Abstract
A disabling impairment of higher-order language function can be seen in patients with Lewy body spectrum disorders such as Parkinson's disease (PD), Parkinson's disease dementia (PDD), and dementia with Lewy bodies (DLB). We focus on script comprehension in patients with Lewy body spectrum disorders. While scripts unfold sequentially, constituent events are thought to contain an internal organization. Executive dysfunction in patients with Lewy body spectrum disorders may interfere with comprehension of this internal structure. We examined 42 patients (30 non-demented PD and 12 mildly demented PDD/DLB patients) and 12 healthy seniors. We presented 22 scripts (e.g., “going fishing”), each consisting of six events. Pilot data from young controls provided the basis for organizing associated events into clusters and arranging them hierarchically into scripts. We measured accuracy and latency to judge the order of adjacent events in the same cluster versus adjacent events in different clusters. PDD/DLB patients were less accurate in their ordering judgments than PD patients and controls. Healthy seniors and PD patients were significantly faster to judge correctly the order of highly associated within-cluster event pairs relative to less closely associated different-cluster event pairs, while PDD/DLB patients did not consistently distinguish between these event-pair types. This relative insensitivity to the clustered-hierarchical organization of events was related to executive impairment and to frontal atrophy as measured by volumetric MRI. These findings extend prior work on script processing to patients with Lewy body spectrum disorders and highlight the potential impact of frontal/executive dysfunction on the daily lives of affected patients.
Keywords: Parkinson's disease, Parkinson's disease dementia, Dementia with Lewy bodies, Frontal cortex, Executive function, Scripts, Organization, Discourse, Volumetric MRI
1. Introduction
The ability to describe a series of events or actions is an essential aspect of communication. Indeed, this is the means by which we tell a friend about the events of the day or describe to a student how to perform a task. A distinction has been made between telling a story (narrative discourse) and describing the steps in a task (procedural discourse). Both types of discourse are ubiquitous in daily conversation. For example, while telling a friend about a dinner party from the previous weekend, we might describe how we made the apple tart served for dessert. In both cases, the speaker and listener must be able to appreciate the organization of a series of events in order for communication to proceed successfully. Both types of discourse rely on linguistic as well as non-linguistic skills, such as attention, working memory, planning and organizing, and decision-making (Ash et al., 2006; Mar, 2004; Xu, Kemeny, Park, Frattali, & Braun, 2005). Moreover, discourse-level processing depends on a large-scale network of brain areas, and difficulties can be seen in patients with a variety of conditions that interfere with this network, including focal brain lesions (Joanette & Goulet, 1990), traumatic brain injury (Coelho, Grela, Corso, Gamble, & Feinn, 2005), and neurodegenerative diseases (Ash et al., 2006; Chapman et al., 2002).
In this study, we examine discourse in patients with Lewy body spectrum disorders, including Parkinson's disease (PD), Parkinson's disease with dementia (PDD), and dementia with Lewy bodies (DLB), in whom communication difficulty is a common and disabling clinical manifestation, using a script comprehension task. Scripts are representations of a sequence of events describing familiar, everyday activities (Schank & Abelson, 1977). The Lewy body spectrum disorders are characterized by dysfunction in frontal–subcortical networks and provide a model for investigating the role of these structures in script processing.
PD is characterized pathologically by deposition of Lewy bodies containing aggregated alpha-synuclein in subcortical and cortical brain regions (Goedert, 2001; Iwatsubo, 2003). Dopamine-producing cells in the substantia nigra pars compacta are prominently affected (Ehringer & Hornykiewicz, 1960), resulting in disruption of nigrostriatal circuitry. Patients exhibit extrapyramidal features including tremor, rigidity, bradykinesia, and abnormal postural reflexes. It is increasingly recognized that many PD patients also have cognitive deficits early in the course of disease (Aarsland, Bronnick, Larsen, Tysnes, & Alves, 2009; Elgh et al., 2009; Foltynie, Brayne, Robbins, & Barker, 2004; Muslimovic, Post, Speelman, & Schmand, 2005). Moreover, approximately 80% of patients with PD eventually develop dementia (PDD) as the disease progresses (Aarsland, Andersen, Larsen, Lolk, & Kragh-Sorensen, 2003; Buter et al., 2008; Hely, Reid, Adena, Halliday, & Morris, 2008). DLB, also marked histopathologically by Lewy body deposition, is characterized by prominent, early cognitive impairment and variable degrees of extrapyramidal motor features, well-formed hallucinations, and fluctuation in level of alertness (McKeith et al., 2005). Patients with PDD and DLB show similar profiles of cognitive impairment, most prominent in frontal/executive and visuospatial domains (Aarsland, Litvan et al., 2003; Horimoto et al., 2003; Mosimann et al., 2004; Weintraub et al., 2005).
In patients with PD and PDD/DLB, the most consistent neuropsychological deficit is frontal/executive dysfunction, affecting attention, initiation of action and thought, working memory, planning and organizing, decision-making, and inhibitory control (Barbas, 2006; Kobayakawa, Tsuruya, & Kawamura, 2010; Muslimovic et al., 2005; Uekermann et al., 2004; Weintraub et al., 2005). Depletion of striatal dopamine likely contributes to executive dysfunction in patients with Lewy body spectrum disorders. Indeed, there is evidence relating executive dysfunction to compromise of frontal-basal ganglia circuits (Lewis, Dove, Robbins, Barker, & Owen, 2003; Zgaljardic, Foldi, & Borod, 2004). Cognitive impairment is also likely related to spread of pathological changes to cortical regions including frontal cortex (Aarsland, Perry, Brown, Larsen, & Ballard, 2005; Gomperts et al., 2008; Hurtig et al., 2000). Functional imaging has shown disturbance of frontostriatal metabolism in these disorders (Lewis et al., 2003; Lozza et al., 2004; Sawamoto et al., 2008). Volumetric MRI studies have shown frontal gray matter loss in PD, although involvement of temporal, occipital, and parietal cortical areas has been demonstrated in PDD/DLB (Burton, McKeith, Burn, Williams, & O'Brien, 2004). Voxel-based diffusion tensor imaging has shown white matter abnormalities in multiple brain regions including the frontal lobes (Lee et al., 2010).
Although aphasia is relatively uncommon in Lewy body spectrum disorders, there can be compromise of more subtle aspects of language function (Berg, Bjornram, Hartelius, Laakso, & Johnels, 2003; Grossman et al., 2000; Lee, Grossman, Morris, Stern, & Hurtig, 2003; McNamara & Durso, 2003; McNamara, Holtgraves, Durso, & Harris, 2010; Monetta & Pell, 2007; Murray, 2000). For instance, patients with PD have greater difficulty processing grammatically complex sentences (e.g., sentences with object-relative center-embedded clauses). This difficulty has been attributed in part to limited executive resources, including attention allocation, working memory, and processing speed. In one study, difficulty with object-relative embedded clauses was correlated with executive measures including reverse digit span and category fluency (Lee et al., 2003). The same group also demonstrated significant decrements in grammatically-mediated sentence comprehension among PD patients when working memory demands were increased (i.e., during concurrent performance of a secondary task) compared to healthy elderly individuals (Grossman et al., 2000). Furthermore, our clinical observation is that patients with Lewy body spectrum disorders can exhibit disjointed, tangential, poorly organized discourse in daily interactions.
The organizational elements of language have been associated in part with executive resources, including planning and organizing processes, mediated by the frontal lobe. From this perspective, it has been proposed that words, phrases, and sentences describing actions or events are organized into higher-level scripts or narratives (Grafman, 2007; Mar, 2004). Evidence for this relationship comes from studies of higher-level language function in patients with disorders affecting frontal regions of the brain. Patients with frontal lobe damage have difficulty generating a sequence of events describing familiar activities (Godbout & Doyon, 1995; Sirigu et al., 1995). Cosentino, Chute, Libon, Moore, and Grossman (2006) performed a study examining processing of four-step scripts describing familiar activities (e.g., “making coffee”) in patients with behavioral-variant frontotemporal dementia (bvFTD), a condition marked by significant frontal/executive impairment (Libon et al., 2007). Patients with bvFTD exhibited difficulty detecting sequencing errors (Cosentino, Chute, Libon, Moore, & Grossman, 2006).
Scripts of familiar activities have also been used to examine the ability of patients with PD to process a series of events. On a script generation task, patients with PD showed more frequent sequencing and perseveration errors, as well as greater intrusion of irrelevant information, relative to controls. These errors were attributed to impairment of temporal ordering, action selection, and maintenance of script actions in working memory related to frontal-striatal dysfunction in PD (Godbout & Doyon, 2000). Moreover, the scripts produced by PD patients contained actions essential to the script, but fewer less central actions that provide contextual information. One explanation proposed by the authors is that retrieval of lower frequency, less central events may be more effortful and therefore more vulnerable to frontal-striatal dysfunction in PD (Godbout & Doyon, 2000). A similar finding was demonstrated in patients with frontal lobe injury (Godbout & Doyon, 1995). Zalla and colleagues also showed that PD patients had difficulty sequencing pre-selected script events and included distractors, a pattern qualitatively similar to that exhibited by patients with frontal lobe injury (Zalla et al., 1998). This group also showed that PD patients had difficulty appreciating the importance of each action with respect to the main goal of the script (Zalla et al., 2000). The performance of PD patients on these script tasks suggests impaired appreciation of the internal structure of a series of events, although the precise basis for this deficit remains to be elucidated empirically.
Like scripts of familiar activities, narrative relies on planning and organizing processes supported by the frontal lobes in order to integrate events into a coherent story. For instance, impaired discourse production has been demonstrated in patients with traumatic brain injury (Coelho et al., 2005). Despite the absence of aphasia, patients with bvFTD asked to narrate a wordless picture story have difficulty producing organized, coherent narratives (Ash et al., 2006; Chapman et al., 2005). Narrative performance was related to executive dysfunction, as well as right frontal and anterior temporal atrophy (Ash et al., 2006). Using the same narrative production task, patients with corticobasal syndrome showed impaired story organization and coherence related to atrophy in frontal and parietal cortex (Gross et al., 2010). Functional MRI (fMRI) studies in healthy subjects using this protocol have also demonstrated the role of frontal structures in producing organized, coherent narratives (Troiani et al., 2008). Studies have also implicated frontal structures in the comprehension of narrative discourse. Patients with frontal lobe injuries had difficulty indicating the pragmatic connection between pairs of successively presented sentences (Ferstl, Guthke, & von Cramon, 2002). In an fMRI study of healthy adults using the same materials, left prefrontal activation was seen during coherence judgments of consecutive sentences (Ferstl & von Cramon, 2001, 2002). In another fMRI study, processing sentences presented as a coherent narrative recruited a bilateral extrasylvian network of structures, including medial frontal regions, relative to activation associated with processing these sentences individually (Xu et al., 2005). A positron emission tomography (PET) study in healthy adults listening to fables showed activation of a network of structures, including right frontal and temporal regions, when participants appreciated the overall theme or moral of the story (Nichelli et al., 1995). Functional MRI showed activation of bilateral prefrontal cortex when healthy subjects listened to brief stories and were asked to integrate inconsistent information into their ongoing understanding of the story (Ferstl, Rinck, & von Cramon, 2005).
