.
RESULTS
The Dll 3′ region is required for leg development
There is no firm functional evidence (such as leg-specific regulatory alleles) showing a requirement for the Dll 5′ regulatory regions during imaginal leg development. However, there is such functional evidence for the 3′ region, suggesting a role of the 3′ region in the control of Dll expression in the leg. The first line involves the DllJ allele (fig. 2A), which is a chromosomal rearrangement that removes the 3′ non-coding region of the locus, but does not affect the transcriptional unit, and is thus a regulatory allele (O’Hara et al., 1993). The transposition breakpoint maps very close to the Dll transcriptional unit, so it removes most of the 3′ non-coding region (Figure 2A). Indeed, the region between the DllB and the DllJ breakpoints contains a regulatory element, ETD6, required for the ventral maxillary expression (O’Hara et al., 1993). DllJ homozygote embryos are lethal and die in late stages showing losses of head organs and other defects, suggesting that the 3′ region is necessary for the development of Dll-dependent organs.
We characterised further the DllJ phenotype. Although DllJ homozygotes die as embryos lacking Keilin organs, we reasoned that the actual lethality may be caused by the secondary chromosomal breakpoint of the transposition, not by the DllJ mutation itself. Thus we crossed DllJ with the null allele DllSA1 , a small deletion that removes the locus (Cohen, 1990), and observed escaper heterozygotes which show a dramatic leg phenotype, with deformities in all medial segments and truncation from the tibia onwards (Fig. 2C, D). Therefore, DllJ behaves as a very strong regulatory allele for the adult leg development too. In other words, the downstream region is required for Dll gene function in imaginal leg development.
The second line involves the Dll minigenes 312 and 313. These minigenes were engineered by fusing a Dll cDNA to the immediate 5′ flanking region covering up to the next coding gene, and both contain the 304, 205/LT, DKO and M enhancers (Fig. 2A) but omitting any sequences downstream of the transcript. It had been previously shown that rescue of DllSA1 homozygotes with either 312 or 313 minigenes restored Keilin organs and all the ventral head sensory organs, but were not reported to produce fully viable flies (Vachon et al., 1992). We therefore repeated these rescues and observed that a few of these animals escape embryonic lethality, continue development until pharate stage and die inside the pupal case or soon after eclosion. Rescued flies show severe truncation and malformation of the medial and distal leg segments (Fig. 2E). The distal tibia and tarsal regions are absent, the femur is shortened and deformed and the trochanter is also deformed. Therefore this second, independent, genetic condition also shows that the regulatory regions upstream of the Dll coding region are not able to sustain the wild type pattern of Dll function throughout leg development but that 3′ sequences are required.
Further indication of the importance of the region 3′of the coding sequence stems from an enhancer trap insertion, Dll1092, which reproduces the pattern of expression of Dll (Fig. 2B) and behaves as a mild Dll mutant alelle (Fig. S1). Dll1092 is described in Flybase (http://flybase.org) as an insertion of a PZ lacZ reporter construct 16.5 kb downstream of the Dll coding region, a localisation that we confirmed by inverse PCR (Fig. 2A). We then performed a mutagenesis by imprecise excision of this P element construct in order to create small deletions of the region (see materials and methods), and recovered jumps with phenotypes ranging from wild type (precise excisions) to strong leg truncations typical of Dll mutant alleles. The strongest allele was DllR28. This mutant had a phenotype that was remarkably similar to the DllJ mutants and the minigene rescues: truncation at the level of distal tibia, shortened and balloon-like femur and malformed trochanter (Figure 2F). Despite some problems relating to a polymorphism in the Dll1092 strain (see methods), we determined the deleted region by flanking PCR amplifications both sides of the original P-element insertion. This showed that the deletion spanned a region of around 2.5 kb (Fig. 1A). Therefore, sequences contained in the tract which is deleted in DllR28 are essential for the function of Dll in leg patterning.
Together, these results clearly show that the 5′ region of the Dll locus does not contain all the elements necessary for the leg development and that some of these elements must reside within the 3′ region.
The downstream region is required for further developmental processes
The mutant phenotype of the 312 and 313 minigene rescues is not restricted to leg truncations, and they present other well described features of Dll mutants. The bristles of the femur and remnant tibia lack bracts, a scale-like structure that accompanies some macrochaetae in the distal leg segments (Fig 2G, H). We have already mentioned that these pharate adults die before or shortly after eclosion, so they do not always have time to extend their wings. In the few expanded wings or after artificially expanding them in NaOH and Hoyer’s mounting medium (Couso et al. 1994), it can be clearly appreciated that they lack the sensory bristles of the wing margin (Fig. 2J, K), as it happens in Dll mutant clones (Campbell and Tomlinson, 1998; Gorfinkiel et al., 1997). Finally, they show a partial antenna to leg transformation (Fig. S1) as encountered with some homozygous hypomorphic and heterozygous dominant alleles of Dll (Cohen and Jurgens, 1989a; Sunkel and Whittle, 1987). The DllR28 mutants also lack bracts (Fig. 2I), but in contrast, they do not have a wing margin phenotype (Figure 2L). These observations indicate that defined portions of the 3′ region of the Dll locus could account for further Dll functions.
Reporter constructs in pPTGal
The only reporter constructs with expression in the larval leg discs described to date are 215/LT, M and the combination of both, LT+M. The only published 3′ construct, ETD6, is expressed in the embryonic ventral maxilla. We decided to analyse the region downstream of the Dll transcription unit in search for new enhancer elements, initially focusing on leg development. The ETD6 element and the breakpoint of the DllJ mutation define the left limit of our region of interest. We analised 17 kb of the 3′ region covering from the ETD6 fragment to beyond the site of insertion of the enhancer trap Dll1092. Six overlapping fragments that cover the whole region were amplified by PCR from a BAC clone obtained from the Berkeley Drosophila Genome Project (BDGP; Fig. 2A). These fragments were cloned into the pPTGal vector, which contains the Gal4 coding sequence and a minimal hsp70 promoter downstream of a multiple cloning site (Sharma et al., 2002). We decided to use this vector because it would allow us to test the expression pattern by combining it with UAS-lacZ or UAS-GFP; and also to attempt functional rescues by driving UAS-Dll in a Dll mutant background (see below). Stable transformants of all fragments were obtained and several independent insertions of each construct were analysed to avoid position effect artefacts.
