Summary
Cogent evidence points to the involvement of neurosteroids in the regulation of dopamine (DA) neurotransmission and signaling, yet the neurobiological bases of this link remain poorly understood. We previously showed that inhibition of 5α-reductase (5αR), a key neurosteroidogenic enzyme, attenuates the sensorimotor gating deficits induced by DA receptor activation, as measured by the prepulse inhibition (PPI) of the acoustic startle reflex. To extend these findings, the present study was aimed at the assessment of the role of other key neurosteroidogenic enzymes in PPI, such as 17α-hydroxylase/C17,20 lyase (CYP17A1), 3α- and 3β-hydroxysteroid dehydrogenase (HSD), in Sprague-Dawley rats. The PPI deficits induced by the DAergic non-selective agonist apomorphine (APO, 0.25 mg/kg, SC) were dose-dependently attenuated by the selective CYP17A1 inhibitor abiraterone (ABI, 10-50 mg/kg, IP) in a fashion akin to that of the 5αR inhibitor finasteride (FIN, 100 mg/kg, IP). These systemic effects were reproduced by intracerebroventricular injection of ABI (1 μg/1 μl), suggesting the involvement of brain CYP17A1 in PPI regulation. Conversely, the PPI disruption induced by APO was not significantly affected by the 3α- and 3β-HSD inhibitors indomethacin and trilostane. Given that CYP17A1 catalyzes androgen synthesis, we also tested the impact on PPI of the androgen receptor (AR) antagonist flutamide (10 mg/kg, IP). However, this agent failed to reverse APO-induced PPI deficits; furthermore, AR endogenous ligands testosterone and dihydrotestosterone failed to disrupt PPI. Collectively, these data highlight CYP17A1 as a novel target for antipsychotic-like action, and suggest that the DAergic regulation of PPI is modulated by androgenic neurosteroids, through AR-unrelated mechanisms.
Keywords: 17α-hydroxylase/C17,20 lyase; dopamine; prepulse inhibition of the startle; neurosteroidogenesis; androgen receptors
1. Introduction
Cogent evidence indicates that several neuropsychiatric disorders are contributed by the excessive activation of dopamine (DA) receptors, including schizophrenia and Tourette syndrome (TS). These disorders, albeit markedly different, share common endophenotypic deficits that have been linked to DAergic hyperactivity, such as impairments of sensorimotor gating and salience mapping (Braff and Geyer, 1990). Notably, these information-processing deficits may play a critical role in the pathophysiology of both disorders, as they interfere with the pre-attentional filtering of perceptual stimuli and the enactment of adaptive responses.
One of the most reliable operational paradigms for the measurement of sensorimotor gating mechanisms is the prepulse inhibition (PPI) of the acoustic startle reflex (ASR). This index is defined as the reduction of the ASR that occurs when the eliciting stimulus is preceded by a weak, non-startling prestimulus (Hoffman and Ison, 1980). PPI is typically impaired in schizophrenia and TS patients (Braff et al., 2001); the neurobiological relation of PPI to these disorders is supported by several lines of evidence: for example, this index is regulated by cortico-striatal-thalamo-cortical (CSTC) loops that have been implicated in both disorders (Swerdlow et al., 2001). In addition, activation of DA receptors in rodents produces marked reductions of PPI (Geyer et al., 2001), which are typically prevented by antipsychotic drugs.
Well-established evidence has shown that both schizophrenia and TS are characterized by a pronounced male predominance (Santangelo et al., 1994; Häfner, 2003). Although PPI deficits are encountered in both genders, this index is differentially modulated in males and females (Lehmann et al., 1999; Kumari et al., 2004). Androgenic steroids may participate in these sex-related variations. Indeed, androgenic steroids have been shown to exert a complex regulation of DA signaling and neurotransmission (Di Paolo, 1994; Sánchez et al., 2010), potentially suggesting that sex-related divergences in neurosteroid and androgen synthesis may contribute to the gender differences in schizophrenia and TS and PPI modulation.
Our group has recently shown that the pharmacological inhibition of 5α-reductase (5αR), the key rate-limiting enzyme in NS and androgen metabolism (Martini et al., 1993; Martini et al., 1996; Paba et al., 2011), counters several behavioral effects of non-selective DAergic receptor agonists in Sprague-Dawley rats, such as the deficits in sensorimotor gating (Bortolato et al., 2008; Devoto et al., 2012). In addition, we have documented that the anti-DAergic actions of the prototypical 5αR inhibitor finasteride (FIN), albeit strikingly akin to those induced by classical antipsychotic agents, are not accompanied by catalepsy (Bortolato et al., 2008). These results have been supported by preliminary clinical observations documenting the efficacy and high tolerability of FIN in adult male, treatment-refractory patients affected by TS (Bortolato et al., 2007; Muroni et al., 2011) and chronic schizophrenia (Koethe et al., 2008).
These premises highlight that the enzymatic machinery for synthesis, metabolism and signaling of androgenic neurosteroids may be a very promising source of novel therapeutic options for schizophrenia and TS. To fully explore this issue, in the present study we analyzed whether the PPI disruption induced by the DAergic receptor agonist apomorphine (APO) may be affected by the blockade of three critical enzymes in neurosteroidogenesis and androgen synthesis (Fig. 1), 17α-hydroxylase/C17,20 lyase (CYP17A1), 3α- and 3β-hydroxysteroid dehydrogenase (HSD), as well as androgen receptors (ARs).
Figure 1.