Higher-order language has also been investigated in PD and related to frontal-striatal dysfunction in these patients. Berg and colleagues demonstrated difficulty processing implied information in patients with PD, including making inferences from brief narratives and comprehending metaphor (Berg et al., 2003). PD patients' narratives of pictured scenes have shown an appropriate amount of verbal output, but this output has been judged to contain fewer informative (i.e., accurate, relevant, novel) utterances (Murray, 2000). PD patients demonstrate impaired pragmatic function in conversational speech (Hall, Ouyang, Lonnquist, & Newcombe, 2011; McNamara & Durso, 2003; McNamara et al., 2010). This difficulty has been related to performance on executive measures (McNamara & Durso, 2003). Studies have also shown difficulty comprehending other non-literal aspects of speech such as irony and metaphor (Monetta, Grindrod, & Pell, 2009; Monetta & Pell, 2007). The authors demonstrated a relationship between impaired working memory and difficulty comprehending metaphor in PD patients (Monetta & Pell, 2007).
At least two hypotheses have been forwarded to characterize the way in which a series of events may be organized: linear-sequential and clustered-hierarchical processing strategies. A linear-sequential structure involves the serial representation of events in a temporally sequenced manner (Hue & Erickson, 1991; van der Meer, Beyer, Heinze, & Badel, 2002). This approach suggests a serial access mechanism directly linked to the chronological order in which events occur, as well as a mechanism that monitors the place in the serial order where each event occurs (Botvinick & Watanabe, 2007). In an fMRI study directly evaluating a linear-sequential approach to event organization, posterior superior frontal activation was seen when subjects were asked to attend to the order of visually presented stimuli (Schubotz & von Cramon, 2001). Moreover, patients with ventral frontal lesions showed difficulty processing information about the sequence of visually presented items (Schubotz, Sakreida, Tittgemeyer, & von Cramon, 2004).
An alternate model emphasizes an internal organization primarily determined by the degree of association between events. According to such models, events within a series can be grouped such that events within the same cluster are more highly associated than events in different clusters (Black & Bower, 1979; Botvinick, 2008; Cooper & Shallice, 2006; Koechlin & Hyafil, 2007; Lichtenstein & Brewer, 1980; Zalla, Pradat-Diehl, & Sirigu, 2003). For instance, in a multi-step activity such as “going fishing,” groups of highly associated events (e.g., “open can of worms” and “place worm on hook”) are clustered tightly together, and they are separated from other events that are more highly associated with each other (e.g., “raise the pole” and “reel in the line”). Some models then posit that event clusters are combined in a hierarchical fashion to create an internally structured series of events (Botvinick, 2008; Cooper & Shallice, 2006; Fiebach & Schubotz, 2006; Tettamanti & Weniger, 2006; van Schie, Toni, & Bekkering, 2006; Zalla et al., 2003). Hierarchical processing is thought to be mediated by areas of prefrontal cortex. Indeed, regions of prefrontal cortex, arrayed anatomically in a caudal–rostral fashion, are thought to process increasingly complex materials involving decisions based on higher levels of abstraction (Badre & D'Esposito, 2007; Fuster, 2004; Koechlin, Ody, & Kouneiher, 2003).
The advantage of such clustered-hierarchical models is adaptability. That is, subroutines within a multi-step task can be managed in a flexible manner in response to external circumstances while the overall goal is maintained. Such models can be extended to procedural discourse, suggesting that the account of a series of events has a hierarchically-structured internal organization based on the degree of association between events. The benefit of adaptability holds: the overall point of the story can be maintained while subsections can be rearranged, elaborated, or adjusted in some way to accommodate listeners' needs. This process of coordination between speaker and listener is a crucial aspect of successful conversation.
Studies have attempted to demonstrate the clustered-hierarchical organization of a series of events in a script. In one report, patients with frontal brain damage were asked to identify the boundaries of “small events” and “large events” within a script (Zalla et al., 2003). Small events were defined as single actions (e.g., “uncap the toothpaste” and “place toothpaste on toothbrush”) that comprise a large, superordinate event (e.g., “brush one's teeth”). Patients had difficulty judging the beginning and end of large events in a script, although they did not differ from controls in judging the boundaries of small events. Although there are other explanations for this finding, greater difficulty judging large events can be interpreted as reflecting a deficit recognizing clusters of associated events, consistent with impaired processing of the internal structure of a series of events (Zalla et al., 2003).
A previous study from our group employed a novel method directly comparing linear-sequential and clustered-hierarchical approaches to processing scripts describing familiar activities such as “making a sandwich” or “going grocery shopping” (Farag et al., 2010). Scripts contained six events with an internal organization defined by clusters of associated events arranged hierarchically. Participants were asked to judge the order of two consecutive events taken either from the same or from different clusters. Healthy seniors were faster and more accurate to judge the order of two events taken from the same cluster relative to two events taken from different clusters, suggesting that they appreciated the clustered-hierarchical organization of events in the script. Using fMRI, healthy controls showed inferior prefrontal activation when judging within-cluster compared to different-cluster event pairs. Patients with bvFTD and progressive nonfluent aphasia (PNFA), both subtypes of FTD with significant frontal disease, did not distinguish between within-cluster and different-cluster event pairs. This insensitivity to internal script organization was related to frontal atrophy as measured by volumetric MRI (Farag et al., 2010). These findings provide evidence for the clustered-hierarchical organization of complex material and underscore the role of frontal cortex in processing this information.
The present study examined brief, multi-event scripts to elucidate the role of impaired planning and organizing processes needed for successful procedural discourse in patients with Lewy body spectrum disorders such as PD and PDD/DLB. We employed the six-event scripts reported previously by our group (Farag et al., 2010). We assessed whether patients are sensitive to the internal organization of the scripts—that is, whether they process differently pairs of highly associated consecutive events taken from the same cluster compared to pairs of less associated consecutive events taken from different clusters. Performance on the script comprehension task was related to measures of executive functioning and to gray matter atrophy as measured by volumetric MRI. We expected that patients with Lewy body spectrum disorders would have difficulty appreciating the special status of within-cluster events and that this would be related to executive dysfunction and frontal atrophy.
2. Methods
2.1. Participants
We examined 42 patients with Lewy body spectrum disorders, including 30 patients with PD and 12 with PDD/DLB (six with PDD and six with DLB). All of these patients were right-handed except for three individuals who were left-handed (two in the PD group and one in the PDD/DLB group). We also assessed 12 age- and education-matched healthy seniors. Patients were recruited from the University of Pennsylvania Health System cognitive neurology and movement disorders clinics. Patients were diagnosed with PD according to published criteria (Hughes, Daniel, Kilford, & Lees, 1992). Patients in the PDD/DLB group likewise met published criteria for these diagnoses (Emre et al., 2007; McKeith et al., 2005). The clinical distinction between PDD and DLB was based on the 1-year rule: DLB is diagnosed if cognitive impairment occurs before or within 1 year of the onset of motor symptoms (McKeith et al., 2005). In addition, patients were classified as having dementia if (1) the Mini-Mental State Exam (MMSE) score was less than or equal to 24, or (2) the MMSE was greater than 24, but the patient performed in the demented range on the Mattis Dementia Rating Scale (DRS; age-adjusted score less than or equal to 5) (Folstein, Folstein, & McHugh, 1975; Lucas et al., 1998; Mattis, 1988). This latter criterion based on the DRS was implemented to increase sensitivity for identifying patients with dementia. Indeed, the DRS is a validated assessment of dementia in PD and is more sensitive than the MMSE to cognitive impairment in Lewy body spectrum disorders, particularly impairment related to executive dysfunction (Dubois et al., 2007; Levy et al., 2002; Llebaria et al., 2008). Exclusion criteria included a primary psychiatric disorder, structural brain lesion, and encephalopathy due to a medical condition. Some patients were taking stable doses of cholinesterase inhibitors, memantine, methylphenidate, non-sedating antidepressants, or low doses of atypical neuroleptic medications as clinically indicated. No patient showed evidence of sedation suggesting over-medication during testing. Control subjects also underwent a screening procedure to ensure absence of any condition or medication that could compromise cognitive performance. In addition, control subjects had to score greater than or equal to 27/30 on the MMSE. This protocol was approved by the University of Pennsylvania Institutional Review Board. Written informed consent was obtained from all participants in this study.
Demographic features, Hoehn and Yahr stage (Hoehn & Yahr, 1967), Unified Parkinson's Disease Rating Scale (UPDRS) motor assessments (Fahn, Elton, & UPDRS Program Members, 1987), and dopaminergic medication use are summarized in Table 1. Dopaminergic medication use is expressed as levodopa equivalents, calculated as follows (Hobson et al., 2002): 100 mg levodopa = 130 mg controlled-release levodopa = 70 mg levodopa + catechol-O-methyl transferase (COMT) inhibitor = 1 mg pergolide = 1 mg pramipexole = 5 mg ropinirole. Other PD medications (e.g., anticholinergics and monoamine oxidase inhibitors) were not included in the determination of levodopa equivalent dose. There were no significant differences in age and education between PD patients and controls or between PDD/DLB patients and controls. When patient groups were compared to each other, PDD/DLB patients were significantly older than PD patients (t(40) = 3.08, p < 0.01), as would be expected for an age-associated dementing condition (Hughes et al., 2000). Patients in the DLB group were younger than patients with PDD (mean (SD) age 72.5 (5.2) and 78.7 (5.5) years, respectively), but this difference did not reach significance (p = 0.074). There were no differences in educational level and disease duration between groups. Mean (SD) MMSE scores were 28.2 (1.4), 21.3 (3.7), and 28.3 (1.0) in the PD, PDD/DLB, and control groups, respectively. The mean MMSE score was significantly lower in PDD/DLB compared to PD patients (t(12) = 6.33, p < 0.001) and controls (t(12) = 6.31, p < 0.001). MMSE scores were lower in patients with DLB relative to those with PDD (19.2 (3.9) versus 23.3 (2.3), t(10) = 2.28, p < 0.05). There was no significant difference in MMSE scores between PD patients and controls. DRS scores were available in 23 patients with Lewy body spectrum disorders (17 PD and 6 PDD/DLB). Mean (SD) age-adjusted DRS scores were 10.8 (2.9) and 5.7 (2.0) in the PD and PDD/DLB groups, respectively. DRS scores were lower in the PDD/DLB group than in the PD group (t(21) = 3.96, p = 0.001). PDD/DLB patients showed a more advanced Hoehn and Yahr stage compared to PD patients (t(35) = 3.27, p < 0.01). There was no difference between the PD and PDD/DLB groups in UPDRS total motor score or dominant upper extremity rigidity and tremor scores. Patients with DLB had greater total motor and Hoehn and Yahr scores than patients with PDD, likely related to greater postural instability in the former group, but these differences were not significant. All but two PD patients and four PDD/DLB patients were taking dopaminergic medications. Review of the data suggests that patients in each group who were not taking dopaminergic medications did not differ in terms of overall response accuracy and/or latency from other members of their respective groups. As measured as levodopa equivalents, use of dopaminergic medications was greater in the PD group compared to PDD/DLB patients, but this difference did not reach significance (t(35) = 1.97, p = 0.057). Correlation analyses did not reveal a relationship between levodopa equivalents and measures of performance on the experimental task. Patients with DLB were taking significantly less dopaminergic medication than those with PDD (t(9) = 4.09, p < 0.01), as such medications were likely precluded by the relatively early and prominent cognitive impairment and hallucinosis characteristic of this condition. A total of six patients (two in the PD group and four in the PDD/DLB group) were taking potentially cognitive-enhancing medications (i.e., cholinesterase inhibitors, memantine, or methylphenidate). Review of the data suggests that patients taking these medications, rather than showing enhanced performance, tended to be among those with the lowest overall accuracies and/or latencies within their respective groups, which is likely a manifestation of the cognitive impairment which originally prompted prescription of these drugs.