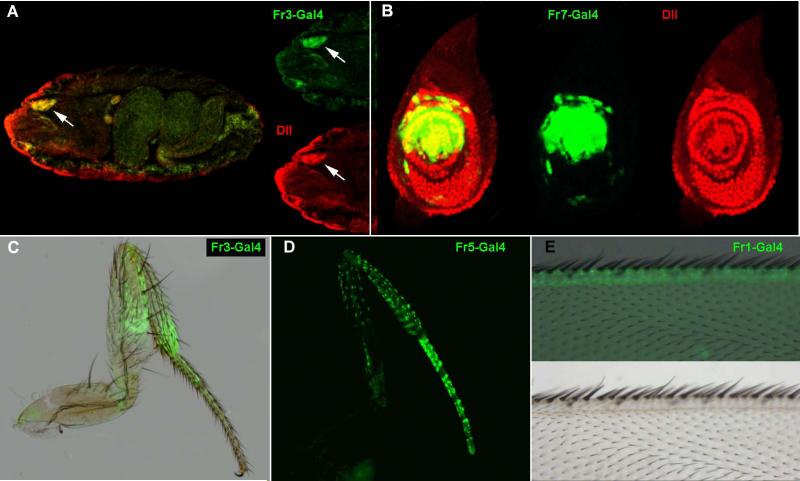
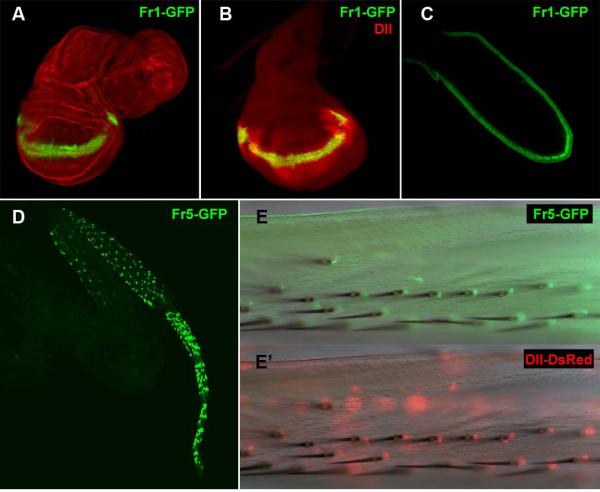
As a positive control for the system we confirmed that Fr3-Gal4 reproduces the published data for ETD6 and drives expression in the embryonic ventral maxillary epidermis (Fig3A). Unexpectedly, none of these pPTGal_constructs could drive expression in the Dll pattern during early or late larval leg development, apart from Fr7-Gal4, which is expressed in a central and dorsal patch in the leg disc contained within the Dll territory (Fig 3B) during late third instar. We then looked for expression during pupal development. In late third instar Dll is expressed in the distal segments, from distal tibia to the pretarsus. Fr3-Gal4 showed expression in these territories, but only in late pupa and pharate stages (Fig. 3C). It also showed weak staining in the wing margin at the same stages (not shown). Fr7-Gal4 gave a similar pattern to Fr3-Gal4 in addition to the already described pattern in the imaginal discs (not shown). Fr4-Gal4 showed no expression in any tisues at any stage. Fr5-Gal4 and Fr6-Gal4 showed expression in some of the bristles of pharate legs, more intensely in distal tibia and femur (Fig. 3D). Finally, Fr1-Gal4 is expressed in late pupa and pharate wing margin, much stronger than Fr3-Gal4 (Fig. 3E).
Figure 3. Pattern of expression of the PTGAL constructs.
A. Fr3-Gal4 stage 16 embryo showing co-expression of Dll (red) and GFP (green) in the ventral maxillary sense organ (arrow). B. Fr7-Gal4, UAS-GFP third instar leg disc showing expression of GFP (green) and Dll (red). C. Fr3-Gal4, UAS-GFP adult leg showing expression of GFP in the femur, tibia and tarsal segments; a bright field image is overlaid to appreciate leg morphology. D. Adult Fr5-Gal4, UAS-GFP leg expression of GFP in a pattern associated to leg bristles. E. Adult Fr1-Gal4, UAS-GFP wing showing GFP expression in the wing margin; a bright field image of the wing is shown in the top panel and the overlay in the bottom panel.
The lack of GFP expression in the leg disc driven by Fr1, Fr5 and Fr6 was particularly surprising since these three fragments cover the region deleted in the DllR28 and Dll3 mutations. We attempted a rescue of the DllR28 mutation by driving expression of UAS-Dll with each of these Gal4 drivers in case the expression was weak but functionally relevant, but we did not see any amelioration of the mutant phenotype. We also failed to see imaginal leg expression in earlier stages.
The genetics of the Dll downstream region clearly indicates that it is required for leg development; but we did not find any enhancers for the leg imaginal discs with this approach. Intriguingly, some of the fragments, namely Fr3 and Fr7, seemed to reproduce the endogenous Dll domain, albeit delayed in development. If we assume that they do contain a genuine imaginal disc enhancer, but that are working below their full efficiency, this could be due to two factors: an inadequacy of the vector employed, or that our change in the positioning of these enhancers, from their 3′ native position to 5′of the reporter gene, does not allow them to work efficiently. In order to circumvent these possible factors we decided to try a vector allowing insertion of putative enhancer sequences 3′ of the reporter gene.
Reporter constructs in pH-stinger
The choice of reporter vectors with 3′ cloning sites is limited, and we decided to use pH-stinger, which has a nuclear GFP gene fusion under the same minimal hsp70 promoter as pPTGal. In addition to a classical multiple cloning site upstream the reporter gene, pH-Stinger has a single cloning site (SpeI) downstream of it (Barolo et al., 2000). We cloned in this vector the fragments that gave pupal or pharate expression in the imaginal disc derivatives: Fr3, Fr7, Fr5 and Fr1. We did not test Fr6 because the Fr6-Gal4 pattern was identical to Fr5-Gal4, with which it overlaps. In addition to their 3′ position with respect to the reporter gene, we cloned the fragments in the same orientation respect to the GFP transcript as they have to the Dll one in their endogenous genomic positions. As we expected, these constructs were now expressed more efficiently and from earlier on. This allowed us to identify several novel enhancers: a wing margin enhancer in Fr1, a leg bract enhancer in Fr5 and, most interestingly, two new leg enhancers. Fr3 and Fr7 contain a late larval leg enhancer, and Fr1 contains an embryonic and larval leg enhancer which maps to a genomic region that is functionally relevant as revealed by the DllR28 mutation.
The LL enhancer
In contrast to the incomplete and late pattern of Fr3-Gal4 and Fr7-Gal4, Their Fr3-GFP and Fr7-GFP counterparts drive expression of GFP in the leg imaginal discs (Fig. 4A, B and Fig. S2). In both cases expression is absent at the beginning of the third instar but becomes activated soon afterwards and continues throughout larval and pupal development. We named this enhancer LL (leg late). Therefore the LL enhancer must lie in the 1.8 kb region where these fragments overlap. In late third instar leg discs the domain of GFP is coincident with that of endogenous protein (Fig. 4C, S3B). GFP expression starts in the wg and dpp-independent stage (Campbell 2002; Galindo et al. 2002), so we wondered if LL may be an autoactivatory enhancer. Indeed, in mutant clones homozygous for DllSA1 induced 48-72h AEL there is a loss of GFP expression, which is completely cell-autonomous (Fig 4D and S2F). Therefore we can conclude that at least part of the self-activation of Dll during larval stages proceeds through the LL enhancer.
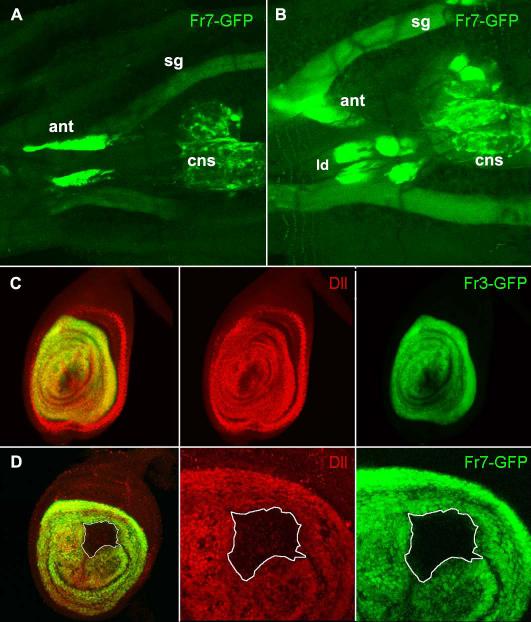
Figure 4. The LL enhancer.
A. ventral view of a Fr7-GFP early third instar larva (72h. AEL) showing GFP expression in the antennal discs (ant), central nervous system (cns) and weakly in the salivary glands (sg). B. In a slightly older larva (90h. AEL) GFP can also be seen in the two anterior pairs of leg discs (ld), while the posterior legs are out of focus. C. In a Fr3-GFP late third instar leg disc, expression of GFP (green) is coincident with Dll (red) in the central domain but not in the peripheral ring (arrowhead). D. Confocal section through a large Dll− clone in a Fr7-GFP leg disc. The white line outlines the cells which lack both Dll (red), and GFP (green).