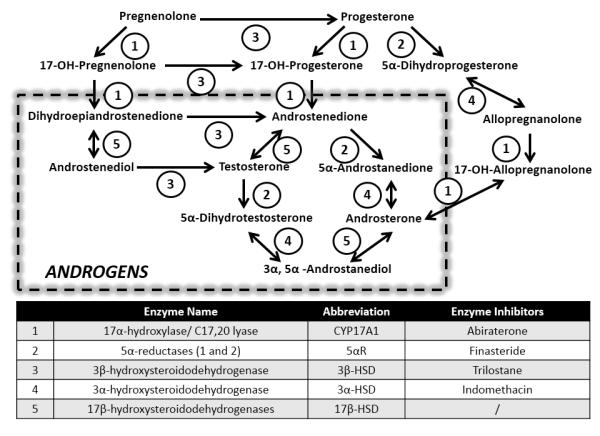
Schematic view of main steroid pathways of androgen synthesis and metabolism. The numerical codes represent the enzymes described in the table. Listed are all enzyme inhibitors used in the present study.
2. Materials and Methods
2.1. Animals
A total of 327 male Sprague—Dawley (Harlan, Italy) rats weighing 250— 300 g were used for this study. Animals were group-housed in cages (n=4) with ad libitum access to food and water. The room was maintained at 22±0.2°C on a 12/12-h dark/light cycle (with lights off at 1900h). Each animal was used only once throughout the study and all efforts were made to minimize animal suffering throughout this study. All experimental procedures were executed in compliance with the National Institute of Health guidelines and approved by the local Animal Use Committees.
2.2. Drugs
The following drugs were used: abiraterone (ABI), finasteride (FIN), testosterone (T), dihydrotestosterone (DHT), flutamide (FLU), trilostane (TRI), indomethacin (INDO) and (R)-(−)-Apomorphine hydrochloride (APO). For systemic injections, ABI, FIN, T, DHT, FLU, TRI, INDO were suspended in a vehicle (VEH) solution containing 5% Tween 80 and 95% saline (SAL; 0.9% NaCl). For intracerebral infusions, ABI was dissolved in hydroxypropyl-β-cyclodextrin/Ringer solution (CDX/R). APO was dissolved in 0.9% SAL containing 0.1% (v/v) of ascorbic acid to prevent oxidation. All solutions were freshly prepared on the day of testing and administered subcutaneously (SC) and intraperitoneally (IP) in an of injection volume of 1 and 2 ml/kg body weight, respectively. The doses of each compound were selected based on preliminary data or on previous reports indicating their biological and behavioral efficacy (Nayebi and Rezazadeh, 2004; Ugale et al, 2004; Ahboucha et al, 2008).
2.3. Stereotaxic surgery and intracerebroventricular (ICV) Infusion procedures
Rats were anaesthetized with Equithesin and placed in a stereotaxic apparatus (Kopf, Tujunga, CA), with blunt ear bars to avoid damage of the tympanic membranes. Under aseptic conditions, rats were shaved and their scalp was retracted. Bilateral craniotomies were performed above the target sites, and stainless steel 22-G guide-cannulae (Plastics One, Roanoke, VA) were lowered slowly into place and implanted using dental cement and two skull screws. The lengths of the cannulae were selected so as to end 1 mm above the targeted areas with the corresponding injector projecting 1 mm beyond guide tip. Cannulae were plugged with wire stylets, and wounds were closed with surgical staples. According to the stereotaxic brain atlas (Paxinos and Watson, 1998), the stereotaxic coordinates for lateral ventricles were: AP = −1 mm, ML = ±1 mm; DV = −3 mm, referring to bregma. Rats were given antibiotic therapy for 5 days (enrofloxacin, Bayer HealthCare, Shawnee Mission, KS) and allowed to recover in their home cages for 7-10 days prior to testing. The day of the test, rats were subjected to ICV administrations through 33-gauge internal cannulae (Plastics One) connected to a 50 μl syringe (Hamilton, Reno, NV, USA) by PE tubing (Intramedic, New York, NY, USA). The rate of infusion (0.5 μl/min) was controlled by microinjection pumps (CMA Microdialysis, Stockholm, Sweden). Injections were confirmed by monitoring movement of liquid in the tubing via a small air bubble. The injectors were left in place for 2 min after infusion, to fully allow diffusion of fluid. PPI testing took place immediately after completion of infusion. On completion of testing, the location of cannula tips was verified by injecting 5 μl of methylene blue dye into the cannula. Rats were euthanized after 5 min and their brain was removed and sliced at the point of cannula entry. Trained operators assessed the diffusion of the dye through the target ventricle into both hemispheres.
2.4. Startle reflex and PPI
Startle and PPI testing were performed as previously described (Bortolato et al., 2004), between 10 AM and 4 PM. Rats were placed in the PPI chamber for a 5-min acclimatization period consisting of 70 dB background white noise, which continued for the remainder of the entire session. The PPI protocol included three consecutive blocks of pulse, pre-pulse+pulse and “no-stimulus” trials. Specifically, unlike the first and the third block, during which rats received only five pulse-alone trials of 115 dB, throughout the second block rats were exposed to a pseudorandom sequence of 50 trials, consisting of 12 pulse-alone trials, 30 trials of pulse preceded by 74, 78 or 82 dB pre-pulses intensities (ten for each level of prepulse loudness) and eight “no stimulus” trials, where the only background noise was delivered. Intertrial intervals were selected randomly between 10 and 15 s. Sound levels were assessed using an A Scale setting. Percent PPI was calculated with the following formula:
with and representing the mean startle amplitudes for all pre-pulse+pulse trials and pulse alone trials, respectively. The first 5 pulse-alone bursts were excluded from the calculation. Except for the intracerebroventricular (ICV) microinjection experiment, all rats received two systemic injections: a pretreatment IP 40 min and a treatment SC immediately before starting PPI session.
