Abstract
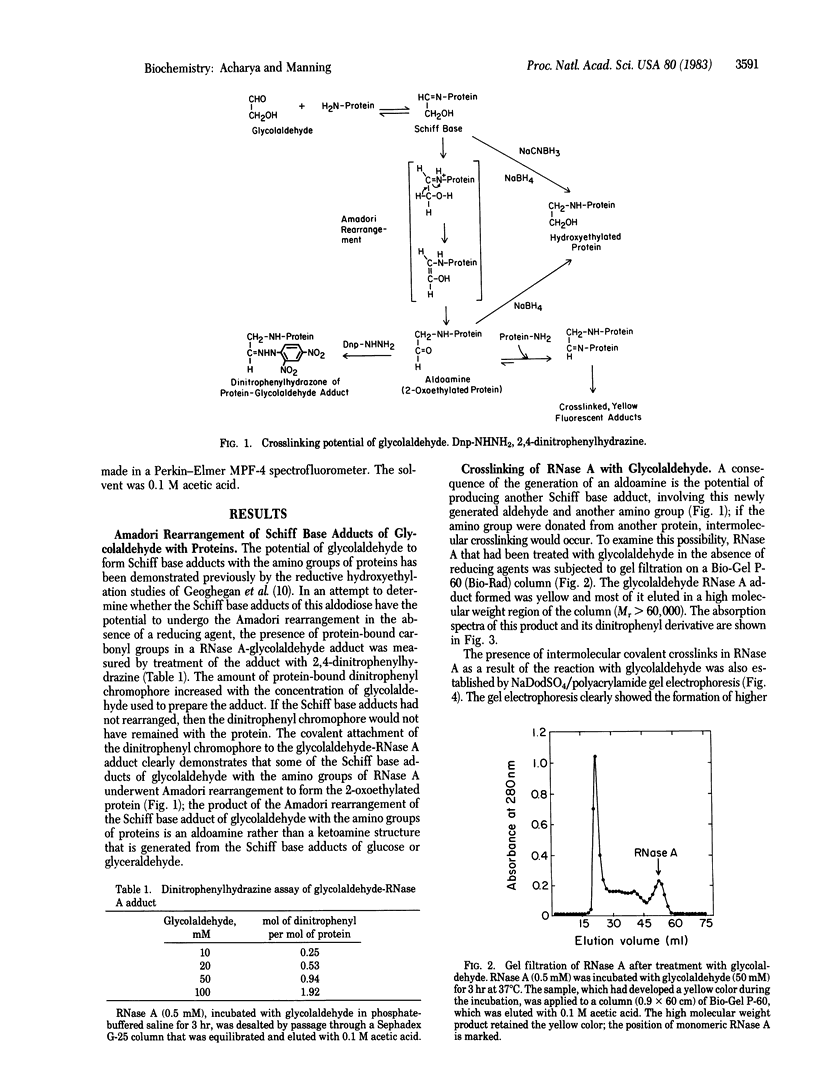
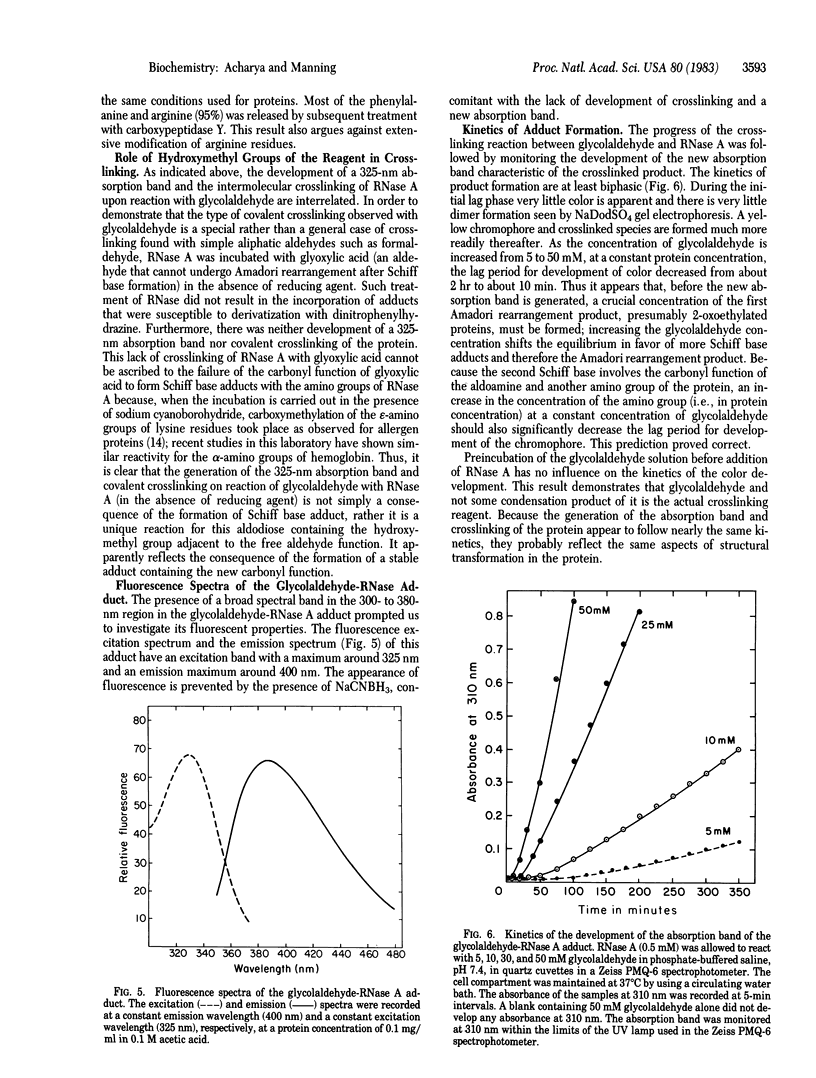
The Schiff base adducts of glyceraldehyde with hemoglobin undergo Amadori rearrangement to form stable ketoamine structures; this reaction is similar to the nonenzymic glucosylation of proteins. In the present studies the analogous rearrangement of the Schiff base adducts of glycolaldehyde with proteins has been demonstrated. However, the Amadori rearrangement of the Schiff base adduct produces a new aldehyde function, an aldoamine, which is generated in situ and is capable of forming Schiff base linkages with another amino group, leading to covalent crosslinking of proteins. Sodium dodecyl sulfate gel electrophoresis of the glycoaldehyde-RNase A adduct showed the presence of dimers, trimers, and tetramers of RNase A, demonstrating the crosslinking potential of this alpha-hydroxyaldehyde. The crosslinked products exhibited an absorption band with a maximum around 325 nm and fluorescence around 400 nm when excited at 325 nm. The crosslinking reaction, the formation of a 325-nm absorption band, and the development of fluorescence were prevented when the incubation was carried out in the presence of sodium cyanoborohydride. This finding indicates that the Amadori rearrangement that generates a new carbonyl function is a crucial step in this covalent crosslinking. Glycolaldehyde could be a bifunctional reagent of unique utility because its crosslinking potential is latent, expressed only upon completion of the primary reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Manning J. M. Amadori rearrangement of glyceraldehyde-hemoglobin Schiff base adducts. A new procedure for the determination of ketoamine adducts in proteins. J Biol Chem. 1980 Aug 10;255(15):7218–7224. [PubMed] [Google Scholar]
- Acharya A. S., Manning J. M. Reactivity of the amino groups of carbonmonoxyhemoglobin S with glyceraldehyde. J Biol Chem. 1980 Feb 25;255(4):1406–1412. [PubMed] [Google Scholar]
- Acharya A. S., Sussman L. G., Manning J. M. Schiff base adducts of glyceraldehyde with hemoglobin. Differences in the Amadori rearrangement at the alpha-amino groups. J Biol Chem. 1983 Feb 25;258(4):2296–2302. [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry. 1969 Jul;8(7):2821–2826. doi: 10.1021/bi00835a019. [DOI] [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Dykes G., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the fibres of sickle cell haemoglobin. Nature. 1978 Apr 6;272(5653):506–510. doi: 10.1038/272506a0. [DOI] [PubMed] [Google Scholar]