Both fragments can also drive expression in the antennal imaginal disc, but the patterns are different and none of them identical to the endogenous Dll (Fig S2G, and Fig. S3C.). Fr7-GFP is expressed in a subset of the Dll-expressing cells, and Fr3-GFP is extensively, but not uniformly, expressed throughout the antennal disc. Both fragments drive expression in other tissues. During embryonic development Fr3-GFP is expressed in the same domain as ETD6 in the ventral maxilla of the embryo, and in addition in the optic lobes, posterior spiracles and epidermis (Fig S2A-D). In late third instar, Fr3-GFP is also expressed in the CNS (FigS2H). Fr7-GFP is expressed in parts of the epidermis in embryo and larva (Fig. S3A). Similar to their Gal4 versions, Fr3-GFP and Fr7-GFP are also expressed in the wing margin, but very weakly compared to the leg expression and only from the end of the pupal development and into pharate stage (not shown). This timing suggests that their contribution to the Dll function in the wing margin is not as important as the WM enhancer that we describe below.
The LP enhancer
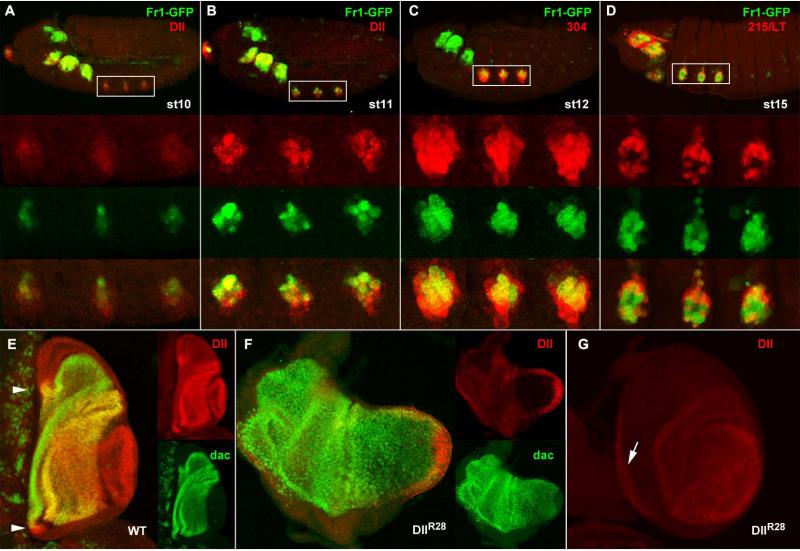
The DllR28 deletion causes a strong leg phenotype, so it was surprising to find that neither Fr1-GFP nor Fr5-GFP showed any leg imaginal disc expression at late third instar. One possibility was that the enhancer responsible for this function was acting earlier in development, so we decided to test for GFP expression from embryonic development. We found that Fr1 contains a leg enhancer that functions earlier than the LL enhancer. According to its expression and putative function, we named it leg primordium (LP) enhancer. Fr1-GFP expression in leg primordia starts soon after the onset of Dll expression, around stage 10 of embryonic development, in a subset of the cells that are already expressing Dll (Fig5A), By late stage 11 the overlap becomes more extensive, although Fr1-GFP is more restricted than Dll, and is stronger in dorsal cells (Fig. 5B). The overall pattern is very similar to the endogenous Dll: the clypeolabral domain is much more reduced than the endogenous Dll territory, but the antennal, maxillary, labial and thoracic ones are very similar. The leg expression remains strong until the end of the first larval instar and then is probably switched off, as GFP is seen to decay during the second instar (not shown).
Figure 5. The LP enhancer.
A-D. Temporal dynamics of the expression of Fr1-GFP (green) during embryo development (anterior to the left, dorsal up). A. Fr1-GFP expression starts in stage 10, soon after Dll protein is detectable (red). B. at stage 11 GFP expression is well established in the same cells where the Dll protein is present, although it is stronger dorsally. C. The expression of GFP at stage 12 is contained within the domain of the early reporter 304-lacZ. At stage 15, when the expression of the late embryonic enhancer 215-lacZ begins, it coincides with Fr1-GFP in the peripheral cells corresponding to the telopodite primordium, but not in the central cells of the Keilin organ primordium, where 215 is repressed. E. Expression of Dll (red) and Dac (green) in a wild type late third instar leg disc in side view (distal to the right, dorsal up) showing the overlap in the trochanter (arrowheads in the dorsal and ventral portions of the ring) and in the cells from distal tibia to proximal tarsus. There is a distal domain where Dac is absent. F. In a DllR28 / Df Dll disc of the same age Dll is much reduced in extent and intensity, as shown with a rabbit anti-Dll antibody, and Dac expression expands distally. G. In another disc of this genotype with a better morphology it can be observed that Fr3-GFP is still expressed, despite the fact that the expression of Dll is weak in the central domain; and in the peripheral ring (arrow), this time using a mouse anti-Dll antibody, which is stronger than the rabbit one.
We compared the early expression of Fr1-GFP with the two known leg reporters in the embryo. As could be expected from the co-expression with the Dll protein, Fr1-GFP expression starts slightly later than 304-lacZ and it is included within its domain of expression, so there are cells that express LacZ but not GFP (Fig. 5C). Expression of Fr1-GFP starts earlier than 215-lacZ, which is only robustly expressed by stage 14 (McKay et al., 2008). By stage 15 215-lacZ and Fr1-GFP are co-expressed in the progenitor cells of the distal leg primordia, but not in the cells of the Keilin organ, which do not express 215-lacZ, but do express Fr1-GFP (Fig. 5D).
It is precisely in the tissues (distal leg) that originate from these Fr1-GFP-expressing cells where the DllR28 mutant flies eventually show an abnormal phenotype (Fig. 2). Moreover, the region deleted in this allele also maps within Fr1. We surmise that the DllR28 deletion removes the LP enhancer and therefore reveals its biological function. Lack of LP-driven Dll expression would then result in the leg phenotype shown in Fig. 2F. Although LP is only active up to first or second instar, in late third instar, DllR28 mutants show a strong reduction of Dll expression, both in extension and intensity (Fig. 5E-G), and an abnormal leg disc morphology, with fewer epithelial folds which sometimes result in an elongated morphology (Fig. 5F). From second instar the dac gene is expressed in medial leg (Mardon et al., 1994) and Dll in the distal segments. During second and early third larval instars, these two territories are maintained by mutual repression of Dll and dac, and by late third instar this repression is overridden and Dll and dac expressions overlap in distal tibia and proximal tarsus (Abu-Shaar and Mann, 1998; Dong et al., 2001, see also Fig. 5E). As a consequence of the reduction in Dll expression in DllR28 the dac gene expression expands distally and occupies most of the disc centre (Fig. 5E, F). This reduction does not only affect the central domain, the ring in the trochanter region is also very faint, as shown by two different anti-Dll antibodies. These two domains of Dll expression, the trochanter and the distal leg, and the intervening femoral leg cells where dac is expressed, constitute the telopodite and therefore derive from the original domain of expression of LP.
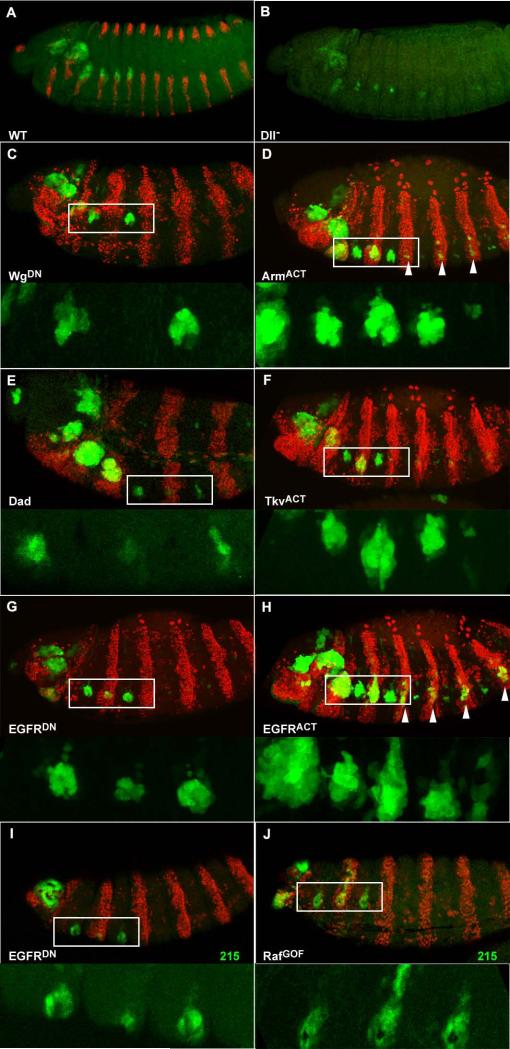
The timing and pattern of expression of LP is different from the other two known leg reporters, 304-lacZ and 215-lacZ. We wondered which regulatory inputs govern the pattern of expression of LP. To test if there is any auto-regulatory effect of Dll mediated by LP we checked its expression in null DllSA1 / Df(2R)ED4065 embryos (Fig. 6A, B). The size of the leg primordia is reduced, as in other Dll mutant embryos (JPC unp. obs.), but expression of GFP can still be observed. Therefore, LP does not merely represent an auto-activatory enhancer. We then tested the three signalling pathways more likely to play a role: the Wg pathway, which activates both 304 and 215, the Dpp pathway, which represses 304 but activates 215, and the EGFR pathway, which represses 304, has no described effect on 215, but is known to have a positive activity on Dll expression (Kubota et al., 2000). To test these pathways we used a strategy that has been employed before to study the regulatory inputs on 215 (McKay et al., 2008). It is based in the use of the driver line prd-Gal4, which can drive expression in mesothoracic leg primordia, but not on prothoracic or metathoracic ones.