2.5. Data analysis
Normality and homoscedasticity of data distribution were verified by using the Kolmogorov-Smirnov and Bartlett’s tests. Analyses were performed by multiple-way ANOVAs, as appropriate, followed by Tukey’s test (with Spjøtvoll-Stoline correction for unequal N whenever required) for post-hoc comparisons of the means. For % PPI analyses, main effects for prepulse levels were consistently found throughout the study, indicating loudness-dependent effects. However, since no interactions between prepulse levels and other factors were found, data relative to different prepulse levels were collapsed. Significance threshold was set at 0.05.
3. Results
3.1 Effects of ABI and FIN on ASR and PPI parameters
The first experiment was aimed at investigating the impact of the selective CYP17A1 inhibitor ABI (10-50 mg/kg, IP), on the effects on startle and PPI mediated by APO (0.25 mg/kg, SC), in comparison with the 5αR inhibitor FIN (100 mg/kg, IP). Neither ABI pretreatment [F(3,67)=1.20, NS] nor APO treatment [F(1,67)=0.16, NS] had significant main effects on startle amplitude. Furthermore, ANOVA did not reveal any significant ABI x APO interactions [F(3, 67)=0.61, NS] (Fig. 2A). In contrast with ABI, FIN significantly reduced baseline startle amplitude [main effect of pretreatment: F(1,35)=32.85, P<0.001]. The combination of FIN and APO, however, did not produce significant effects on startle parameters [pretreatment x treatment interaction: F(1,35)=1.03, NS]. The analysis of PPI revealed a significant pre-treatment x treatment interaction, indicating that the dose of 25 mg/kg of ABI significantly reduced the PPI-disruptive properties of APO {[F(3,67)=3.16, P<0.05]; P<0.05 for comparison VEH+APO vs ABI 25+APO; Tukey’s}, albeit only partially (Ps<0.01 for all comparisons between SAL- and APO-treated rats) (Fig. 2B). In line with our previous results, FIN significantly countered APO-mediated PPI disruption [F(1,35)=4.33, P<0.05] (Fig. 2B).
Figure 2.
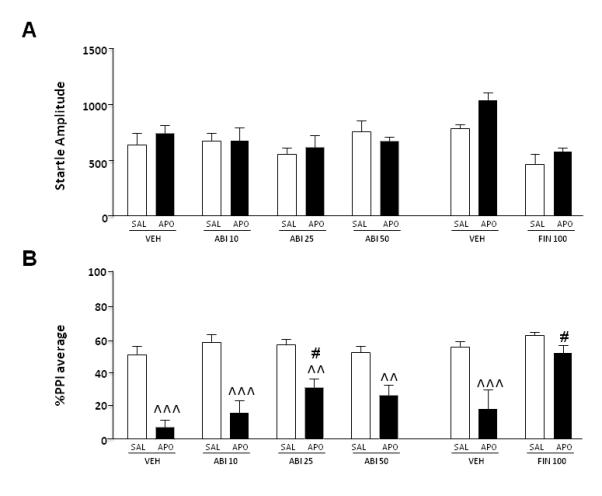
Effects of systemic finasteride (FIN, 100 mg/kg, IP) and abiraterone (ABI, 10-50 mg/kg, IP) on startle reflex (A) and %PPI (B) in relation to the gating deficits induced by systemic apomorphine (APO, 0.25 mg/kg, SC). FIN and ABI doses are indicated in mg/kg. VEH, vehicle of FIN and ABI; SAL, saline. Values are expressed as mean ± S.E.M. N= 8-14/group. ^^, P<0.01; ^^^, P<0.001 vs rats treated with the same pre-treatment and SAL (pretreatment x treatment interaction); #, P<0.05 vs rats treated with VEH and APO (pretreatment x treatment interaction). Main effects are not indicated; for further details, see text.
To verify the involvement of brain structures in the PPI-ameliorating properties of systemic ABI, we studied the effects of ICV injections of this compound (1 μg/1 μl) on startle reflex and PPI parameters. Startle analyses did not disclose significant main effects of ABI [F(1, 30)=3.80, NS] or APO [F(1,30)=0.46, NS]. The interaction of the two treatments also failed to affect startle amplitude [F(1,30)=0.41, NS] (Fig. 3A). In addition, we found a significant pre-treatment x treatment interaction with respect to PPI values, indicating that ICV ABI infusions significantly ameliorated APO-mediated PPI deficits [F(1, 30)=3.63, P<0.05] (Fig. 3B).
Figure 3.
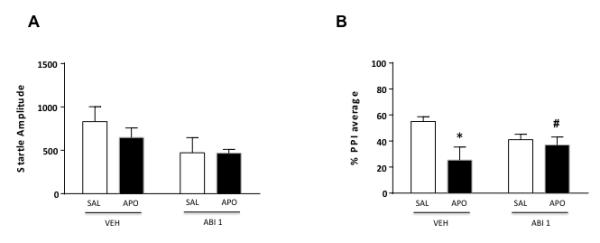
Effects of intracerebroventricular abiraterone (ABI, 1 μg/μl) on startle reflex (A) and %PPI (B) in relation to the gating deficits induced by systemic apomorphine (APO, 0.25 mg/kg, SC). ABI doses are indicated in μg (in 1μl of solution). VEH, vehicle of ABI (Hydroxypropyl β-Cyclodextrin/Ringer solution, 20% w/v). Values are expressed as mean ± S.E.M, N= 8-9/group. *, P<0.05 vs rats treated with VEH and saline (pretreatment x treatment interaction); #, P<0.05 vs rats treated with VEH and APO. For further details, see text.