Table 1.
Mean (SD) demographic and clinical features of patients with Lewy body spectrum disorder and healthy elderly controls.
| PD | PDD | DLB | PDD/DLB | Controls | |
|---|---|---|---|---|---|
| Age | 67.0 (8.9) | 78.7 (5.5) | 72.5 (5.2) | 75.6 (6.0)* | 71.3 (8.9) |
| Education | 15.6 (3.3) | 14.8 (2.4) | 16.2 (1.6) | 15.5 (2.1) | 14.9 (3.0) |
| Disease duration | 6.8 (4.3) | 7.5 (3.9) | 6.0 (3.8) | 6.8 (3.8) | – |
| MMSE | 28.2 (1.4) | 23.3 (2.3) | 19.2 (3.7)† | 21.3 (3.7)** | 28.3 (1.0) |
| DRS | 10.8 (2.9) | 5.67 (2.0) | – | 5.7 (2.0)** | – |
| Hoehn and Yahr stage | 2.2 (0.6) | 2.7 (0.4) | 2.9 (0.2) | 2.8 (0.3)* | – |
| UPDRS total motor score | 25.5 (9.0) | 25.8 (12.0) | 29.3 (12.5) | 27.7 (11.8) | – |
| UPDRS rigidity (dominant arm) | 1.3 (0.9) | 1.2 (0.8) | 0.5 (0.5) | 0.7 (0.4) | – |
| UPDRS tremor (dominant arm) | 0.7 (1.0) | 0.0 (0.0) | 0.7 (1.2) | 0.4 (0.9) | – |
| Levodopa equivalent dose | 633.4 (380.1) | 700.0 (351.8) | 75.0 (125.5)†† | 359.1 (404.9) | – |
PD = Parkinson's disease; PDD = Parkinson's disease dementia; DLB = dementia with Lewy bodies; MMSE = Mini-Mental State Exam; DRS = Dementia Rating Scale (age-adjusted score); UPDRS = Unified Parkinson's Disease Rating Scale.
p < 0.01 (relative to PD).
p ≤ 0.001 (relative to PD and/or controls).
p < 0.05 (relative to PDD).
p < 0.01 (relative to PDD).
2.2. Materials
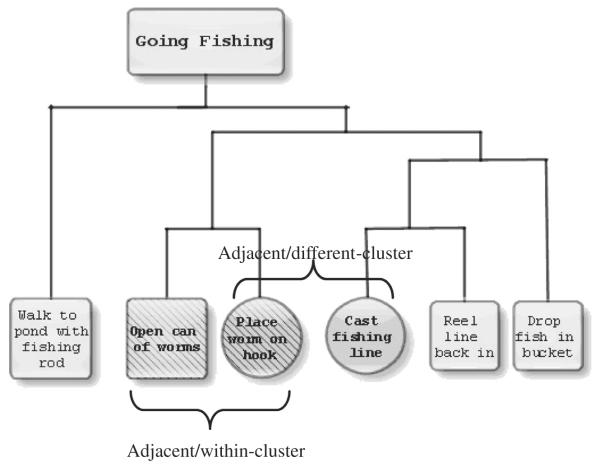
We created 22 scripts, each composed of six events, describing familiar activities such as “going fishing” or “making a sandwich.” The development of the scripts used in this study has been described previously (Farag et al., 2010). Briefly, the associativity of events in each script was determined based on judgments from a group of 10 young, healthy individuals. Pilot subjects were given a series of six 5″ × 8″ index cards, each with a script event typed and centered in black 14-point Times New Roman font. Pilot subjects were asked to order script events chronologically, which all accomplished successfully. They were then asked to cluster events into groups such that events felt to be most closely associated with each other would be clustered together. Subjects were instructed to cluster each event only once. These pilot data allowed us to group events by associativity strength and arrange these clusters into hierarchical structures. Fig. 1 illustrates an example of a clustered-hierarchical script structure.
Fig. 1.
An example of the clustered-hierarchical structure for the script “Going Fishing” showing within-cluster (WC) and different-cluster (DC) event pairs.
We created event pairs (also illustrated in Fig. 1) involving adjacent events contained within the same cluster (WC) and adjacent events from different clusters (DC), where one DC event was the same as an event from the WC pair. We analyzed those event pairs that included non-initial, non-terminal script events, as participants could be biased toward a linear approach to script processing cued by the initial or terminal event (Franklin, Smith, & Jonides, 2007). We used as filler items nonadjacent event pairs (separated by one event), as well as both adjacent and non-adjacent event pairs involving an initial or terminal script event. Half of all event pairs were shown in the correct order (“open can of worms”/“place worm on hook”), and half were shown in the incorrect order (“place worm on hook”/“open can of worms”). Participants were shown a total of 198 event pairs. These stimuli were randomly distributed over six runs and intermixed with events presented in the incorrect order, resulting in a total of 33 items per run, with each type of event pair type presented anywhere from two to five times per run.
2.3. Script procedure
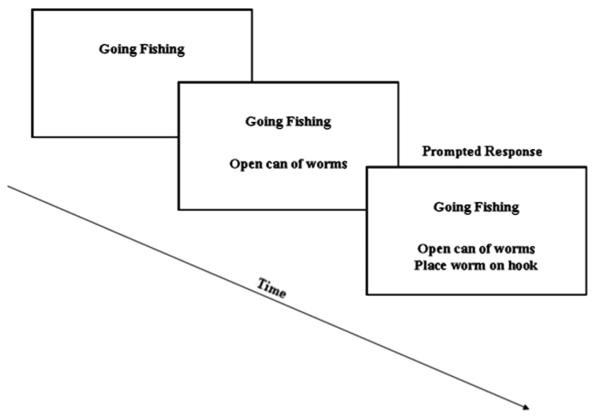
All stimuli were displayed using a Dell Inspiron 1100 laptop. E-Prime v1.4.1 presentation software recorded response accuracy and latency. For each trial, a script title and pair of events were displayed vertically on the monitor in black 18-point Arial font. The script title was presented at the top of the screen for 3000 ms, followed by a blank screen for 200 ms. Next, the first script event was shown below the previously displayed script title for 4000 ms, again followed by a blank screen for 200 ms. Finally, the second script event was shown below the title and first event for 4000 ms. The title was presented first and then maintained during event presentation to provide context, thereby encouraging participants to process event pairs as part of a larger conceptual whole. Relatively long stimulus presentation intervals were used to ensure that patients had sufficient time to read and understand the text. We repeated the script title with the first event, as well as the title and first event with the second event, to minimize reliance on memory function during the task. Participants were then asked to judge whether the two events were shown in the correct order for the script. They were given as much time as needed to make these ordering judgments, but were instructed to work as quickly and as accurately as possible. “Yes” or “no” responses were recorded by pressing one of two buttons on the computer keyboard. Fig. 2 illustrates stimulus presentation used in this study. Prior to the experiment, participants were given a practice run consisting of six scripts. During the practice trials, incorrect answers were corrected and explained by the experimenter to ensure comprehension of task instructions. Participants (n = 3) were excluded if they provided incorrect answers on more than two practice script sessions.
Fig. 2.
Illustration of stimulus presentation for the script “Going Fishing.”
We recorded the accuracy and response latency of each ordering judgment. Only response latencies to event pairs that had been presented in the correct order were analyzed, as we felt that many factors other than the organization of script events could contribute to long latency to respond to event pairs shown in the reverse order (e.g. exhaustive search through long-term memory for candidate experiences before responding “no”). Response latencies for accurate ordering judgments were included in the analysis after eliminating outliers (less than 500 ms or greater than 14,000 ms) that would unduly bias the statistical distribution of response latencies during statistical analyses. These cut-offs were based on patients' performance on the task, where responses faster than 500 ms were very unlikely to be physiologically plausible (e.g., could represent responses to the previous trial) and responses longer than 14,000 ms occurred very rarely. After removing these outliers, an individualized 2.5 standard deviation filter for latencies was then employed to normalize responses based on each participant's own distribution of reaction times. Overall, 7.5% of responses were excluded on average across participants. Two patients with PDD/DLB were excluded from the latency analysis due to inability to press the response keys reliably. The experimenter pressed the response key for these individuals, thus their responses were able to be included in the accuracy analysis.
2.4. Neuropsychological assessment
We examined performance in all patients on measures of executive function. We selected verbally-mediated measures available in all patients to minimize risk of confounding by task modality. Digit span was measured in the forward direction to assess sequencing and in the reverse direction to assess working memory (Wechsler, 1987). Verbal fluency was assessed by asking participants to name as many unique words as possible in 1 min beginning with each of the letters, F, A, and S (Benton & Hamsher, 1976). Subjects were also asked to list as many animals as possible in 1 min. These measures assessed mental planning and organization in a semantic and a non-semantic context. It should be noted that patients' clinical charts did not indicate that dysarthria, when present, was severe enough to compromise verbal fluency performance. In addition to the executive battery just described, participants completed the Boston Naming Test (BNT) to measure lexical retrieval involving semantic memory (Kaplan, Goodglass, & Weintraub, 1983). Individual patients' scores on each neuropsychological task were converted to Z scores. Z scores were based on the neuropsychological task performance of a separate, well-characterized set of age- and education-matched controls (n = 24). PD patients were not impaired relative to controls on any test. PDD/DLB patients were significantly impaired on category fluency and the BNT relative to controls (Z < −1.96, p < 0.05). Performance was significantly worse in the PDD/DLB group relative to the PD group on all cognitive tests (p < 0.01 for all comparisons; see Table 2). Performance was similar in PDD versus DLB patients, except that DLB patients had significantly greater difficulty with category verbal fluency (t(10) = 2.49, p < 0.05) and on the BNT (t(10) = 3.20, p = 0.01).