Figure 6. Regulation of the LP enhancer.
All embryos oriented anterior left, dorsal up. A. wild type stage 14 embryo with normal expression of Fr1-GFP (green), at the edge of the ventral stripes of wg-lacZ (red). B. DllSA1 / Df Dll stage 14 embryo still has Fr1-GFP expression in the reduced leg primordia. C-H. Co-expression of different UAS constructs and nuclear DsRed as a marker (red) under the control of prd-Gal4, which drives expression in alternate segments, to see the effect on Fr1-driven GFP. Top panels show whole embryos and bottom panels are magnifications of the boxed area without the marker. Unless specified, all the embryos are stage 13 or 14. C. Expression of the dominant negative WgΔc produces a complete repression of Fr1-GFP. D. In contrast, expression of the activated ArmS10 produces a dorsal expansion of the domain and some ectopic expression in more posterior segments (arrowheads). E. Down-regulating Dpp signalling by expression of Dad produces a reduction in the early Fr1-GFP expression in a stage 11 embryo. F. Expression of an activated form of the Tkv receptor TkvQ199D results in ventral expansion of GFP. G. The presence of a dominant negative EGFRDN.B induces a reduction in GFP expression. H. EGFRλ4.2, a constitutively activated form, produces both expansion of the endogenous GFP domain and ectopic activation in other segments (arrowheads). I,J. The same strategy was applied to study EGFR regulation of 215-lacZ (green) in stage 16 embryos. I. Expression of the dominant negative EGFR produces a reduction of the GFP domain. J. Activation of the EGFR pathway by expression of Rafgof produces a dorsal expansion of the 125/LT domain.
Alterations in the Wg pathway had a very striking effect, especially expression of a dominant negative version of Wg, which abolishes GFP expression completely (Fig. 6C). Expression of a constitutively active Armadillo shows a slight expansion of GFP expression, and can also drive ectopic expression in more posterior prd-Gal4 expressing segments (Fig. 6D). Expression of UAS Dad, which down-regulates dpp signalling, results in a delay of the onset of expression of Fr1-GFP (Fig. 6E). This is a relatively mild effect, probably due to the fact that this is a weak UAS line. More convincingly, ectopic activation of the Dpp pathway by expression of an activated form of the receptor Tkv produces an expansion of the GFP domain towards the ventral side (Fig. 6F).Interestingly, we also found EGFR to have a positive effect on the expression of GFP: expression of a dominant negative form of the EGFR receptor shows a reduction in the expression of GFP (Fig. 6G), and an activated form of the same receptor can induce increased expression of GFP in the endogenous domain, and also ectopic expression in more posterior segments (Fig. 6H). In summary, all three pathways have a positive effect on the LP enhancer. The most distinct result was the positive effect of EGFR on GFP expression, so we wondered if this signalling pathway has the same effect on 215/LT. Indeed, blocking the EGFR pathway produced some reduction in the expression of 215-lacZ (Fig. 6I), and activation by means of expression of an activated form of Raf induces a dorsal expansion of the lacZ domain (Fig. 6J), although no ectopic induction of 215 in posterior segments (as it was the case with Fr1-GFP). This weaker effect on 215 could be interpreted as secondarily due to the auto-maintenance activity of Dll, rather than to a direct input from EGFR, and thus these results map the activatory, unexplained repeat from EGFR of Kubota et al. to LP.
Therefore, Fr1 contains a new embryonic leg enhancer different from the two previously described. Although the regulatory inputs of Fr1 have similarities with 215/LT, namely the effect of the Wg, Dpp and EGFR pathways, there are also important differences: Dll is required for 215/LT but not for LP, and proneural proteins inhibit 215/LT but not LP in the Keilin organ primordium. In addition, both reporters differ in the timing of their expression, with LP switching off.
The WM enhancer
In addition of the already described expression in the embryonic and early larval leg primordium that define the LP enhancer, Fr1-GFP is expressed in the wing margin from third larval instar up to pharate adult stage (Fig. 7A, B), so we named this putative enhancer WM. The WM domain represents a subset of the wing pattern of Dll (Fig. 7C) comprising only the most distal cells of the margin itself.
Figure 7. The WM and BR enhancers.
A.Late third instar wing and leg imaginal discs stained with phalloidin-TRITC showing expression of Fr1-GFP in the wing margin anlage, but not in the leg disc. B. Expression of Fr1-GFP (green) and Dll (red) in the wing disc. C. Fr1-GFP expression in the wing margin of a late pupal wing. D. Pharate leg showing spotted expression of Fr6-GFP in medial and distal leg. E, E′. Overlay of a bright field and in vivo fluorescence images of the leg cuticle at a higher magnification show that Dll-Gal4-driven Ds-Red (E) and Fr6-GFP (E′) are both expressed in individual cells at the base of the bristle, which form the bracts associated to these bristles.
Since the DllR28 deletion overlaps Fr1, we wondered whether this mutation could also affect Dll function in the wing margin? However DllR28 mutants usually display no obvious morphological defects of the wing margin (Fig.2I). In addition, in these mutants we observe no apparent defect in the Dll expression in the wing margin (not shown). These observations suggest that the WM enhancer is not covered by the DllR28 deletion and confirm that the LP and WM enhancers are independent of each other.
The BR enhancer
We mentioned above that Fr5-Gal4 and Fr6-Gal4 could drive expression in pharate legs near bristles of the distal leg. Fr5-GFP is also expressed in this pattern (Fig. 7D). Detection of the Dll protein with the anti-Dll antibody in whole leg at this stage is very difficult due to the presence of the cuticle. Instead we investigated co-expression with a Dll-Gal4 insert driving a nuclear form of the fluorescent protein DsRed. Both Dll-Gal4 and Fr5-GFP are expressed in the cells at the base of the bristles that form the bract (Fig. 7E-E′), so we have called this control element BR. This expression is necessary for bract development, because these structures are missing in Dll mutants (Campbell and Tomlinson, 1998; Gorfinkiel et al., 1997). We have also observed a phenotype of lack of bracts in the minigene rescues and in our DllR28 mutants (Fig. 2J-L). Therefore, the BR enhancer must be located in Fr5, in the region affected by the DllR28 deletion. It would overlap with Fr6, which had a similar expression pattern, but not with Fr1.