3.2 Effects of INDO on startle and PPI parameters
In the third set of experiments, we tested the effects of the 3α-HSD inhibitor INDO (5 mg/kg, IP), on the PPI disruption mediated by apomorphine (APO, 0.25 mg/kg, SC). The analysis of startle amplitude revealed a significant interaction between pretreatment and treatment [F(1, 31)=5.47, P<0.05] (Fig. 4A). Post-hoc comparisons disclosed that this effect reflected a statistical trend for a difference between INDO+SAL vs INDO+APO (P<0.10; Tukey’s test).
Figure 4.
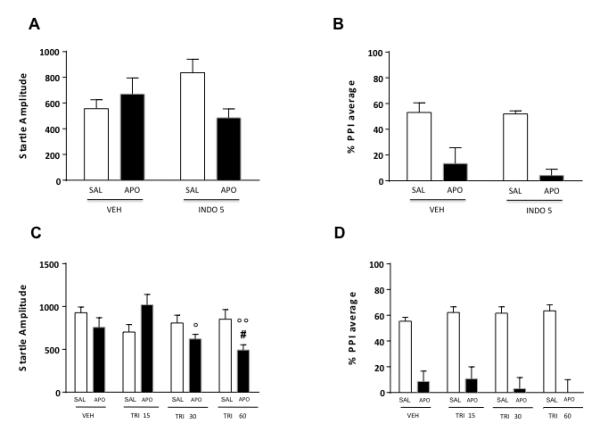
Effects of systemic indomethacin (INDO, 5 mg/kg, IP) and trilostane (TRI, 15-60 mg/kg, IP) on startle reflex (A-C) and %PPI (B-D) in relation to the gating deficits induced by systemic apomorphine (APO, 0.25 mg/kg, SC). INDO and TRI doses are indicated in mg/kg. VEH, vehicle of TRI and INDO; SAL, saline. Values are expressed as mean ± S.E.M., N= 7-17/group. #, P<0.05 vs rats treated with VEH and APO; °, P<0.05, °°, P<0.01 vs rats treated with TRI 15 and APO (pretreatment x treatment interaction). Main effects are not indicated. For further details, see text.
PPI analyses disclosed a significant main effect for APO [F(1, 31)=37.52, P<0.001], but not for INDO [F(1, 31)=0.53, NS] treatment. Finally, no significant pretreatment x treatment interactions were found [F(1, 31)=0.31, NS], indicating that INDO was not able to counter the PPI disruption mediated by APO (Fig. 4B).
3.3 Effects of TRI on startle and PPI parameters
To investigate the role of 3β-HSD in the DAergic regulation of PPI, we then measured the impact of different doses of TRI (15-60 mg/kg, IP), the selective inhibitor of this enzyme, in association with APO (0.25 mg/kg, SC). The analysis of startle amplitude detected a significant pretreatment x treatment interaction [F(3,72)=4,56, P<0.01] (Fig. 4C). Post-hoc analyses evidenced that the combination of APO and the higher dose of TRI significantly decreased startle amplitude in comparison with the association of VEH and SAL (P<0.05, Tukey’s). PPI analyses did not reveal a significant main effect for pretreatment [F(3, 72)=0.19, NS]. As expected, however, APO produced a marked PPI deficit [main treatment effect F(1, 72)=109.2, P<0.001], which was not reversed by TRI pretreatment [pretreatment x treatment interaction: F(3,72)=0.64, NS] (Fig. 4D).
3.4 Effects of AR ligands on startle and PPI parameters
For the evaluation of the role of AR on startle reflex and gating functions, we studied the effect of its natural ligands (T and DHT; 100 mg/kg, IP) and antagonist (FLU, 10 mg/kg, IP). As shown in Fig. 5A, startle amplitude was not modified by either T pretreatment [F(1,22)=0.81, NS] or APO treatment [F(1,22)=0.04, NS]. A statistical trend was identified for pretreatment x treatment interaction [F(1,22)=3.34; P=0.07]. PPI analyses (Fig. 5B) revealed that T did not modify PPI, while APO significantly reduced gating [F(1,22)=26.08, P<0.001], even though this effect was not prevented by T pretreatment [F(1, 22)=0.01, NS].
Figure 5.
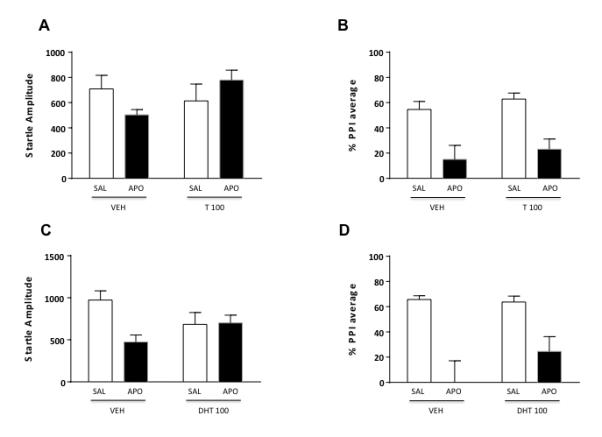
Effects of systemic testosterone (T, 100 mg/kg, IP) and dihydrotestosterone (DHT, 100 mg/kg, IP) on startle reflex (A-C) and %PPI (B-D) in relation to the gating deficits induced by systemic apomorphine (APO, 0.25 mg/kg, SC). VEH, vehicle of T and DHT; SAL, saline. Values are expressed as mean ± S.E.M. N= 6-10/group. Main effects are not indicated. For further details, see text.