Table 2.
Mean (SD) Z scores for performance on selected cognitive tasks in patients with Lewy body spectrum disorders.
| Cognitive task | PD | PDD | DLB | PDD/DLB |
|---|---|---|---|---|
| Forward digit span | 0.2 (0.7) | −0.6 (0.5) | −0.6 (1.2) | −0.6 (0.9)* |
| Reverse digit span | 0.0 (1.1) | −1.4 (1.1) | −1.4 (1.5) | −1.4 (1.2)** |
| Letter fluency | −0.2 (1.0) | −1.5 (0.8) | −2.0 (0.6) | −1.7 (0.7)** |
| Category fluency | −0.6 (1.0) | −2.2 (0.5) | −2.9 (0.4)† | −2.5 (0.6)** |
| Boston naming test | −0.2 (0.9) | −1.4 (0.9) | −3.8 (1.6)†† | −2.6 (1.7)** |
PD = Parkinson's disease; PDD = Parkinson's disease dementia; DLB = dementia with Lewy bodies.
p < 0.01 (relative to PD).
p ≤ 0.001 (relative to PD).
p < 0.05 (relative to PD).
p = 0.01 (relative to PDD).
2.5. Script statistical analysis
Independent sample t-tests were used to compare the overall accuracy of ordering judgments, as well as the overall response latency for accurate ordering judgments, between (1) PD patients and controls, (2) PDD/DLB patients and controls, and (3) between PD and PDD/DLB patients. Unadjusted linear regression analyses using overall accuracy or response latency as the dependent variable and diagnosis as the independent variable were performed to obtain effect size estimates (beta coefficient). Comparisons of task performance in patients with PDD and DLB were performed, but must be interpreted with great caution due to small sample sizes. Pooled-variance t-tests were used except when group variances were not equal according to Levene's test. In such cases, separate-variance t-tests were employed. Paired t-tests were used to compare within groups accuracy and latency to respond to WC versus DC event pairs. Differential response to WC versus DC events would provide support for clustered-hierarchical script organization. We therefore compared WC and DC event types within groups on the assumption that if Lewy body spectrum disorder patients have impaired processing of script organization, they would fail to differentiate between these two event-pair types. Two-tailed t-tests were planned for all comparisons. Significance was determined at the p < 0.05 level for these analyses. Correlation analyses between performance on the experimental task and executive measures were performed across the combined Lewy body spectrum disorder group after examining data for outliers. We consider the degree of executive dysfunction to lie along a continuum across patients with Lewy body spectrum disorders, and we performed the correlations this way in order to capture the statistical variance across the entire spectrum of PD and PDD/DLB patients. Correlation analyses between the experimental task and executive measures were corrected for multiple comparisons using a Bonferroni procedure (significance threshold p < 0.01). All patients completed cognitive testing within 1 year of the script comprehension task (median time between cognitive testing and the experimental task was 2.0 months). Regression analyses were carried out between experimental measures and each cognitive measure using the time interval between the experimental task and cognitive testing as a covariate. All statistical analyses were performed using SPSS 12.0 (SPSS Inc., Chicago, IL).
2.6. Imaging procedure and analysis
Thirteen patients with Lewy body spectrum disorders, including seven patients with PD and six patients with PDD/DLB (2 PDD, 4 DLB), underwent brain MRI within 1 year of the script comprehension task. The median time interval between MRI scanning and the experimental task was 5.0 months. These patients were demographically representative of the entire group of patients and reflected performance of the entire group on the experimental task. For three patients and 36 age- and education-matched controls, images were collected using a SIEMENS Trio 3.0T scanner with 1-mm slice thickness and a 195 × 256 matrix. Ten patients had MRI scans acquired using a GE 1.5T scanner with 1.2-mm slice thickness and a 144 × 256 matrix. Images from both scanners were normalized to a standard space and segmented using the Pipe-Dream interface (http://sourceforge.net/projects/neuropipedream/) to the ANTS toolkit (ANTS, http://www.picsl.upenn.edu/ANTS/). The ANTS toolkit implements a diffeomorphic and symmetric registration method (Avants & Gee, 2004; Avants et al., 2010), which was recently evaluated against other freely-available tools (Klein et al., 2009) and found to be most reliable overall in processing a variety of data sets.
We used the standard ANTS-based imaging pipeline provided by PipeDream. Prior to running the pipeline, a local T1 template of 1 mm3 resolution is built using ANTS, and a transformation is computed that maps this local template to Montreal Neurological Institute (MNI) template space. The pipeline begins by registering the subject image to the local template, after which the subject space can be mapped directly to MNI space by combining the subject-to-template and template-to-MNI transformations. Next, three-tissue (gray matter, white matter, and cerebrospinal fluid) segmentation was performed in subject space using the Atropos tool in ANTS. Prior probability images for each tissue class, previously defined in the local template, were warped into the subject image to guide the segmentation. Post-segmentation, gray matter probability images were transformed into MNI space for statistical analysis and smoothed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) using a 4-mm full-width half-maximum (FWHM) Gaussian kernel to minimize individual gyral variations.
In SPM5, a two-sample t-test, with a covariate for each scanner, contrasted gray matter probability between patients with Lewy body spectrum disorders and healthy controls to identify regions of significant atrophy. For this atrophy analysis, an explicit mask was defined by generating a mean gray matter image from the healthy controls in order to limit the analysis to voxel-wise comparisons within gray matter. We used a 200-voxel extent and a statistical height threshold of p < 0.05. We accepted clusters containing a peak with Z score greater than 3.09 (p < 0.001) and FDR-corrected at the p < 0.05 level.
The regression module in SPM5 then was used to relate gray matter density to (1) the accuracy difference and (2) the latency difference between WC and DC event pairs. We used a statistical height threshold of p < 0.05 and a 150-voxel extent. We accepted clusters containing a peak with Z score greater than 3.09 (p < 0.001). We next restricted the regression analysis to regions known to be atrophied from the prior analysis using an explicit mask, a statistical height threshold of p < 0.05, and a 50-voxel extent. We again accepted clusters containing a peak with Z score greater than 3.09 (p < 0.001). This was done in order to test the relationship between task performance and brain areas known to be significantly atrophied from the prior analysis. We also ran the regression analysis between script performance and gray matter density using time interval between the experimental task and the MRI as a covariate. We report coordinates in Talairach space (Talairach, 1988) generated using a nonlinear transformation (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
3. Results
3.1. Behavioral results
Accuracies of ordering judgments are summarized in Table 3. Mean (SD) overall accuracy of ordering judgments was 93% (5%) among healthy seniors, 96% (3%) among non-demented PD patients, and 79% (14%) among PDD/DLB patients. PDD/DLB patients were significantly less accurate in their ordering judgments compared to both non-demented PD patients (t(11) = 4.45, p = 0.001) and controls (t(14) = 3.34, p < 0.01). Moreover, these accuracy differences remained between groups after covarying for age. Both non-demented PD patients and control subjects performed with high task accuracy. The difference in accuracy between these groups reached statistical significance (t(14) = 2.39, p < 0.05). Unadjusted linear regression analysis using overall accuracy as the dependent variable and diagnosis as the independent variable provided an effect size estimate: t = 6.85, β = 18% for the PDD/DLB – PD comparison, t = 3.34, β = 14% for the PDD/DLB – control group comparison, and t = 3.00, β = 3.7% for the PD – control group comparison. A within-group analysis was then used to compare the accuracy of participants' responses to WC and DC event pairs. Healthy seniors were significantly more accurate in their ordering judgments for WC event pairs relative to DC event pairs (t(11) = 3.92, p < 0.01). Both non-demented PD patients (t(29) = 4.13, p < 0.001) and PDD/DLB patients (t(11) = 2.64, p < 0.05) were significantly more accurate in their responses to WC versus DC event pairs.
Table 3.
Mean (SD) percent accuracy and latency for ordering judgments in patients and controls.
| PD | PDD | DLB | PDD/DLB | Controls | |
|---|---|---|---|---|---|
| Overall accuracy | 96 (3) | 0.82 (0.17) | 0.75 (0.11) | 79 (14) | 93 (5) |
| WC events | 98 (2) | 0.90 (0.07) | 0.81 (0.14) | 86 (12) | 99 (3) |
| DC events | 95 (5) | 0.77 (0.23) | 0.73 (0.13) | 75 (18) | 89 (9) |
| Overall latency | 3668 (1489) | 5091 (1933) | 7507 (2213) | 6057 (2294) | 3553 (1081) |
| WC events | 3635 (1452) | 4758 (1578) | 7527 (2352) | 5866 (2296) | 3331 (1048) |
| DC events | 3784 (1518) | 5021 (2137) | 7788 (2738) | 6128 (2660) | 3572 (1108) |
PD = Parkinson's disease; PDD/DLB = Parkinson's disease dementia/dementia with Lewy bodies; WC = within-cluster; DC = different-cluster.
Response latencies for accurate ordering judgments are also summarized in Table 3. In terms of overall response latency, PD patients did not differ from controls. In contrast, PDD/DLB patients were slower to respond overall than PD patients (t(38) = 3.82, p < 0.001) and controls (t(12) = 3.17, p < 0.01). These latency differences remained between groups after covarying for age. Unadjusted linear regression analysis using overall response latency as the dependent variable and diagnosis as the independent variable provided an effect size estimate: t = 3.82, β = 2389 ms for the PDD/DLB – PD comparison, t = 3.37, β = 2505 ms for the PDD/DLB – control group comparison. A within-group analysis was then used to compare participants' latencies to respond to WC and DC event pairs. Healthy seniors were significantly faster to make ordering judgments for WC event pairs relative to DC event pairs (t(11) = 3.86, p < 0.01). Non-demented PD patients were also significantly faster to judge the order of WC versus DC events (t(29) = 2.52, p < 0.05), suggesting that like controls, these patients assign a special status to highly associated events within a script. In contrast, PDD/DLB patients did not show a significant difference in their latencies to judge WC and DC event pairs (t(9) = 1.25, p = 0.24), suggesting that performance in this group is not facilitated by greater associativity between events in the WC condition.