Sequence conservation backs-up our experimental characterization of downstream enhancers
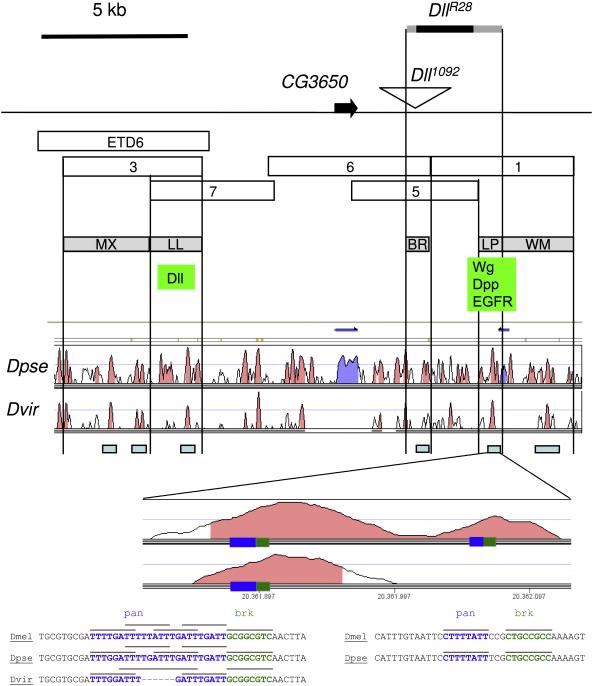
We have performed a comparison with the syntenic regions in two other Drosophila species through the Vista genome browser. We chose Drosophila virilis because it is one of the most distant among the sequenced Drosophila species, so the presence of conserved stretches of DNA is more evident. Unfortunately, the genome of this species is not completely sequenced and there are gaps in the Dll locus, so we have also included the alignment with Drosophila pseudoobscura. Several conserved blocks appear throughout the region, and there is at least one such block in each of our new enhancers. In addition, there are other conserved blocks that could contain more conserved regions for Dll expression in tissues or organs that have not been looked into in the present study, such as the central and peripheral nervous systems. We show in figure 8 the Vista plot, and we indicate the most probable locations for these control elements, based on the overlap (or lack of) of the different fragments among themselves and with the DllR28 deletion. We have tried to substantiate the putative regulatory nature of these conserved regions by searching binding consensi for the relevant transcription factors. The most striking result concerns two clusters of putative binding sites for the Wnt pathway effector Pangolin and for the Dpp antagonist Brinker. The first cluster is present in all three species and consists in 4 copies of the Pangolin consensus in D. melanogaster and D. pseudoobscura, or three in D. virilis, followed by a Brinker consensus. The second cluster comprises a Pangolin consensus followed by a Brinker consensus, and is present only in the first two species. The structure of these cluster is fully compatible with the regulatory logic proposed by Estella and Mann (2008) for the activation of the other Wg and Dpp-responsive element, 215/LT.
Figure 8. Map of the new Dll downstream enhancers.
The probable location of the downstream enhancers is mapped according to the overlaps of the different genomic fragments among themselves, and with the deletion DllR28. Underneath the map, the regions conserved in Drosophila pseudoobscura and Drosophila virilis obtained with the Vista genome browser are shown. Conserved sequences that fall within the new enhancers are indicated with a light blue rectangle. The conserved region within the LP enhancer is magnified and the location of the putative binding site clusters is indicated by rectangles. The sequence alignment of these clusters is depicted underneath, with particular binding sites for pangolin (pan) and brinker (brk) indicated by lines.
DISCUSSION
New enhancers in the Dll downstream region
The genomic region immediately downstream of the Dll transcription unit has remained virtually unexplored since the gene was first characterised at the molecular level. The only enhancer described in this region was a maxillary enhancer which requires Dfd to drive Dll expression. This enhancer was contained within the ETD6 fragment, affected in the DllB allele, but not in DllJ (O’Hara et al., 1993). Beyond the ETD6 fragment lie several kilobases of genomic DNA without any major transcripts. In contrast, the genomic region upstream of Dll had been extensively investigated in search for control regions involved in the leg expression of Dll. In this work we have identified at least four new enhancers in the Dll downstream region than can drive expression in the embryonic and early larval leg primordia (LP), late larval leg disc primordia (LL), leg bracts (BR) and wing margin (WM). In addition, our results have helped refine the location of the maxillary enhancer (MX) (figure 8).
Control of Dll expression in the leg
The known control elements for leg expression included an early embryo enhancer (304), a late embryo and early larval enhancer (215/LT), a Keilin organ enhancer (DKO) and a self-maintenance element (M) (McKay et al., 2008; Vachon et al., 1992). These spread over 20 kb upstream of the Dll transcription unit, and together they seemed to account for the whole pattern of expression of Dll (Estella et al., 2008). This conclusion would be based mostly on the pattern of expression they could impose on reporter genes. The only functional information available on regulatory regions was the rescue experiments with the 312 and 313 minigenes, and these suggested that the upstream region was able to rescue the lack of Keilin organs in a Dll null mutant. We have revisited and extended these rescues, and we observe that a few of the rescued individuals can develop into pharate adults displaying a severe leg phenotype, which indicates that this 5′ region is not enough to support a complete Dll gene expression pattern in leg development. This conclusion is supported by the phenotypes of the DllJ and Dll1092 regulatory mutants and, most strikingly, by the newly induced DllR28 mutant. This mutant is a relatively small deletion and its phenotype is remarkably similar to the minigene rescues, which suggests that the enhancer that it affects accounts for the most crucial part of the leg function of the 3′ region.
The crucial regulatory element disrupted by DllR28 is the LP enhancer. The deleted region is well covered by our reporter fragments 5 and 1, with extensive overlap among them, and the only leg enhancer is LP, present only in fragment 1. Therefore, it is unlikely that any other leg enhancer which is covered by the DllR28 mutation has passed unnoticed, and we can map the LP enhancer to a 0.8Kb interval up to some 3kb downstream of the Dll1092 insertion site. Impairing the function of this enhancer has dramatic consequences resulting in deformities in medial leg and truncation of the distal segments. These regions derive from cells that fell within the LP-expressing territory up to first instar. We have observed that in late third instar the morphology of the DllR28 imaginal discs is abnormal, expression of Dac is extended distally and the expression of Dll is weakened both in the central domain and in the peripheral ring. Therefore, the activity the LP enhancer is required for the early determination of leg PD fates and the subsequent efficient distal expression of Dll. The LP enhancer is not only functionally different from 304 and 215/LT, but also the other two embryonic leg enhancers. In addition, its timing and the regulation of its expression are also different. LP starts to work in stage 11, soon after 304 and earlier than 215. It integrates positive effects from three main signalling pathways: Wg, Dpp and EGFR, and it does not absolutely require Dll for its own expression.
LP is the only Dll enhancer described to date with any functional significance in leg development. The combination of 215/LT+M can drive expression of lacZ in a central domain in the leg disc which is coincident with the endogenous Dll (Estella et al., 2008, Castelli-Gair et al., 1994), but to date no leg-specific regulatory mutation has been mapped to this region. Another major caveat against the central role attributed to the combination of the 215/LT and M enhancers comes from the fact that both this combination and 215/LT itself are dependent on Dll expression (Estella et al., 2008) and therefore they may represent an autoactivatory input to reinforce the expression of Dll, rather than the actual trigger of Dll expression in the leg. It is possible that 215/LT contains a ‘shadow’ leg enhancer whose functionality would be required to reinforce and maintain the activity of the downstream leg enhancers in extreme physiological conditions (Frankel et al., 2010; Hong et al., 2008) or during certain developmental periods (see below).
We have found a further leg enhancer, expressed from early-mid third instar in leg imaginal discs which we have called LL. LL is an autoregulatory enhancer, which autonomously requires Dll. Its pattern of expression coincides with the endogenous Dll domain, and in this respect it is similar to the other autoregulatory enhancer described to date, the M enhancer. Thus, it would seem then that Dll expression may require a variety of enhancers with an autoactivatory component: 215/LT (Castelli-Gair et al., 1994, Estella et al., 2008); M (Estella et al., 2008); and LL (this work).