The systemic effects of DHT (Fig. 5C-D) were similar to those observed after T injection, with no overall effects on startle amplitude [F(1,27)=0.05, NS]. In addition, statistical trends were found for the main effect of APO [F(1,27)=3.38, P=0.07] and for pretreatment x treatment interactions [F(1,27)=3.87, P=0.06]. Furthermore, DHT did not significantly affect PPI [F(1, 27) = 0.05, NS], while APO significantly reduced this parameter [F(1, 27)=36.33, P<0.001]. Finally, the administration of DHT was not able to reverse APO-mediated PPI impairments [F(1, 27)=2,46, NS].
The last series of experiments investigated the effects of AR blockade by systemic injections of FLU, on startle and PPI parameters. ANOVA detected a significant pretreatment x treatment interaction on startle amplitude [F(1, 28)=30.82, P<0.001] (Fig. 6A). Post-hoc analyses conducted with Tukey’s test revealed significant differences between VEH+SAL and FLU+SAL (P<0.001), VEH+APO and FLU+SAL, (P<0.01), VEH+APO and FLU+APO (P<0.05), FLU+SAL and FLU+APO (P<0.001). Finally, PPI analysis detected a significant main effect for APO treatment [F(1, 28)=31.30, P<0.001], but not for FLU pretreatment [F(1, 28)=0.20, NS]. No pretreatment x treatment interactions were found [F(1, 28)=0.72, NS] (Fig. 6B).
Figure 6.
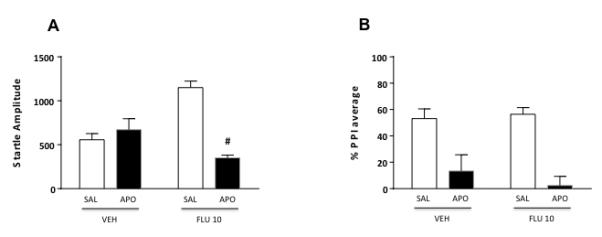
Effects of systemic flutamide (FLU, 10mg/kg, IP) on startle reflex (A) and %PPI (B) in relation to the gating deficits induced by systemic apomorphine (APO, 0.25 mg/kg, SC). VEH, vehicle of FLU; SAL, saline. Values are expressed as mean ± S.E.M. N= 8/group. #, P<0.05 vs rats treated with VEH and APO. Main effects are not indicated. For further details, see text.
4. Discussion
The major result of this study is that ABI, the selective CYP17A1 inhibitor, led to a dose-dependent reversal of the PPI deficits induced by the direct DA receptor agonist APO. Notably, these effects were observed after both systemic and ICV injections, and were not paralleled by changes in startle amplitude. CYP17A1 is a member of the cytochrome P450 enzyme family, responsible for the biosynthesis of androgens from C21 steroids (such as progesterone and pregnenolone; Fig.1). The enzyme catalyzes two separate reactions, consisting in the hydroxylation of the 17α terminal (17α-hydroxylase) and the cleavage of the bond between carbons 17 and 20 (C17,20 lyase), respectively (for a review on the enzyme, see Porubek, 2013). In particular, the latter component is responsible for the synthesis of androgenic steroids; accordingly, males with a selective C17,20 lyase deficiency exhibit reduced sex hormone levels and severe undervirilization (Miller, 2012). These premises, together with our previous evidence on the antipsychotic-like actions of 5αR inhibitors (Bortolato et al., 2008; Devoto et al., 2012), point to the implication of androgen synthesis in the modulation of DAergic control of information processing. In addition, these data may help account for the male predominance of schizophrenia, TS and other neuropsychiatric disorders featuring gating deficits and DAergic perturbations.
The effects of ICV infusion of ABI were comparable with those ensuing systemic treatment, suggesting the involvement of brain CYP17A1 in the antipsychotic-like effects of ABI. Although CYP17A1 expression is most abundant in the adrenal cortex, this enzyme has also been documented in several brain regions by immunoreactivity, such as hippocampus, hypothalamus, brainstem and cerebellum (Stromstedt and Waterman, 1995; Yamada et al., 1997; Kohchi et al., 1998; Hojo et al., 2004). In addition, in situ hybridization studies have documented the presence of CYP17A1 mRNA across key regions for the DAergic regulation of PPI, such as the cortex, striatum and hippocampus (Allen Atlas; http://mouse.brain-map.org/gene/show/12855; Swerdlow et al, 2001). While our experiments do not allow for a characterization of the specific brain regions involved in the anti-DAergic effects of ABI, it is worth noting that APO-induced PPI deficits are supported by multiple areas of the CSTC circuitry, including the nucleus accumbens core, medial prefrontal cortex and ventral subiculum (Hart et al., 1998; Swerdlow et al., 2001).
Our results showed that ABI and FIN elicited analogous effects on PPI regulation, although the effects of the latter drug were more marked. It is possible that the antipsychotic-like properties of these agents may be partially supported by similar substrates. With respect to this hypothesis, it is worth noting that the systemic effects of FIN were reproduced by local injections into the shell and core of the nucleus accumbens, but not other brain regions (Devoto et al., 2012). These findings suggest that the nucleus accumbens may be responsible for the contribution of neurosteroids in sensorimotor gating modulation, and point to the implication of this region in the antipsychotic-like properties of ABI.