As shown in Table 3, we also compared task performance between patients with a clinical diagnosis of PDD and those with DLB. Mean (SD) overall accuracy of ordering judgments was 82% (17%) among PDD patients and 75% (11%) among DLB patients. In the accuracy analysis, both PDD and DLB patients were more accurate to judge the order of WC relative to DC events. However, given small sample sizes (n = 6 in each group for the accuracy analysis), differences were not significant. There were no significant differences in response latencies for WC versus DC events in either group.
We then examined correlations between script performance and executive measures in patients with Lewy body spectrum disorders. Correlations with executive measures were corrected for multiple comparisons using a Bonferroni procedure since we had no specific hypothesis about the role of a particular executive measure in script comprehension. Accuracy to judge the order of WC event pairs was correlated with tests of mental organization: letter-guided verbal fluency (r = 0.49, p = 0.001) and category-guided verbal fluency (r = 0.63, p < 0.001). Latency for correct ordering judgments of WC event pairs was significantly correlated with tests of mental organization and working memory: letter-guided verbal fluency (r = 0.49, p = 0.001), category-guided verbal fluency (r = 0.71, p < 0.001), and reverse digit span (r = 0.47, p < 0.01).
Response accuracy was related to performance on the BNT (r = 0.45, r < 0.01). There was no correlation between BNT performance and latency to respond to WC events. Across the combined patient group, the accuracy and latency to respond to WC event pairs was correlated with the MMSE (r = 0.74, p < 0.001 and r = 0.62, p < 0.001, respectively) and DRS (r = 0.44, p < 0.05 and r = 0.49, p < 0.05, respectively). Finally, regression analyses were carried out between experimental measures and each cognitive measure. In no case did a consideration of the time interval between the experimental task and cognitive testing change the statistical relationship between variables.
3.2. Imaging results
Areas of significant atrophy are displayed in Table 4 and Fig. 3. In the atrophy analysis, a large cluster (with peak at −23, −20, −14) was identified containing 116,048 voxels. Given numerous subpeaks within this cluster, we only report in Table 4 maxima that lie in clearly distinct anatomical regions. We found significant atrophy in frontal, temporal, parietal, occipital, and basal ganglia regions bilaterally. A regression analysis was used to relate gray matter density to (1) the difference in response accuracy for WC versus DC event pairs, and (2) the difference in response latency for WC versus DC event pairs. The results of unmasked (whole-brain) regression analyses are shown in Figs. 4 and 5, as well as in Supplemental Table A1. We then report results using an explicit mask to perform a regression between task performance and brain areas known to be significantly atrophied from the prior analysis. As shown in Table 5 and Fig. 4, the difference in accuracy to respond to WC versus DC events was significantly related to atrophy in frontal cortex bilaterally (including Brodmann areas (BA) 10, 11, and 47). The accuracy difference was also significantly related to atrophy in left cingulate, right temporal, left insula, right thalamus, and bilateral hippocampal structures. The latency analysis revealed that difficulty appreciating the special status of WC event pairs was significantly related to atrophy in left frontal cortex (BA 11; Table 5 and Fig. 5). Finally, we re-ran the regression analysis between task performance and atrophy using the time lapse between the experimental task and the MRI as a covariate, which yielded similar results. This finding suggests that atrophy in regions associated with task performance cannot be attributed to change over time with disease progression.
Table 4.
Volumetric MRI analysis showing areas of significant gray matter atrophy in patients with Lewy body spectrum disorders relative to controls.
| Anatomic locus (Brodmann area) | Coordinates |
Z score* | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| L hippocampus† | −32 | −20 | −14 | 6.38 | 116048 |
| R amygdala | 18 | −6 | −12 | 5.97 | – |
| L amygdala | −21 | −5 | −12 | 5.86 | – |
| R posterior cingulate (29/30) | 8 | −49 | 11 | 5.47 | – |
| L parahippocampal gyrus (30) | −23 | −38 | 4 | 5.37 | – |
| R hippocampus | 29 | −22 | −10 | 5.34 | – |
| R medial globus pallidus | 18 | −6 | −8 | 5.32 | – |
| R anterior cingulate (24) | 5 | 26 | 18 | 5.15 | – |
| R fusiform gyrus (37) | 40 | −44 | −8 | 5.11 | – |
| L parahippocampal gyrus (28) | −22 | −15 | −21 | 5.10 | – |
| L pulvinar | −12 | −26 | 16 | 4.96 | – |
| R parahippocampal gyrus (36/30) | 26 | −37 | −6 | 4.88 | – |
| R inferior temporal gyrus (20/21) | 59 | −12 | −16 | 4.76 | – |
| R parahippocampal gyrus (28) | 21 | −14 | −14 | 4.75 | – |
| R cingulate gyrus (24/31) | 5 | −19 | 39 | 4.70 | – |
| L putamen | −15 | 14 | −10 | 4.67 | – |
| L anterior cingulate (32) | −3 | 23 | −8 | 4.66 | – |
| L caudate | −6 | 13 | 5 | 4.61 | – |
| L inferior temporal (20) | −59 | −12 | −29 | 5.12 | 419 |
| L inferior frontal (47) | −38 | 13 | −12 | 4.91 | 9356 |
| R parietal-occipital (19/39) | 28 | −64 | 30 | 4.74 | 1406 |
| R superior frontal (10) | 19 | 56 | 21 | 4.60 | 701 |
| R medial frontal (10) | 3 | 49 | 12 | 4.56 | 246 |
| R fusiform gyrus (37) | 42 | −58 | −6 | 4.54 | 472 |
| L inferior parietal (39) | −58 | −60 | 24 | 4.22 | 6060 |
| L precentral (4) | −23 | −25 | 53 | 4.12 | 1561 |
| R paracentral lobule (5) | 20 | −26 | 51 | 4.02 | 4056 |
| R middle frontal (8) | 20 | 28 | 38 | 3.98 | 3008 |
| R inferior parietal (40) | 35 | −44 | 39 | 3.91 | 704 |
| R middle temporal (21/37) | 42 | −59 | 4 | 3.81 | 1165 |
| L middle frontal (10) | −40 | 49 | 19 | 3.74 | 884 |
| R superior parietal (7) | 34 | −71 | 46 | 3.74 | 1243 |
| L inferior frontal (47) | −38 | 23 | −19 | 3.72 | 350 |
| R inferior frontal (47) | 50 | 26 | −10 | 3.65 | 1001 |
| L cerebellum | −46 | −51 | −20 | 3.50 | 2488 |
| R middle frontal (8) | 31 | 35 | 40 | 3.50 | 250 |
| L superior temporal (22) | −46 | −38 | 9 | 3.49 | 291 |
| L middle frontal (8) | −22 | 23 | 42 | 3.47 | 2271 |
| R superior temporal (22) | 57 | 5 | −4 | 3.45 | 2924 |
| L inferior frontal (44) | −45 | 3 | 17 | 3.36 | 422 |
| R inferior frontal (44) | 33 | 13 | 23 | 3.36 | 892 |
| R inferior frontal (46) | 48 | 40 | 13 | 3.29 | 1125 |
| L inferior parietal (7/40) | −29 | −46 | 38 | 3.28 | 850 |
| L inferior occipital (18) | −15 | −84 | −3 | 3.22 | 242 |
| R superior temporal (22) | 63 | −53 | 17 | 3.20 | 1056 |
| R middle occipital (18/19) | 30 | −80 | 3 | 3.15 | 385 |
| R precuneus (31) | 18 | −65 | 24 | 3.14 | 211 |
| R middle temporal (39) | 56 | −65 | 12 | 3.11 | 226 |
p < 0.05 (false discovery rate corrected) for all clusters.
Due to the large number of subpeaks in this cluster, we only report maxima that lie in clearly different anatomical regions from others within the cluster.
Fig. 3.
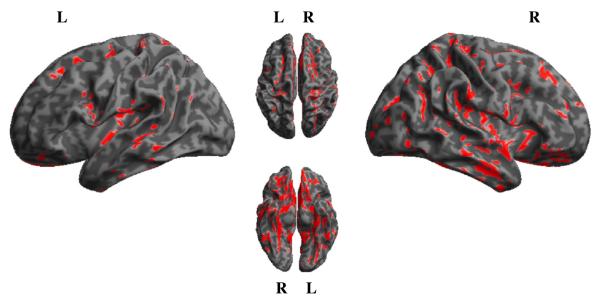
Volumetric MRI showing areas of significant gray matter atrophy in patients with Lewy body spectrum disorders. Red = atrophied regions relative to controls.
Fig. 4.
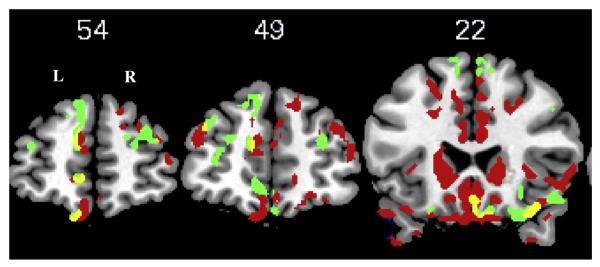
Results of regression analysis showing areas of atrophy in bilateral frontal cortex (including Brodmann areas 10, 11, and 47) significantly related to the difference in accuracy to respond to within-cluster (WC) versus different-cluster (DC) event pairs. Red = atrophied regions relative to controls; green = significant regions from unmasked regression analysis; yellow = significant regions from masked regression analysis. Y coordinates are shown above the corresponding image.
Fig. 5.
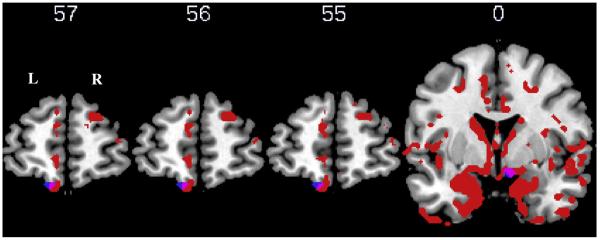
Results of regression analysis showing areas of atrophy in left frontal cortex (Brodmann area 11) significantly related to the difference in latency to judge correctly the order of within-cluster (WC) versus different-cluster (DC) event pairs. Red = atrophied regions relative to controls; blue = significant regions from unmasked regression analysis; pink = significant regions from masked regression analysis. Y coordinates are shown above the corresponding image.
Table 5.