An integrated model of the regulation of Dll in the legs would be as follows (see also Fig. 1): At stage 10, Dll expression is activated in the single primordium for the Keilin’s organ (the vestigial larval leg), the leg and the wing imaginal disc, and is required for the formation of these three structures. This activation of 304 is achieved by Wg, while Dpp, EGFR and the Hox genes Ubx and AbdA act as repressors; hence this mixed appendage primordium is located in the thoracic segments only and at the dorsal edge of the ventral stripe of wg expression. Slightly later, at stage 11, 304 ceases to act, the wing primordium loses Dll expression, and separates and moves away dorsally. Dll expression remains in the leg and Keilin primordia but is now driven by LP, which interprets inputs differently than 304: thus, while LP is similarly activated by Wg, is also activated by Dpp and EGFR, which were repressors of 304. Later yet, during stages 12 and 13, Dll215/LT/M becomes active and collects activatory inputs from Wg, Dpp and Dll itself to reinforce the action of LP. This mode of regulation remains during first instar, and is responsible for the specification of most of the imaginal leg (the telopodite), giving raise to trochanter, femur, tibia, and tarsus. At the first to second instar transition, the activity of LP ceases, the leg imaginal disc separates from the Keilin organ (Anderson) and the expression of Dll disappears from the presumptive femur and distal tibia, which acquire the expression of dac (Mardon, Abu-Saar). Expression of Dll remains in the distal part of the leg (tibia and tarsus), presumably driven by 215/LT. At early third instar, the expression of Dll becomes independent of Wg and Dpp and seems to rely exclusively on autoactivatory maintenance driven by 215/LT+M and the new 3′ autoactivatory enhancer described here, LL. This self-mantained expression remains until the late pupa, when sensory-organ specific expression driven by the BR enhancer appears in the bracts of the leg bristles.
While this model accounts for Dll regulation in Drosophila, and presumably other holometabolous insects with separate larval and imaginal leg primordia, is likely that a very similar mechanism operates in less derived hemimetabolous insects and other arthropods, which develop their legs directly at embryogenesis. These less derived arthropods also display a dynamic Dll expression including events like the dissapareance of Dll expression from the presumptive medial leg (femur and tibia in insects) (prpic damen, Kaufmann), which in Drosophila we show to correlate with inactivation of the activatory 3′ enhancer LP. This reduction in Dll expression does not occur in the antenna of any of these species, and this differential regulation contributes to the different pattern and morphology of these appendages (Cummins et al., 2003, Dong et al., 2002).
Control of Dll expression in other organs
It was already suspected that the Dll wing margin enhancer had to lie in the downstream region, since the upstream region could not drive any expression in the wing imaginal disc (Estella and Mann, 2008; Estella et al., 2008). In this work we show that the minigene rescues with the 312 and 313 fragments produce pharate adults in which the wing margin has a typical Dll phenotype of lack of bristles. In consequence, there must be a wing enhancer in this downstream region. We have found two regions that can drive GFP expression in the wing margin. The first one, shared by fragments 3 and 7 may be the same as the LL enhancer, and it is active in the wing margin late in pupal development. It could be a manifestation of the self-regulatory enhancer LL in the wing margin, but in any case it is probably irrelevant since the expression of Dll is required for the determination of the wing margin bristles earlier, in late third instar (Campbell and Tomlinson, 1998). The second one, the enhancer that we have called WM is most likely the missing wing margin enhancer. WM is contained in Fr1, like LP, but probably 3′ of it since the DllR28 deletion does not affect the wing margin. We have observed that WM can only drive GFP expression in a narrow line of cells at the presumptive wing margin itself, while Dll protein expression is stronger in the wing margin, but then decays gradually in the wing pouch, in what has been shown to be a graded response to wg (Zecca, 1996). The most likely explanation is that this enhancer may need to act in conjunction with another element elsewhere in the Dll locus, most likely an autoactivatory enhancer. Thus, cells in the early wing disc close to the margin would switch Dll expression on, but as the disc grows some of these cells will find themselves further away from the margin, and outside of the functional Wg gradient. In these cells, some weaker Dll expression would still remain thanks to the self-maintenance activity of Dll. In this scenario, the gradient of Dll protein observed in the wing disc (strong levels near the margin, weaker in the blade, would be the result of the life history of the disc cells, while the pattern of Fr1-GFP would just represent a snapshot of the cells currently exposed to Wg. It would be interesting to test this possibility in the context of recent re-assesments of the Wg gradient hypothesis (Piddini and Vincent 2009 ; Zecca and Struhl, 2010).
Finally, we have found a BR enhancer that is co-expressed with Dll-Gal4 and probably represents the driver required for the function of Dll in the leg bracts. Bracts are determined by directional EGFR signalling from the bristle (del Alamo et al., 2002; Held, 2002). It was long known that a typical phenotype in different combinations of Dll mutant alleles and in Dll- somatic clones was the lack of the bracts, which are characteristic of medial and distal leg segments (Hannah-Alava, 1958). We have shown through the small deletion in DllR28 that this phenotype maps to the downstream region of Dll, and we have identified the corresponding control region in the overlap of Fr5 and Fr6 since both fragments can drive expression of reporter genes in the bracts.
Topology and function of enhancers
Two cautionary lessons could be obtained from our results. First, some enhancers may have specific positional requirements with respect to the coding region in order to function efficiently. In this respect, the LP, LL and WM enhancers did not work or worked much less efficiently when placed 5′ of the Gal4 transcription unit, but did drive expression of GFP when placed downstream of the transcription unit. Since the objective of the present work was not to study the positional specificity of enhancers, our results do not permit a completely watertight interpretation, but some of the alternatives can be discarded on close inspection.
The nature of the vector backbone is unlikely to be the cause of the difference, since both use the same hsp70 minimal promoter, which is standard for many Drosophila vectors. In addition, PTGal has been used in the characterization of several regulatory regions, with at least 14 publications listed by Pubmed. Finally, this positional effect was not present in the MX or BR enhancers, which both could drive correct expression either upstream or downstream of both reporters. In the case of MX, this fragment works in three different constructs (lacZ, Gal4 and GFP). The difference between LP, LL and WM 5′ and 3′ reporters could also be due to a specific requirement for these enhancers to be situated at a minimal distance from the promoter; this minimal spacing would be achieved more easily when situated 3′ of the transcription unit. However, the size of our cloned fragments is between 4 and 5 kb, while the size of the GFP ORF is just 1,2 kb. therefore the position of an enhancer proximal or distal to the ORF is less important than the location within the fragment containing it. In any case, any argument based on distances fades if we consider that the distance of LP to the endogenous Dll promoter is nearly 40 kb.
Still, other possibilities cannot be yet discarded, such as the presence in our fragments 7 and 1 of insulators, located 3′ to the actual LP and LL enhancers. To definitely prove this 3′ position effect, cloning of the LP and LL enhancers 5′ of the hsp70 promoter and the GFP reporter in the pH-Stinger vector would be required.
Regardless of the precise basis of this position effect, its functional significance may reflect some constraint in the control of the transcription of Dll, or it may help to prevent the ectopic activation of genes further downstream, and therefore represent a more general safety mechanism in the control of gene expression. In a similar study, the downstream region of the wingless gene was investigated and regulatory regions for the eye, wing and ventral (leg and antenna) imaginal discs (Pereira, 2006). Although the patterns of expression of the reporter genes closely resembled endogenous Wg, some details in their pattern and activation timing differed with respect to the endogenous protein (Pereira, 2006; F. Casares pers. comm.; JPC unp. obs). Small differences like these have been usually disregarded, but may stem from the fact that regulatory regions have been largely characterised in reporter constructs in which the genomic region was cloned upstream of the lacZ reporter gene, even if their native position is downstream of the coding region. These results beg further research that might challenge the prevalent view that the 5′ or 3′ positioning of enhancers is not as important as distance to the promoter, and may illuminate new models of enhancer-promoter communication.
From our results, a second prevention arises. Even if the pattern of expression of a reporter construct is similar to the endogenous gene product, one cannot necessarily conclude that the DNA region cloned in such construct is either absolutely required or fully sufficient to control the expression of the gene. Similarly, in vitro binding assays inform of the potential ability of DNA fragments to bind certain proteins, not of the functional outcome in vivo. Multiple enhancers, either similar or unrelated, can contribute towards the final output in both normal and extreme conditions (Frankel et al., 2010; Hong et al. 2008; this work). Therefore, expression data of reporter constructs should be complemented with functional information in order to obtain meaningful insights into the regulation of the genes under study.