- Fields R., Dixon H. B. Micro method for determination of reactive carbonyl groups in proteins and peptides, using 2,4-dinitrophenylhydrazine. Biochem J. 1971 Feb;121(4):587–589. doi: 10.1042/bj1210587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galembeck F., Ryan D. S., Whitaker J. R., Feeney R. E. Reaction of proteins with formaldehyde in the presence and absence of sodium borohydride. J Agric Food Chem. 1977 Mar-Apr;25(2):238–245. doi: 10.1021/jf60210a019. [DOI] [PubMed] [Google Scholar]
- Geoghegan K. F., Ybarra D. M., Feeney R. E. Reversible reductive alkylation of amino groups in proteins. Biochemistry. 1979 Nov 27;18(24):5392–5399. doi: 10.1021/bi00591a021. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- King T. P., Kochoumian L., Lichtenstein L. M. Preparation and immunochemical properties of methoxypolyethylene glycol-coupled and N-carboxymethylated derivatives of ragweed pollen allergen, antigen E. Arch Biochem Biophys. 1977 Jan 30;178(2):442–450. doi: 10.1016/0003-9861(77)90214-4. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- Martin C. J., Marini M. A. Spectral detection of the reaction of formaldehyde with the histidine residues of alpha-chymotrypsin. J Biol Chem. 1967 Dec 25;242(24):5736–5743. [PubMed] [Google Scholar]
- Means G. E., Feeney R. E. Reductive alkylation of amino groups in proteins. Biochemistry. 1968 Jun;7(6):2192–2201. doi: 10.1021/bi00846a023. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Namiki M., Hayashi T., Ohta Y. Novel free radicals formed by the amino-carbonyl reactions of sugars with amino acids, amines, and proteins. Adv Exp Med Biol. 1977;86B:471–501. doi: 10.1007/978-1-4757-9113-6_28. [DOI] [PubMed] [Google Scholar]
- Nigen A. M., Manning J. M. Inhibition of erythrocyte sickling in vitro by DL-glyceraldehyde. Proc Natl Acad Sci U S A. 1977 Jan;74(1):367–371. doi: 10.1073/pnas.74.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971 Jun;43(7):880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Shapiro R., McManus M. J., Zalut C., Bunn H. F. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980 Apr 10;255(7):3120–3127. [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]