Regression analysis showing areas of atrophy significantly related to (1) the difference in accuracy and (2) the difference in latency to respond to within-cluster (WC) versus different-cluster (DC) event pairs in patients with Lewy body spectrum disorders.
| Anatomic locus (Brodmann area) | Coordinates |
Z score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Accuracy analysis | |||||
| L medial frontal (9/10) | −12 | 51 | 14 | 4.10 | 229 |
| L cingulate (24/32) | −11 | 6 | 38 | 4.02 | 97 |
| L middle frontal (11) | −19 | 34 | −20 | 3.84 | 57 |
| R fusiform gyrus (20/36) | 39 | −25 | −22 | 3.68 | 4239 |
| R thalamus | 6 | −5 | 6 | 3.51 | 976 |
| R inferior frontal (47) | 15 | 16 | −20 | 3.47 | 2332 |
| R parahippocampal gyrus (30) | 8 | −46 | 3 | 3.39 | 96 |
| L superior frontal (11) | −11 | 53 | −23 | 3.35 | 279 |
| L parahippocampal gyrus (35/36) | −25 | −28 | −15 | 3.31 | 675 |
| R middle temporal (37) | 44 | −59 | 8 | 3.29 | 67 |
| Linsula (13) | −39 | −17 | 16 | 3.29 | 438 |
| L medial frontal (10) | −8 | 55 | −4 | 3.21 | 110 |
| R inferior temporal (20) | 64 | −27 | −15 | 3.18 | 171 |
| R middle temporal (21) | 54 | 0 | −29 | 3.18 | 74 |
| L cingulate (29/30) | −11 | −46 | 12 | 3.17 | 422 |
| L superior frontal (10) | −35 | 50 | 22 | 3.14 | 99 |
| Latency analysis | |||||
| R subcallosal gyrus (25/34) | 8 | 0 | −13 | 3.39 | 228 |
| R middle occipital (19) | 31 | −56 | −3 | 3.38 | 183 |
| L inferior frontal (11) | −11 | 53 | −22 | 3.20 | 90 |
4. Discussion
In this study, we examined script comprehension in patients with Lewy body spectrum disorders, including PD and PDD/DLB. While previous studies have shown impaired script processing in this population (Godbout & Doyon, 2000; Zalla et al., 1998, 2000), we sought to examine in more depth the planning and organizing processes that are compromised during comprehension of multi-event scripts in these patients. Specifically, we assessed two competing claims about the processing of a series of events. One hypothesis is that events are processed in a linear-sequential manner (Botvinick & Watanabe, 2007; Hue & Erickson, 1991; van der Meer et al., 2002). If participants were applying a linear-sequential processing strategy in this study, we would expect judgments about a pair of consecutive events to be comparable to judgments of any other pair of consecutive events. However, an alternate hypothesis is that events in a series are organized such that some events are preferentially clustered together because they are more highly associated than other events (Black & Bower, 1979; Botvinick, 2008; Cooper & Shallice, 2006; Koechlin & Hyafil, 2007; Lichtenstein & Brewer, 1980; Zalla et al., 2003). If this hypothesis is true, then the order of consecutive events clustered together in a script should be judged differently than the order of consecutive events that are less tightly associated.
We compared participants' accuracies and latencies when asked to judge the order of more highly associated WC events compared to less associated DC events. First, we found that controls and both patient groups were more accurate in their judgments of WC events relative to DC events. The accuracy analysis was not sufficiently sensitive to capture difficulty among PDD/DLB patients in appreciating the special status of WC events. However, we found that healthy seniors and non-demented PD patients responded more quickly to WC relative to DC events. The differential treatment of WC and DC events is consistent with the claim that controls and non-demented PD patients perceive an intrinsic clustered-hierarchical script organization and take advantage of this during procedural discourse in a statistically reliable manner. In contrast, PDD/DLB patients did not consistently respond more quickly to WC events, suggesting relative insensitivity to internal script structure. Among patients with Lewy body spectrum disorders, difficulty processing the clustered-hierarchical structure of a script was related to impaired performance on executive measures. Finally, imaging analysis in a subset of patients suggested a relation between frontal atrophy and the difference in response accuracy and latency to judge the order of WC versus DC event pairs. These findings implicate frontal/executive dysfunction in relative difficulty processing the clustered-hierarchical organizational structure of multi-event scripts.
Our findings are consistent with previous studies demonstrating the clustered-hierarchical organization of complex, multi-step scripts. A study of patients with frontal lobe injury showed difficulty judging the boundary of “large events” in a script compared to “small events,” although these patients performed similarly to controls when judging the boundary of small events (Zalla et al., 2003). An interpretation of this finding is that “large events” in fact consist of multiple “small events,” and patients have difficulty appreciating how constituent events are related to each other and organized into the superordinate “large event.” However, the study could not exclude the possibility of a linear-sequential processing impairment. Moreover, large events by definition contain more components and may have involved greater working memory demands relative to small events. A subsequent study by Farag et al. (2010) demonstrated more explicitly that scripts feature a clustered-hierarchical organizational scheme. The authors showed differential treatment of highly associated WC events relative to less closely associated DC events in young controls, healthy seniors, and patients with semantic dementia and Alzheimer's disease. In contrast, patients with bvFTD and PNFA were relatively insensitive to this internal script organization and responded similarly to the two types of event pairs. The latter two groups have executive difficulty and disease in frontal brain regions, and this was related to their poor performance judging highly associated pairs of events. Thus, using different patient populations that are marked by prominent executive deficits, this previous report and the current study provide converging evidence consistent with the claim that complex multi-event materials like scripts have a clustered-hierarchical structure and that processing this structure relies in part on intact frontal function.
The PD patients participating in this study were carefully screened for any cognitive deficits. As a group, PD patients included in this study were unimpaired on tests of frontal/executive function. In contrast, the PDD/DLB group exhibited deficits relative to controls on executive measures. Other work has demonstrated executive deficits in patients with Lewy body spectrum disorders (Aarsland, Litvan et al., 2003; Barbas, 2006; Kobayakawa et al., 2010; Muslimovic et al., 2005; Troster, 2008; Uekermann et al., 2004; Weintraub et al., 2005). In this study, difficulty processing the clustered-hierarchical organization of scripts was related to executive measures, including tasks that depend in part on mental organization and working memory (i.e., verbal fluency and reverse digit span). Thus across the combined group of patients with Lewy body spectrum disorders, patients with greater executive dysfunction showed greater difficulty appreciating the special status of closely-associated WC event pairs. Executive dysfunction can disrupt the ability to plan and organize in a wide range of circumstances (Grafman, 2007). The current report suggests that such difficulty extends to deficits processing the organizational structure of discourse. Indeed, impairments of both procedural and narrative discourse have been demonstrated in patients with Lewy body spectrum disorders and other conditions affecting the frontal lobes (Ash et al., 2006; Berg et al., 2003; Cosentino et al., 2006; Godbout & Doyon, 1995, 2000; Murray, 2000; Zalla et al., 2000). In addition to the relationship between performance on the script task and executive function, accuracy judging the order of WC event pairs was associated with performance on the BNT. We discuss this in greater detail below.
We observed significant prefrontal disease in a subset of patients with Lewy body spectrum disorders who underwent brain MRI, consistent with previous imaging studies (Burton, McKeith, Burn, Williams, & O'Brien, 2004). Moreover, in the current report, the difference in response accuracy and latency between correctly judged WC and DC events pairs was related directly to atrophy in frontal cortex. This observation helps guide interpretation of our behavioral findings that frontal cortex contributes to processing the clustered-hierarchical organization of scripts. Using the same stimuli, an fMRI study of healthy controls showed greater bilateral inferior frontal activation in judgments of WC relative to DC event pairs (Farag et al., 2010). In a structural MRI analysis, these authors also showed that patients with PNFA and bvFTD, who did not appreciate the special status of WC events, have significantly more atrophy relative to controls in a region of interest corresponding to the left inferior frontal activation seen in the fMRI study (Farag et al., 2010). Other functional imaging studies have also shown involvement of prefrontal cortex using a variety of discourse-level tasks, including judging the coherence of consecutive sentences (Ferstl & von Cramon, 2001, 2002; Ferstl et al., 2002), processing sentences set in a narrative context (Xu et al., 2005), extracting the theme or moral of stories (Nichelli et al., 1995), and integrating information into an ongoing narrative (Ferstl et al., 2005). Production studies have also implicated inferior frontal areas in discourse-level organization. One study examined narratives produced by patients with frontal disease due to bvFTD. A detailed analysis of patients' narratives revealed poor organization of story elements and limited ability to maintain the story theme, which was related to significant frontal (including inferior frontal) and anterior temporal atrophy (Ash et al., 2006). In a study of healthy adults using the same task, Troiani and colleagues used arterial spin labeling perfusion fMRI to show involvement of left inferior frontal cortex in organizational aspects of narrative production (Troiani et al., 2008).
Based on the finding that script comprehension performance was correlated with executive measures and frontal lobe atrophy, we propose that relative insensitivity to the clustered-hierarchical organization of scripts is related in part to frontal/executive dysfunction in mildly demented patients with PDD/DLB. However, other possible explanations for the lack of difference in latencies to respond to WC and DC event pairs in the PDD/DLB group should be considered. Although imaging analysis did not reveal correlation between task performance and subcortical structures, we cannot rule out the possibility that subcortical dysfunction contributed to impaired script processing in patients with Lewy body spectrum disorder. Indeed, these disorders are known to affect the basal ganglia and frontal-striatal networks, which have been implicated in such relevant processes as temporal sequencing and selection of competing actions (Da Cunha, Gomez, & Blaha, xxxx; Tinaz, Schendan, Schon, & Stern, 2006; Tinaz, Schendan, & Stern, 2008). Moreover, across the combined patient group, accuracy and latency to respond to WC event pairs was related to performance on the MMSE and DRS. These measures of general cognitive status contain tests of executive function. Nevertheless, it remains plausible that general cognitive decline contributes to script task performance. Another possible explanation is that inattention among PDD/DLB patients compromised performance on the script task. Evidence of adequate attentiveness comes from the fact that stimuli were excluded because of outliers (responses that were too rapid or too slow due to inattention) no more often in the PDD/DLB group than in controls and non-demented PD patients. In addition, patients with PDD/DLB performed normally on the forward digit span task (mean (SD) Z score relative to age-and education-matched controls = −0.6 (0.9)). These findings are consistent with the observation of trained examiners that all study participants were alert, cooperative, and able to remain on task. Nonetheless, to exclude the potential confound of inattention, future investigations of script performance in PDD/DLB patients would benefit from more formal testing of attention (e.g., using a continuous performance task).