The possibility that ABI and FIN elicit similar effects is particularly interesting, in view of the translational potential of our results. Indeed, our preclinical findings on the antipsychotic-like properties of FIN (Bortolato et al., 2008) were matched by therapeutic effects of this agent in patients affected by Tourette syndrome (Bortolato et al., 2007; Muroni et al., 2011) as well as schizophrenia (Koethe et al., 2008) and other disorders (Paba et al., 2011). Notably, ABI is already approved for clinical use as a chemotherapeutic agent for the treatment of castration-resistant prostate cancer (Attard et al., 2009; de Bono et al., 2011).
Inhibitions of 5αR and CYP17A1 share a number of similar outcomes with respect to neurosteroidogenesis. Indeed, both enzymes lead to a significant reduction of 5α-reduced androstane derivatives, such as androsterone and 5α-androstanedione. It is worth noting that the latter compound is the metabolic product of the combined enzymatic actions of 5αR and CYP17A1 on progesterone, and may therefore be directly implicated in the anti-DAergic effects of FIN and ABI. Furthermore, the greater antipsychotic-like effect of FIN in comparison with ABI suggests that sensorimotor gating may be partially regulated by nonandrogenic neurosteroids (not directly affected by ABI), such as allopregnanolone. Accordingly, recent studies support the implication of this compound in PPI modulation (Darbra and Pallares, 2010; Darbra et al, 2012; 2013). Nevertheless, the lack of neurochemical analyses in the present study does not allow us to fully exclude that the anti-DAergic effects of these antiandrogenic drugs may be supported by differential underpinnings; indeed, only FIN produced a significant reduction of startle amplitude, likely reflecting partially divergent mechanisms of the two drugs on behavioral reactivity.
We found that the inhibition of 3α-HSD and 3β-HSD by INDO and TRI, respectively, did not elicit significant effects on sensorimotor gating. Both enzymes have been described in the brain of humans and rodents (Dupont et al., 1994; Khanna et al., 1995a; 1995b; Yu et al., 2002), where they are posited to play key roles in neurosteroid biosynthesis, by catalyzing the conversion of Δ5-hydroxysteroids into Δ4-ketosteroids. Nevertheless, it should be noted that the failure to elicit effects may signify that INDO and TRI are competitive inhibitors and their activity may therefore be limited by substrate accumulation. Thus, a conclusive assessment of the role of 3α-HSD and 3β-HSD in PPI regulation awaits the development of novel, highly selective non-competitive inhibitors for these enzymes.
The lack of effects of FLU suggests that the effects of androgens in PPI might not be mediated by AR. The existence of membrane-bound receptors has been postulated by several authors, based on the ability of androgens to rapidly modulate the activity of ion channels and intracellular calcium levels (Heinlein and Chang, 2002). Non-genomic effects are likely mediated through membrane androgen receptors or act through SHBG or the c-Src kinase-AR complex. Interestingly, a human membrane receptor for progesterone has been cloned (Gerdes et al., 1998; Bernauer et al., 2001). Furthermore, new membrane receptors for other steroid hormones have been recently identified (Fernández-Pérez et al., 2008).
Notably, FLU has been used as a potential therapy for Tourette syndrome (Peterson et al., 1998), yielding very limited and short-lived effects. Thus, while both FIN and FLU are generally labeled as “anti-androgenic” drugs, the contrast in effects between these two agents may reveal a greater implication of 5AR in TS pathophysiology. Future double-blind, placebo-controlled clinical trials on FLU will help settle this interesting issue.
Systemic administrations of both T and DHT did not exert any intrinsic PPI-disrupting properties, in further support that these potent activators of AR may not be directly responsible for the effects of FIN and ABI on DAergic responses. Interestingly, our data complement previous results by Van den Buuse and Eikelis (2001), who documented that, in female rats, T and estradiol, but not DHT, enhanced PPI. These divergent effects suggest the existence of sex-specific differences in the metabolic fate of T, which may result in different roles of this steroid with respect to the regulation of PPI. In addition, our results do not exclude the possibility that, in males, T may still play a facilitatory role for DAergic impairments. Acute T has been reported to increase DA levels in the neostriatum and in the nucleus accumbens (de Souza Silva et al., 2009), while chronic T may increase DA metabolism and turnover, but not content (Thiblin et al., 1999). In addition, recent findings indicate that T and DHT increase the expression of biosynthetic and metabolic DA enzymes in the substantia nigra of male rats (Purves-Tyson et al., 2012). Future studies are warranted to analyze whether the combination of these steroids with sub-threshold doses of DA releasers (such as d-amphetamine) may result in PPI impairments.
Although the inability of T, DHT and FLU to affect APO-induced PPI deficits may seemingly contradict our findings on the anti-DAergic properties of ABI and FIN, it should be noted that, unlike T and DHT, most 5α-reduced androgenic neurosteroids do not exert their neuroactive properties through ARs. For example, 3β,5α-androstanediol has been shown to be a potent agonist of β estrogen receptors (Kuiper et al., 1997), while 3α,5α-androstanediol acts as a positive modulator of GABA-A receptors (Reddy and Jian, 2011). Furthermore, androsterone has been shown to activate farnesoid receptors (Wang et al., 2006). The implication of these receptors in information processing awaits further investigations.
Several limitations in the present study should be acknowledged. First, our analyses were restricted to males, thereby limiting our ability to predict whether the observed antipsychotic-like effects of CYP17A1 inhibition may be applicable to females. Second, another important caveat of our study lies in the lack of available selective 3α- and 3β-HSD inhibitors. Indeed, both INDO and TRI are known to elicit their actions also through other mechanisms, namely the inhibition of prostaglandin synthesis and the activation of β-estrogen receptors, respectively (Vane and Botting, 1998; Espallergues et al., 2011). Third, the conclusions that INDO and FLU did not protect from the APO-induced PPI deficits are limited by the lack of dose-response curves; although the doses for both compounds were selected based on a careful literature search, we cannot completely rule out that higher concentrations (or chronic treatments) may have elicited ameliorative effects in sensorimotor gating. Fourth, our interpretation of the reported effects of neurosteroidogenic inhibitors on startle and PPI is restricted by the lack of accompanying measurements of their effects on steroid profile; nevertheless, it should be clarified that this limitation may not be easily overcome at present, since the levels of most neurosteroids (particularly among 3α,5α- and 3β,5α-androstane derivatives) cannot be accurately detected in rat brain regions, due to their relatively low content.