MATERIALS AND METHODS
Flies
The null allele DllSA1 (Cohen 1993), DllJ (Cohen and Jurgens 1989b), the reporters UAS-lacZ. 304-lacZ and 215-lacZ, and the minigene constructs Dll312 and Dll313 (Vachon et al. 1992) were obtained from Stephen Cohen. Dllmd743-Gal4 (Calleja et al., 1996) was obtained from M. Calleja. The following transgenic constructs were used the enhancer activation studies: UAS-Efgrλ4.2, UAS-EgfrDN.B, UAS-rlSEM, UAS-wgΔc, UAS-armS10, UAS-tkvQ199D, P{EP}DadEP3196, prd-GAL4, UAS-RedStinger, UAS-vg::sdTEA.
For the generation of mitotic clones, the following stocks were used:
w; FRT42D, DllSA1 / SM6aTM6B / Fr3
w; FRT42D, DllSA1 / SM6aTM6B / Fr7
w, hsFLP; FRT42D, πM, M(2)531 / CyO
Other strains used in this study were Oregon-R, P{PZ}Dll01092, Df(2R)ED4065 (referred to as Df Dll in the text), UAS-GFP, obtained from the Bloomington stock centre.
Somatic clones
To study the expression of Fr3 and Fr7 in Dll clones were induced in flies of the following genotype, and the equivalent one with Fr7-GFP.
w hsFLP; FRT42D DllSA1 / FRT42D, πM, M(2)531; Fr3-GFP / +
Dll clones tend to grow slowly and tend to segregate from the plane of the epithelium, so it is difficult to obtain large clones. 48-72 h. AEL larvae were heat shocked and allowed to develop until late third instar. Mutant cells were identified by the absence of a nuclear version of the myc tag.
Mutagenesis
We generated new mutant alleles by excision of the PZ enhancer trap element present in the Dll1092 stock. Dll1092 was generated in the Gene Disruption Project of the Berkeley Drosophila Genome Project, and contains a PZ element inserted 16.5 kb downstream of the Dll coding region. Dll1092 females were crossed to males carrying a transposase source. Double heterozygous males were crossed to ry506 females and the progeny was scored for loss of the ry+ marker carried by the PZ element. Single individuals were crossed to balanced partners in order to make stocks. Complementation tests with other Dll alleles showed that, as expected, most of the excision events were precise and produced no phenotype in the heterozygote. Five new Dll alleles of varying strength were found, and we chose the strongest one of them, DllR28 to study the genetics of the region. A plasmid rescue experiment revealed that in the Dll1092 strain, the reporter PZ element is inserted within a natural transposable element, an opus element (also called yoyo), which is a polymorphism with respect to the canonical genomic sequence. Therefore, because we did not know the genomic sequence of the host strain, we could not design primers flanking the insertion site. Instead, we narrowed down the extent of the deletion by nested PCR products approaching the insertion point from each side. With this strategy we determined the deleted fragment, with a region of indetermination on each side, between the nearest primer that amplified and the nearest primer that failed to amplify.
Amplification of the genomic fragments
The genomic fragments for the search of enhancers were amplified by PCR from the BAC clone BACR27P17. This clone was obtained from the Berkeley Drosophila Genome Project and contains the canonical sequence used in the genome sequencing and annotation. To obtain the different fragments the following primers were employed: Fr1 forward TGG GGG TCA GGG GTC ACA AAG GTA AGG; Fr1 reverse TAG CCG GCC AGT CAG TCA GGA GGA TAA GTC; Fr3 forward CGG AAG AAA GAA AGC GTA AGC G; Fr3 reverse GAG ATC TGG GTG CAA CAT AGT CCC; Fr4 forward CGC ACC TCC GCA CAT CCG TCT GA; Fr4 reverse GGT TTG GGT CTT GGA CCT TAG CCT TGC CT; Fr5 forward GAC ACG CTC ACC GCC TCC ACC TTC T; Fr5 reverse ATC GCT CCA CTC GCA CTT TAC GGC AAC; Fr6 forward GAG TGT CGT CAG CCA TCT TAC CAG CC; Fr6 reverse GGA ATT ACA ACA GCC ACC CCT TAC CTT T; Fr7 forward ACC TTT TGT CCT GTC CCC TTC ATT C; Fr7 reverse TCT CAC TAA TCA AAA CCT CAA CCC ACA T. The PCR fragments were cloned directly using the TOPOTA kit (Invitrogen, Carlsbad CA) into either the pCR2.1, pCR4 or pCR-XL vectors.
Cloning into reporter vectors
For the Gal4 reporter constructs the fragments were subcloned into the pPT-Gal vector (Sharma et al.). The inserts were excised using the appropriate restriction enzymes and cloned into the polylinker of pPT-Gal. For the nuclear eGFP reporter constructs some of the fragments were subcloned into pH-stinger (Barolo et al., 2000). For this we used a single SpeI cloning site 3′ of the eGFP sequence. The PCR fragments were excised with SpeI-compatilble enzimes and cloned into SpeI-linearised pH-stinger. The orientation of the insert with respect to the eGFP open reading frame was tested by restriction mapping and subsequent sequencing from the vector.
Stable transgenic lines were obtained by germ line transformation using standard methods. We used the Vanedis Drosophila transgenesis service (Oslo, Norway) for the injection of the embryos, and we did the selection of positive transformants and the mapping of the insertions. At least ten independent transformants for each construct were tested to avoid position effects.
Immunohistochemistry and microscopy
The following primary antibodies were employed: mouse monoclonal anti-Dll (1:2000, from I. Duncan), rabbit polyclonal anti-Dll (1:250, from S. Carroll), mouse anti-Dac (1:240, developed by Mardon and Rubin, obtained from the DSHB, University of Iowa), rabbit polyclonal anti-GFP (1:500, Molecular Probes), anti-β-galactosidase (1:1000, Promega), mouse monoclonal anti-myc 9E10 (1:200, Sigma). Secondary antibodies conjugated to FITC or rhodamine from Jackson Immunochemicals were used at 1:200 dilution. Phalloidin-rhodamine (Invitrogen) was used as a probe for the actin cytoskeleton.
Embryos and imaginal discs were fixed in 4% paraformaldehyde in PBS and stained according to standard procedures (Couso et al. 1994). Confocal images were obtained in Zeiss LMS 510 and Leica TCS SL microscopes, and analysed with the ImageJ program.
Adult cuticles, mainly adult and pharate legs and wings, were dissected, treated and mounted as described in Couso et al. 1994. They were photographed in a Leica DM RXA2 microscope.
Bioinformatics
Pairwise alignments of the Dll locus between Drosophila melanogaster and the other two species, D. pseudoobscura and D. virilis, were obtained through the Vista Genome Browser (http://pipeline.lbl.gov). The curves shown in the graph follow the default parameters: display regions with 50-100% identity over a 100 bp window, highlight areas with over 70% identity. Putative ind sites consensi were searched with the web-based Jaspar application (http://jaspar.cgb.ki.se/).
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by a Wellcome Trust Senior Fellowship to JPC (ref. 057730), and a Wellcome Prize PhD studentship to DFG. MIG is a Ramón y Cajal Fellow. We are indebted to Stephen Cohen and Manuel Calleja for Drosophila stocks and to Ian Duncan and Sean Carroll for anti-Dll antibodies. We would also like to thank Richard Mann and Claudio Alonso for comments and discussions.