Another possibility is that deficits of lexical retrieval and/or semantic knowledge may have contributed to patients' performance on the script task. In a script comprehension study involving patients with frontal disease due to bvFTD, Cosentino and colleagues showed difficulty detecting sequencing errors compared to semantic errors, suggesting that there is an organizational component to script processing which is separable from knowledge of script content (Cosentino et al., 2006). Previous studies of script processing in PD have suggested impaired representation of script knowledge (Godbout & Doyon, 2000). The authors propose degraded representations of familiar actions as an explanation for some aspects of impaired script processing in PD, such as generating scripts with fewer contextual elements. Recent work implicating a semantic deficit in discourse comes from clever priming studies. When faced with lexical ambiguities at the end of a sentence or paragraph, PD patients have difficulty selecting the context-appropriate meaning in the setting of long inter-stimulus intervals. Difficulty priming over this prolonged time course in PD was thought to reflect a limitation in more controlled or strategic forms of context-based semantic activation mediated by executive resources (Copland, Chenery, & Murdoch, 2000, 2001). More recently, these investigators showed that PD patients have difficulty suppressing an incongruous meaning of a homophone during a second presentation of the priming pair, which they attributed to impairment of controlled semantic activation related to limitation of executive resources, such as working memory needed to maintain meaning in an active state during discourse (Copland, Sefe, Ashley, Hudson, & Chenery, 2009). This group has also provided evidence for delayed lexical activation in PD, as well as for the influence of dopaminergic medications on semantic processing in these patients (Angwin, Chenery, Copland, Murdoch, & Silburn, 2007; Angwin, Copland, Chenery, Murdoch, & Silburn, 2006). The behavioral analysis provided mixed evidence, where BNT performance correlated with accuracy, but not latency, to respond to WC event pairs. Imaging analysis revealed correlation between task performance and posterior regions, such as the fusiform gyrus and other occipital and temporal areas. These regions have been implicated in visually-based semantics (Heath et al., 2012), and it is possible that these regions support patients' generation of a mental image of the script. Additional work is needed to assess the role of lexical retrieval and semantic memory in script comprehension in patients with Lewy body spectrum disorders. Another issue is whether use of dopaminergic medications could have affected performance on the experimental task. All but six of 42 patients (2 PD, 4 PDD/DLB) were taking dopaminergic medications. Indeed, differential use of dopaminergic medications may be expected in patients with PD versus those with PDD/DLB, as individuals in the latter group would be more vulnerable to cognitive and/or psychiatric side effects, making clinicians less likely to prescribe them for these patients. There was a trend toward greater levodopa equivalents in the PD group, although this difference did not reach statistical significance. The patients in each group who were not taking dopaminergic medications did not differ in terms of overall response accuracy and/or latency from other members of their respective groups. Moreover, correlation analyses did not reveal a relationship between levodopa equivalents and measures of performance on the experimental task.
In this study, PDD/DLB patients were significantly older than PD patients, consistent with work demonstrating age as a risk factor for the development of dementia in PD (Hughes et al., 2000). Little is known about the effects of aging on script processing. Age is thought to be a factor in some aspects of cognitive performance, particularly on measures of verbal fluency (Loonstra, Tarlow, & Sellers, 2001), which were related to task performance in this study. Nevertheless, differences in task performance between patients in the PD and PDD/DLB groups remained after covarying for age. In our population, age was related to accuracy and latency to respond to WC and DC events, as well as with performance on letter- and category-guided verbal fluency and the BNT. However, regression analyses were carried out between experimental measures and each related cognitive measure. In no case did a consideration of age substantially change the relationship between variables. These findings suggest that greater age cannot fully explain impaired task performance in the PDD/DLB group.
5. Conclusion
In the current study, we investigated processing of script structure in patients with Lewy body spectrum disorders. In addition to our discussion above, several caveats should be kept in mind when considering our findings. We examined small numbers of patients, and additional work is needed with larger numbers of patients to confirm our observations. Future studies would also benefit from more extensive executive function testing to appreciate more fully what aspects of executive function are needed for script order judgments. Moreover, it would be valuable in future studies, where executive measures are available for control participants, to determine if relationships between executive function and script processing are qualitatively different between patients and healthy adults. An additional limitation of this study is the relatively long interval between the experimental task and cognitive testing or imaging among some patients with Lewy body spectrum disorders. The ecological validity of our experimental measure remains to be examined with measures of functional status, communication efficacy, and quality of life. With these caveats in mind, we found that mildly demented patients with PDD/DLB showed relatively impaired appreciation of the special status of highly associated WC script events. Relative insensitivity to the clustered-hierarchical organization of scripts was related to executive dysfunction and frontal lobe atrophy, consistent with a wider body of work demonstrating the crucial role of frontal cortex in the organization of complex human behavior. Our observations underscore a real-world consequence of prefrontal disease in patients with PDD/DLB, where difficulty processing a series of events compromises communication with substantial impact on patients in their daily lives.
Supplementary Material
Acknowledgments
This work was supported by the Morris K. Udall Parkinson's Disease Research Center of Excellence and the National Institutes of Health (NS53488, AG15116, AG17586, NS44266). In addition, R.G.G. received support from an American Academy of Neurology Foundation grant, C.T.M. from NIH grant HD060406, P.C. by NIH Grant T32NS054575, and A.S. from a health research grant awarded by the Department of Health of the Commonwealth of Pennsylvania (SAP4100027296) from the Tobacco Master Settlement Agreement under Act 2001-77. The authors would like to thank the individuals who participated in this study.
Footnotes
Portions of this work were presented at the American Academy of Neurology Annual Meeting, Seattle, WA, in April 2009 and at the Movement Disorders Society Annual Meeting, Paris, France in June 2009.
Appendix A. Supplementary material Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bandl.2013.02.006.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: Comparison with progressive supranuclear palsy and Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74(9):1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: A prospective, community-based study. Annals of Neurology. 2005;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- Angwin AJ, Chenery HJ, Copland DA, Murdoch BE, Silburn PA. The speed of lexical activation is altered in Parkinson's disease. Journal of Clinical and Experimental Neuropsychology. 2007;29(1):73–85. doi: 10.1080/13803390500507188. [DOI] [PubMed] [Google Scholar]
- Angwin AJ, Copland DA, Chenery HJ, Murdoch BE, Silburn PA. The influence of dopamine on semantic activation in Parkinson's disease: Evidence from a multipriming task. Neuropsychology. 2006;20(3):299–306. doi: 10.1037/0894-4105.20.3.299. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66(9):1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Avants BB, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23(Suppl. 1):S139–150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Barbas NR. Cognitive, affective, and psychiatric features of Parkinson's disease. Clinics in Geriatric Medicine. 2006;22(4):773–796. v–vi. doi: 10.1016/j.cger.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. 2nd ed. AJA Associates, Inc.; Iowa City: 1976. [Google Scholar]
- Berg E, Bjornram C, Hartelius L, Laakso K, Johnels B. High-level language difficulties in Parkinson's disease. Clinical Linguistics and Phonetics. 2003;17(1):63–80. doi: 10.1080/0269920021000055540. [DOI] [PubMed] [Google Scholar]
- Black JB, Bower GH. Episodes as chunks in narrative memory. Journal of Verbal Learning and Verbal Behavior. 1979;18:309–318. [Google Scholar]
- Botvinick MM. Hierarchical models of behavior and prefrontal function. Trends in Cognitive Sciences. 2008;12(5):201–208. doi: 10.1016/j.tics.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Watanabe T. From numerosity to ordinal rank: A gain-field model of serial order representation in cortical working memory. Journal of Neuroscience. 2007;27(32):8636–8642. doi: 10.1523/JNEUROSCI.2110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: A comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: A 12-year population study. Neurology. 2008;70(13):1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Bonte FJ, Wong SB, Zientz JN, Hynan LS, Harris TS, et al. Convergence of connected language and SPECT in variants of frontotemporal lobar degeneration. Alzheimer Disease and Associated Disorders. 2005;19(4):202–213. doi: 10.1097/01.wad.0000189050.41064.03. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Zientz J, Weiner M, Rosenberg R, Frawley W, Burns MH. Discourse changes in early Alzheimer disease, mild cognitive impairment, and normal aging. Alzheimer Disease and Associated Disorders. 2002;16(3):177–186. doi: 10.1097/00002093-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Coelho CA, Grela B, Corso M, Gamble A, Feinn R. Microlinguistic deficits in the narrative discourse of adults with traumatic brain injury. Brain Injury. 2005;19(13):1139–1145. doi: 10.1080/02699050500110678. [DOI] [PubMed] [Google Scholar]
- Cooper RP, Shallice T. Hierarchical schemas and goals in the control of sequential behavior. Psychological Review. 2006;113(4):887–916. doi: 10.1037/0033-295X.113.4.887. discussion 917–831. [DOI] [PubMed] [Google Scholar]
- Copland DA, Chenery HJ, Murdoch BE. Understanding ambiguous words in biased sentences: Evidence of transient contextual effects in individuals with nonthalamic subcortical lesions and Parkinson's disease. Cortex. 2000;36(5):601–622. doi: 10.1016/s0010-9452(08)70541-0. [DOI] [PubMed] [Google Scholar]
- Copland DA, Chenery HJ, Murdoch BE. Discourse priming of homophones in individuals with dominant nonthalamic subcortical lesions, cortical lesions and Parkinson's disease. Journal of Clinical and Experimental Neuropsychology. 2001;23(4):538–556. doi: 10.1076/jcen.23.4.538.1233. [DOI] [PubMed] [Google Scholar]