Irrespective of the specific mechanisms, the present set of results point to CYP17A1 as a potential target for certain behavioral disorders characterized by gating disturbances, such as schizophrenia and TS. In particular, following our discovery of 5αR inhibitors as antipsychotic-like agents using the rat model of APO-induced PPI disruption, we have pursued the possibility that 5αR inhibitors may have therapeutic efficacy for these conditions (Bortolato et al., 2007; Koethe et al., 2008; Muroni et al., 2011). Following this perspective, it is possible that CYP17A1 inhibitors may be novel therapeutic avenues for mental disorders featuring alterations of sensorimotor gating. In particular, this possibility is highlighted by the recent development of drugs that inhibit more specifically C17,20 lyase and spare 17α-hydroxylase, such as orteronel (TAK-700; Hara et al., 2013). These next-generation drugs, which induce fewer side effects due to the lack of effects on glucocorticoid synthesis, may have important implications in the therapy of psychiatric disorders.
Acknowledgments
We are grateful to Alessandra Pardu for her technical assistance.
The present study was supported by National Institute of Health grant R21HD070611 (to MB), Research Grant from the Tourette Syndrome Association (to MB and PD), Bank of Sardinia Foundation (BSF) and Autonomous Region of Sardinia (ARS) (to PD, RF and VB). This study was also partially supported by sub-awards (to MB) from the NIH grants P20 GM103638 and UL1 TR000001 (formerly UL1RR033179), awarded to the University of Kansas and University of Kansas Medical Center. We are grateful to Alessandra Pardu for her valuable support in the execution of the experiments. No conflicts of interest were declared by any authors. None of the institutions had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of interest The Authors certify that there is no actual or potential conflict of interest in relation to this article.
Contributors RF designed the experiments, monitored data collection, analyzed behavioral data and performed statistical analyses, drafted and revised the manuscript. VB, performed behavioral tests and statistics. RP, GP and PS performed the behavioral tests. PD discussed and revised the paper. MB supervised the experimental design and execution, monitored data collection, performed statistical analyses, wrote and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahboucha S, Jiang W, Chatauret N, Mamer O, Baker GB, Butterworth RF. Indomethacin improves locomotor deficit and reduces brain concentrations of neuroinhibitory steroids in rats following portacaval anastomosis. Neurogastroenterol Motil. 2008;20:949–57. doi: 10.1111/j.1365-2982.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–40. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- Bernauer S, Wehling M, Gerdes D, Falkenstein E. The human membrane progesterone receptor gene: genomic structure and promoter analysis. DNA Seq. 2001;12:13–25. doi: 10.3109/10425170109042047. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Aru GN, Orru M, Gessa GL. Baclofen reverses the reduction in prepulse inhibition of the acoustic startle response induced by dizocilpine, but not by apomorphine. Psychopharmacology. 2004;171:322–330. doi: 10.1007/s00213-003-1589-5. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette’s syndrome with finasteride. Am J Psychiatry. 2007;164:1914–1915. doi: 10.1176/appi.ajp.2007.07060978. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru M, Bourov Y, Marrosu F, Mereu G, Devoto P, Gessa GL. Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology. 2008;33:3146–3156. doi: 10.1038/npp.2008.39. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Darbra S, Mòdol L, Pallarès M. Allopregnanolone infused into the dorsal (CA1) hippocampus increases prepulse inhibition of startle response in Wistar rats. Psychoneuroendocrinology. 2012;37:581–585. doi: 10.1016/j.psyneuen.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Darbra S, Mòdol L, Vallée M, Pallarès M. Neonatal neurosteroid levels are determinant in shaping adult prepulse inhibition response to hippocampal allopregnanolone in rats. Psychoneuroendocrinology. 2013;38:1397–1406. doi: 10.1016/j.psyneuen.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Darbra S, Pallarès M. Alterations in neonatal neurosteroids affect exploration during adolescence and prepulse inhibition in adulthood. Psychoneuroendocrinology. 2010;35:525–535. doi: 10.1016/j.psyneuen.2009.08.020. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Silva MA, Mattern C, Topic B, Buddenberg TE, Huston JP. Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. Eur Neuropsychopharmacol. 2009;19:53–63. doi: 10.1016/j.euroneuro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Devoto P, Frau R, Bini V, Pillolla G, Saba P, Flore G, Corona M, Marrosu F, Bortolato M. Inhibition of 5alpha-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2012;37:1630–1645. doi: 10.1016/j.psyneuen.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Dupont E, Simard J, Luu-The V, Labrie F, Pelletier G. Localization of 3 beta-hydroxysteroid dehydrogenase in rat brain as studied by in situ hybridization. Mol Cell Neurosci. 1994;5:119–123. doi: 10.1006/mcne.1994.1014. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Temsamani J, Laruelle C, Urani A, Maurice T. The antidepressant-like effect of the 3β-hydroxysteroid dehydrogenase inhibitor trilostane involves a regulation of β-type estrogen receptors. Psychopharmacology. 2011;214:455–63. doi: 10.1007/s00213-010-2053-y. [DOI] [PubMed] [Google Scholar]