References
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Bachmann A, Knust E. Dissection of cis-regulatory elements of the Drosophila gene Serrate. Development Genes and Evolution. 1998;208:346–351. doi: 10.1007/s004270050190. [DOI] [PubMed] [Google Scholar]
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726–732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Campbell GL. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature. 2002;418:781–785. doi: 10.1038/nature00971. [DOI] [PubMed] [Google Scholar]
- Campbell GL, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- Camprodón FJ, Castelli-Gair JE. Ultrabithorax protein expression in breakpoint mutants: localization of single, co-operative and redundant cis regulatory elements. Development Genes and Evolution. 1994;203:411–421. doi: 10.1007/BF00188690. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J, Akam M. How the Hox gene Ultrabithorax specifies two different segments: the significance of spatial and temporal regulation within metameres. Development. 1995;121:2973–2982. doi: 10.1242/dev.121.9.2973. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J, Greig S, Micklem G, Akam M. Dissecting the temporal requirements for homeotic gene function. Development. 1994;120:1983–1995. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature. 1990;343:173–177. doi: 10.1038/343173a0. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Imaginal disc development. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Vol. 2. Cold Spring Harbor Laboratory Press; New York: 1993. pp. 747–841. [Google Scholar]
- Cohen SM, Bronner G, Kuttner F, Jurgens G, Jackle H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989;338:432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jurgens G. Proximal-distal pattern formation in Drosophila graded requirement for Distal-less gene activity during limb development. Roux Arch. dev. Biol. 1989a;198:157–169. doi: 10.1007/BF02438941. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jurgens G. Proximal-distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 1989b;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso JP, Bishop SA. Proximo-distal development in the legs of Drosophila. Int J Dev Biol. 1998;42:345–52. [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez Arias A. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- Cummins M, Pueyo JI, Greig SA, Couso JP. Comparative analysis of leg and antenna development in wild-type and homeotic Drosophila melanogaster. Dev Genes Evol. 2003;213:319–327. doi: 10.1007/s00427-003-0326-8. [DOI] [PubMed] [Google Scholar]
- del Alamo D, Terriente J, Diaz-Benjumea FJ. Spitz/EGFr signalling via the Ras/MAPK pathway mediates the induction of bract cells in Drosophila legs. Development. 2002;129:1975–1982. doi: 10.1242/dev.129.8.1975. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- Dong PD, Chu J, Panganiban G. Proximodistal domain specification and interactions in developing Drosophila appendages. Development. 2001;128:2365–2372. doi: 10.1242/dev.128.12.2365. [DOI] [PubMed] [Google Scholar]
- Dong PD, Dicks JS, Panganiban G. Distal-less and homothorax regulate multiple targets to pattern the Drosophila antenna. Development. 2002;129:1967–1974. doi: 10.1242/dev.129.8.1967. [DOI] [PubMed] [Google Scholar]
- Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12:1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C, Mann RS. Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg. Development. 2008;135:627–636. doi: 10.1242/dev.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C, McKay DJ, Mann RS. Molecular Integration of Wingless, Decapentaplegic, and Autoregulatory Inputs into Distalless during Drosophila Leg Development. Developmental Cell. 2008;14:86–96. doi: 10.1016/j.devcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C, Rieckhof G, Calleja M, Morata G. The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development. 2003;130:5929–5941. doi: 10.1242/dev.00832. [DOI] [PubMed] [Google Scholar]
- Frankel N, Davis GK, Vargas D, Wang S, Payre F, Stern DL. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature. 2010;466:490–493. doi: 10.1038/nature09158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo MI, Bishop SA, Couso JP. Dynamic EGFR-Ras signalling in Drosophila leg development. Developmental Dynamics. 2005;233:1496–1508. doi: 10.1002/dvdy.20452. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297:256–259. doi: 10.1126/science.1072311. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Pueyo JI, Fouix S, Bishop SA, Couso JP. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. Plos Biology. 2007;5:e106. doi: 10.1371/journal.pbio.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes and Development. 1997;11:2259–2271. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Sánchez L, Guerrero I. Drosophila terminalia as an appendage-like structure. Mechanisms of Development. 1999;86:113–123. doi: 10.1016/s0925-4773(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Goto S, Hayashi S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development. 1997;124:125–132. doi: 10.1242/dev.124.1.125. [DOI] [PubMed] [Google Scholar]
- Hannah-Alava A. Developmental Genetics of the Posterior Legs in Drosophila Melanogaster. Genetics. 1958;43:878–905. doi: 10.1093/genetics/43.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held LI., Jr Bristles induce bracts via the EGFR pathway on Drosophila legs. Mech Dev. 2002;117:225–34. doi: 10.1016/s0925-4773(02)00212-5. [DOI] [PubMed] [Google Scholar]
- Hong J-W, Hendrix DA, Levine MS. Shadow Enhancers as a Source of Evolutionary Novelty. Science. 2008;321:1314. doi: 10.1126/science.1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst K, Kunes S. Pattern formation in the visual centers of the Drosophila brain: wingless acts via decapentaplegic to specify the dorsoventral axis. Cell. 1994;78:437–448. doi: 10.1016/0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Kassis JA. Spatial and temporal control elements of the Drosophila engrailed gene. Genes Dev. 1990;4:433–443. doi: 10.1101/gad.4.3.433. [DOI] [PubMed] [Google Scholar]
- Klein T, Arias AM. Different spatial and temporal interactions between Notch, wingless, and vestigial specify proximal and distal pattern elements of the wing in Drosophila. Dev Biol. 1998;194:196–212. doi: 10.1006/dbio.1997.8829. [DOI] [PubMed] [Google Scholar]
- Kojima T. The mechanism of Drosophila leg development along the proximodistal axis. Dev. Growth Differ. 2004;46:115–129. doi: 10.1111/j.1440-169X.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Kojima T, Sato M, Saigo K. Formation and specification of distal leg segments in Drosophila by dual Bar homeobox genes, BarH1 and BarH2. Development. 2000;127:769–778. doi: 10.1242/dev.127.4.769. [DOI] [PubMed] [Google Scholar]
- Kubota K, Goto S, Eto K, Hayashi S. EGF receptor attenuates dpp signaling and helps to distinguish the wing and leg cell fates in Drosophila. Development. 2000;127:3769–3776. doi: 10.1242/dev.127.17.3769. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388:139–45. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- McKay DJ, Estella C, Mann RS. The origins of the Drosophila leg revealed by the cis-regulatory architecture of the Distalless gene. Development. 2009;136:61–71. doi: 10.1242/dev.029975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara E, Cohen B, Cohen SM, McGinnis W. Distal-less is a downstream gene of Deformed required for ventral maxillary identity. Development. 1993;117:847–856. doi: 10.1242/dev.117.3.847. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Rubenstein JLR. Developmental functions of the Distalless/Dlx homeobox genes. Development. 2002;129:4371–4386. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- Pereira PS, Pinho S, Johnson K, Couso JP, Casares F. A 3prime cis-regulatory region controls wingless expression in the Drosophila eye and leg primordia. Developmental Dynamics. 2006;235:225–234. doi: 10.1002/dvdy.20606. [DOI] [PubMed] [Google Scholar]
- Piddini E, Vincent JP. Interpretation of the wingless gradient requires signaling-induced self-inhibition. Cell. 2009;136:296–307. doi: 10.1016/j.cell.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Pueyo JI, Couso JP. Parallels between the proximal-distal development of vertebrate and arthropod appendages: homology without an ancestor? Current Opinion in Genetics and Development. 2005;15:439–446. doi: 10.1016/j.gde.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes and Development. 2002;16:1089–1101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y, Cheung U, Larsen EW, Eberl DF. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis. 2002;34:115–118. doi: 10.1002/gene.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre SE, Galindo MI, Couso JP, Thor S. Control of Drosophila imaginal disc development by rotund and roughened eye: differentialy expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development. 2002;129:1273–1281. doi: 10.1242/dev.129.5.1273. [DOI] [PubMed] [Google Scholar]
- Sunkel CE, Whittle JRS. Brista: A gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Roux’s Archives of Developmental Biology. 1987;196:124–132. doi: 10.1007/BF00402034. [DOI] [PubMed] [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- Werner T, Hammer A, Wahlbuhl M, Bosl MR, Wegner M. Multiple conserved regulatory elements with overlapping functions determine Sox10 expression in mouse embryogenesis. Nucl. Acids Res. 2007;35:6526–6538. doi: 10.1093/nar/gkm727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.