- Copland DA, Sefe G, Ashley J, Hudson C, Chenery HJ. Impaired semantic inhibition during lexical ambiguity repetition in Parkinson's disease. Cortex. 2009;45(8):943–949. doi: 10.1016/j.cortex.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Chute D, Libon D, Moore P, Grossman M. How does the brain support script comprehension? A study of executive processes and semantic knowledge in dementia. Neuropsychology. 2006;20(3):307–318. doi: 10.1037/0894-4105.20.3.307. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gomez AA, Blaha CD. The role of the basal ganglia in motivated behavior. Rev. Neurosci. 2012;23(5–6):747–767. doi: 10.1515/revneuro-2012-0063. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson's disease dementia: Recommendations from the movement disorder society task force. Movement Disorders. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: A population-based study. European Journal of Neurology. 2009;16(12):1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R, UPDRS Program Members . Unified Parkinson's disease rating scale. In: Fahn S, Marsden C, Goldstein M, Calne D, editors. Recent developments in Parkinson's disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, et al. Hierarchical organization of scripts: Converging evidence from fMRI and frontotemporal degeneration. Cerebral Cortex. 2010;20(10):2453–2463. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl EC, Guthke T, von Cramon DY. Text comprehension after brain injury: Left prefrontal lesions affect inference processes. Neuropsychology. 2002;16(3):292–308. doi: 10.1037//0894-4105.16.3.292. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Rinck M, von Cramon DY. Emotional and temporal aspects of situation model processing during text comprehension: An event-related fMRI study. Journal of Cognitive Neuroscience. 2005;17(5):724–739. doi: 10.1162/0898929053747658. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. The role of coherence and cohesion in text comprehension: An event-related fMRI study. Cognitive Brain Research. 2001;11(3):325–340. doi: 10.1016/s0926-6410(01)00007-6. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. What does the frontomedian cortex contribute to language processing: Coherence or theory of mind? Neuroimage. 2002;17(3):1599–1612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schubotz RI. Dynamic anticipatory processing of hierarchical sequential events: A common role for Broca's area and ventral premotor cortex across domains? Cortex. 2006;42(4):499–502. doi: 10.1016/s0010-9452(08)70386-1. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Smith EE, Jonides J. Distance effects in memory for sequences: Evidence for estimation and scanning processes. Memory. 2007;15(1):104–116. doi: 10.1080/09658210601149702. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Upper processing stages of the perception–action cycle. Trends in Cognitive Sciences. 2004;8(4):143–145. doi: 10.1016/j.tics.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Godbout L, Doyon J. Mental representation of knowledge following frontal-lobe or postrolandic lesions. Neuropsychologia. 1995;33(12):1671–1696. doi: 10.1016/0028-3932(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Godbout L, Doyon J. Defective representation of knowledge in Parkinson's disease: Evidence from a script-production task. Brain and Cognition. 2000;44(3):490–510. doi: 10.1006/brcg.2000.1213. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nature Reviews Neuroscience. 2001;2:492–510. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J. Planning and the brain. In: Miller BL, Cummings JL, editors. The human frontal lobes: Functions and disorders. The Guilford Press; New York: 2007. pp. 249–261. [Google Scholar]
- Gross RG, Ash S, McMillan CT, Gunawardena D, Powers C, Libon DJ, et al. Impaired information integration contributes to communication difficulty in corticobasal syndrome. Cognitive Behavioral Neurology. 2010;23(1):1–7. doi: 10.1097/WNN.0b013e3181c5e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Kalmanson J, Bernhardt N, Morris J, Stern MB, Hurtig HI. Cognitive resource limitations during sentence comprehension in Parkinson's disease. Brain and Language. 2000;73(1):1–16. doi: 10.1006/brln.2000.2290. [DOI] [PubMed] [Google Scholar]
- Hall D, Ouyang B, Lonnquist E, Newcombe J. Pragmatic communication is impaired in Parkinson disease. International Journal of Neuroscience. 2011;121(5):254–256. doi: 10.3109/00207454.2010.550389. [DOI] [PubMed] [Google Scholar]
- Heath S, McMahon K, Nickels L, Angwin A, MacDonald A, van Hees S, et al. Priming picture naming with a semantic task: An fMRI investigation. PLoS ONE. 2012;7(3):e32809. doi: 10.1371/journal.pone.0032809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: The inevitability of dementia at 20 years. Movement Disorders. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: A survey by the Canadian Movement Disorders Group. JAMA. 2002;287(4):455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Horimoto Y, Matsumoto M, Nakazawa H, Yuasa H, Morishita M, Akatsu H, et al. Cognitive conditions of pathologically confirmed dementia with Lewy bodies and Parkinson's disease with dementia. Journal of the Neurological Sciences. 2003;216(1):105–108. doi: 10.1016/s0022-510x(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Hue CW, Erickson JR. Normative studies of sequence strength and scene structure of 30 scripts. American Journal of Psychology. 1991;104:229–240. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology. 2000;54(8):1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54(10):1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. Aggregation of alpha-synuclein in the pathogenesis of Parkinson's disease. Journal of Neurology. 2003;250(Suppl. 3):11–14. doi: 10.1007/s00415-003-1303-x. [DOI] [PubMed] [Google Scholar]
- Joanette Y, Goulet P. Narrative discourse in right-brain-damaged right-handers. In: Joanette Y, Brownell HH, editors. Discourse ability and brain damage: Theoretical and empirical perspectives. Springer Verlag; New York: 1990. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa M, Tsuruya N, Kawamura M. Parkinsonism and Related Disorders. 2010. Sensitivity to reward and punishment in Parkinson's disease: An analysis of behavioral patterns using a modified version of the Iowa gambling task. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lee C, Grossman M, Morris J, Stern MB, Hurtig HI. Attentional resource and processing speed limitations during sentence processing in Parkinson's disease. Brain and Language. 2003;85(3):347–356. doi: 10.1016/s0093-934x(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park HJ, Park B, Song SK, Sohn YH, Lee JD, et al. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson's disease dementia and dementia with Lewy bodies. Journal of Neurology, Neurosurgery and Psychiatry. 2010;81(3):320–326. doi: 10.1136/jnnp.2009.184747. [DOI] [PubMed] [Google Scholar]
- Levy G, Jacobs DM, Tang MX, Cote LJ, Louis ED, Alfaro B, et al. Memory and executive function impairment predict dementia in Parkinson's disease. Movement Disorders. 2002;17(6):1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Neuroscience. 2003;23(15):6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68(5):369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Lichtenstein EH, Brewer WF. Memory for goal-directed events. Cognitive Psychology. 1980;12:412–445. [Google Scholar]
- Llebaria G, Pagonabarraga J, Kulisevsky J, Garcia-Sanchez C, Pascual-Sedano B, Gironell A, et al. Cut-off score of the Mattis Dementia Rating Scale for screening dementia in Parkinson's disease. Movement Disorders. 2008;23(11):1546–1550. doi: 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. Applied Neuropsychology. 2001;8(3):161–166. doi: 10.1207/S15324826AN0803_5. [DOI] [PubMed] [Google Scholar]
- Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Human Brain Mapping. 2004;22(3):236–245. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, et al. Normative data for the Mattis Dementia Rating Scale. Journal of Clinical and Experimental Neuropsychology. 1998;20(4):536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neuropsychology of narrative: Story comprehension, story production and their interrelation. Neuropsychologia. 2004;42(10):1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McNamara P, Durso R. Pragmatic communication skills in patients with Parkinson's disease. Brain and Language. 2003;84(3):414–423. doi: 10.1016/s0093-934x(02)00558-8. [DOI] [PubMed] [Google Scholar]
- McNamara P, Holtgraves T, Durso R, Harris E. Social cognition of indirect speech: Evidence from Parkinson's Disease. Journal of Neurolinguistics. 2010;23(2):162. doi: 10.1016/j.jneuroling.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monetta L, Grindrod CM, Pell MD. Irony comprehension and theory of mind deficits in patients with Parkinson's disease. Cortex. 2009;45(8):972–981. doi: 10.1016/j.cortex.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Monetta L, Pell MD. Effects of verbal working memory deficits on metaphor comprehension in patients with Parkinson's disease. Brain and Language. 2007;101(1):80–89. doi: 10.1016/j.bandl.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Mather G, Wesnes KA, O'Brien JT, Burn DJ, McKeith IG. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2004;63(11):2091–2096. doi: 10.1212/01.wnl.0000145764.70698.4e. [DOI] [PubMed] [Google Scholar]
- Murray LL. Spoken language production in Huntington's and Parkinson's diseases. Journal of Speech, Language and Hearing Research. 2000;43(6):1350–1366. doi: 10.1044/jslhr.4306.1350. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R. Where the brain appreciates the moral of a story. NeuroReport. 1995;6(17):2309–2313. doi: 10.1097/00001756-199511270-00010. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain. 2008;131(Pt 5):1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Schank R, Abelson R. Scripts, plans, goals, and understanding: An inquiry into human knowledge structures. Erlbaum; Hillsdale, NJ: 1977. [Google Scholar]
- Schubotz RI, Sakreida K, Tittgemeyer M, von Cramon DY. Motor areas beyond motor performance: Deficits in serial prediction following ventrolateral premotor lesions. Neuropsychology. 2004;18(4):638–645. doi: 10.1037/0894-4105.18.4.638. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Interval and ordinal properties of sequences are associated with distinct premotor areas. Cerebral Cortex. 2001;11(3):210–222. doi: 10.1093/cercor/11.3.210. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Zalla T, Pillon B, Grafman J, Dubois B, Agid Y. Planning and script analysis following prefrontal lobe lesions. Annals of the New York Academy of Sciences. 1995;769:277–288. doi: 10.1111/j.1749-6632.1995.tb38145.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tettamanti M, Weniger D. Broca's area: A supramodal hierarchical processor? Cortex. 2006;42(4):491–494. doi: 10.1016/s0010-9452(08)70384-8. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Schon K, Stern CE. Evidence for the importance of basal ganglia output nuclei in semantic event sequencing: An fMRI study. Brain Research. 2006;1067(1):239–249. doi: 10.1016/j.brainres.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Stern CE. Fronto-striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiology of Aging. 2008;29(3):397–407. doi: 10.1016/j.neurobiolaging.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Fernandez-Seara MA, Wang Z, Detre JA, Ash S, Grossman M. Narrative speech production: An fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40(2):932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson's disease with dementia: Differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychology Review. 2008;18(1):103–119. doi: 10.1007/s11065-008-9055-0. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Bielawski M, Muhlack S, Peters S, Przuntek H, et al. Differential executive control impairments in early Parkinson's disease. Journal of Neural Transmission. 2004;Suppl(68):39–51. doi: 10.1007/978-3-7091-0579-5_5. [DOI] [PubMed] [Google Scholar]
- van der Meer E, Beyer R, Heinze B, Badel I. Temporal order relations in language comprehension. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2002;28(4):770–779. doi: 10.1037//0278-7393.28.4.770. [DOI] [PubMed] [Google Scholar]
- van Schie HT, Toni I, Bekkering H. Comparable mechanisms for action and language: Neural systems behind intentions, goals, and means. Cortex. 2006;42(4):495–498. doi: 10.1016/s0010-9452(08)70385-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler memory scale – Revised. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Katz IR, Stern MB. Dimensions of executive function in Parkinson's disease. Dementia and Geriatric Cognitive Disorders. 2005;20(2–3):140–144. doi: 10.1159/000087043. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: Emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25(3):1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zalla T, Pradat-Diehl P, Sirigu A. Perception of action boundaries in patients with frontal lobe damage. Neuropsychologia. 2003;41(12):1619–1627. doi: 10.1016/s0028-3932(03)00098-8. [DOI] [PubMed] [Google Scholar]
- Zalla T, Sirigu A, Pillon B, Dubois B, Agid Y, Grafman J. How patients with Parkinson's disease retrieve and manage cognitive event knowledge. Cortex. 2000;36(2):163–179. doi: 10.1016/s0010-9452(08)70522-7. [DOI] [PubMed] [Google Scholar]
- Zalla T, Sirigu A, Pillon B, Dubois B, Grafman J, Agid Y. Deficit in evaluating pre-determined sequences of script events in patients with Parkinson's disease. Cortex. 1998;34(4):621–627. doi: 10.1016/s0010-9452(08)70519-7. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Foldi NS, Borod JC. Cognitive and behavioral dysfunction in Parkinson's disease: Neurochemical and clinicopathological contributions. Journal of Neural Transmission. 2004;111(10–11):1287–1301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.