- Fernández-Pérez L, Flores-Morales A, Chirino-Godoy R, Díaz-Chico JC, Díaz-Chico BN. Steroid binding sites in liver membranes: interplay between glucocorticoids, sex steroids, and pituitary hormones. J. Steroid Biochem. Mol. Biol. 2008;109:336–43. doi: 10.1016/j.jsbmb.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379:907–911. doi: 10.1515/bchm.1998.379.7.907. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hara T, Kouno J, Kaku T, Takeuchi T, Kusaka M, Tasaka A, Yamaoka M. Effect of a novel 17,20-lyase inhibitor, orteronel (TAK-700), on androgen synthesis in male rats. J. Steroid Biochem. Mol. Biol. 2013;134:80–91. doi: 10.1016/j.jsbmb.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Hart S, Zreik M, Carper R, Swerdlow NR. Localizing haloperidol effects on sensorimotor gating in a predictive model of antipsychotic potency. Pharmacol. Biochem. Behav. 1998;61:113–119. doi: 10.1016/s0091-3057(98)00079-3. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna M, Qin KN, Cheng KC. Distribution of 3 alpha-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J Steroid Biochem Mol Biol. 1995a;53:41–46. doi: 10.1016/0960-0760(95)00019-v. [DOI] [PubMed] [Google Scholar]
- Khanna M, Qin KN, Wang RW, Cheng KC. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3 alpha-hydroxysteroid dehydrogenases. J. Biol. Chem. 1995b;270:20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- Koethe D, Bortolato M, Piomelli D, Leweke FM. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry. 2008;41:115–116. doi: 10.1055/s-2008-1058110. [DOI] [PubMed] [Google Scholar]
- Kohchi C, Ukena K, Tsutsui K. Age- and region-specific expressions of the messenger RNAs encoding for steroidogenic enzymes p450scc, P450c17 and 3beta-HSD in the postnatal rat brain. Brain Res. 1998;801:233–238. doi: 10.1016/s0006-8993(98)00585-x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr. Res. 2004;69:219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav. Brain Res. 1999;104:113–117. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Martini L, Celotti F, Melcangi RC. Testosterone and progesterone metabolism in the central nervous system: cellular localization and mechanism of control of the enzymes involved. Cell Mol Neurobiol. 1996;16:271–282. doi: 10.1007/BF02088095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL. P450 oxidoreductase deficiency: a disorder of steroidogenesis with multiple clinical manifestations. Sci. Signal. 2012;5:11. doi: 10.1126/scisignal.2003318. [DOI] [PubMed] [Google Scholar]
- Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord. 2011;26:2146–2147. doi: 10.1002/mds.23810. [DOI] [PubMed] [Google Scholar]
- Nayebi AR, Rezazadeh H. Involvement of serotoninergic mechanism in analgesia by castration and flutamide, a testosterone antagonist, in the rat formalin test. Pharmacol Biochem Behav. 2004;77:9–14. doi: 10.1016/j.pbb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M. Steroid 5alpha-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des. 2011;17:151–167. doi: 10.2174/138161211795049589. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Peterson BS, Zhang H, Anderson GM, Leckman JF. A double-blind, placebo-controlled, crossover trial of an antiandrogen in the treatment of Tourette’s syndrome. J Clin Psychopharmacol. 1998;18:324–331. doi: 10.1097/00004714-199808000-00013. [DOI] [PubMed] [Google Scholar]
- Porubek D. CYP17A1: A Biochemistry, Chemistry, and Clinical Review. Curr. Top. Med. Chem. 2013;13:1364–1384. doi: 10.2174/1568026611313120002. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Handelsman DJ, Double KL, Owens SJ, Bustamante S, Weickert CS. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 2012 doi: 10.1186/1471-2202-13-95. doi: 10.1186/1471-2202-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J. Pharmacol. Exp. Ther. 2011;334:1031–41. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:43–71. doi: 10.1111/j.1755-5949.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo SL, Pauls DL, Goldstein JM, Faraone SV, Tsuang MT, Leckman JF. Tourette’s syndrome: what are the influences of gender and comorbid obsessive-compulsive disorder? J Am Acad Child Adolesc Psychiatry. 1994;33:795–804. doi: 10.1097/00004583-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Strömstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res. Mol. 1995;34:75–88. doi: 10.1016/0169-328x(95)00140-n. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Brit J Pharmacol. 1999;126:1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugale RR, Hirani K, Morelli M, Chopde CT. Role of neuroactive steroid allopregnanolone in antipsychotic-like action of olanzapine in rodents. Neuropsychopharmacology. 2004;29:1597–1609. doi: 10.1038/sj.npp.1300460. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Eikelis N. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur. J. Pharmacol. 2001;425:33–41. doi: 10.1016/s0014-2999(01)01139-6. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998;104:2–8. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- Wang S, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology. 2006;147:4025–4033. doi: 10.1210/en.2005-1485. [DOI] [PubMed] [Google Scholar]
- Yamada H, Kominami S, Takemori S, Kitawaki J, Kataoka Y. Immunohistochemical localization of cytochrome P450 enzymes in the rat brain, considering the steroid-synthesis in the neurons. Acta Histochem Cytochem. 1997;30:609–616. [Google Scholar]
- Yu L, Romero DG, Gomez-Sanchez CE, Gomez-Sanchez EP. Steroidogenic enzyme gene expression in the human brain. Mol Cell Endocrinol. 2002;190:9–17. doi: 10.1016/s0303-7207(02)00041-2. [DOI] [PubMed] [Google Scholar]


