Abstract
An important component of barley cell walls, particularly in the endosperm, is (1,3;1,4)-β- glucan, a polymer that has proven health benefits in humans and that influences processability in the brewing industry. Genes of the cellulose synthase-like (Csl) F gene family have been shown to be involved in (1,3;1,4)-β-glucan synthesis but many aspects of the biosynthesis are still unclear. Examination of the sequence assembly of the barley genome has revealed the presence of an additional three HvCslF genes (HvCslF11, HvCslF12 and HvCslF13) which may be involved in (1,3;1,4)-β-glucan synthesis. Transcripts of HvCslF11 and HvCslF12 mRNA were found in roots and young leaves, respectively. Transient expression of these genes in Nicotiana benthamiana resulted in phenotypic changes in the infiltrated leaves, although no authentic (1,3;1,4)-β-glucan was detected. Comparisons of the CslF gene families in cereals revealed evidence of intergenic recombination, gene duplications and translocation events. This significant divergence within the gene family might be related to multiple functions of (1,3;1,4)-β-glucans in the Poaceae. Emerging genomic and global expression data for barley and other cereals is a powerful resource for characterising the evolution and dynamics of complete gene families. In the case of the CslF gene family, the results will contribute to a more thorough understanding of carbohydrate metabolism in grass cell walls.
Background
Interest in barley as a food component has been increasing due to the comparatively high levels of mixed linkage (1,3;1,4)-β-glucan found in the grain. In 2006, the U.S. Food and Drug Administration (FDA) approved health-related claims stating that the intake of 3 grams of soluble β-glucan (from oat or barley) per day helps to effectively lower blood total and LDL cholesterol [1], [2]. The (1,3;1,4)-β-glucan functions as soluble dietary fibre and has additional health benefits in reducing the risk of cardiovascular disease (CVD), type II diabetes and colorectal cancer [1], [2]. In the gastrointestinal tract, (1,3;1,4)-β-glucan is believed to form a gel matrix that increases bile acid excretion and delays glucose absorption into the blood, thus lowering insulin levels. The health properties of (1,3;1,4)-β-glucan are thus dependent on its molecular weight (MW) and solubility [3]. However, in the brewing and distilling industries, high levels of (1,3;1,4)-β-glucan are undesirable, causing problems with filtration and decreasing processability. Similarly, (1,3;1,4)-β-glucans are classified as anti-nutrients in animal feed formulations, where they reduce growth rates of monogastric animals [4].
Although commonly found in walls of the graminaceous monocotyledons, (1,3;1,4)-β-glucan is generally absent from dicotyledon cell walls. The polymer is a major constituent of the primary cell wall and more minor component of secondary cell walls in most members of the Poaceae, including the common cereals wheat, barley and oat [5]. In seeds, (1,3;1,4)-β-glucan may play a role in energy storage and it is believed to have a growth-related function in vegetative tissues, although significant levels of (1,3;1,4)-β-glucan also occur in mature tissues of rice and some other grasses [6]. The first functional identification of a gene capable of synthesising (1,3;1,4)-β-glucan came from Burton et al. [7] who transformed the dicot Arabidopsis thaliana with a cellulose synthase-like CslF2 gene from rice (Oryza sativa) and demonstrated the subsequent presence of a small amount of (1,3;1,4)-β-glucan in the dicot cell walls. In 2009, Doblin et al. introduced a CslH gene from barley into Arabidopsis and this gene also promoted synthesis of detectable amounts of (1,3;1,4)-β-glucan; thus it appears that two different gene families could be involved in the synthesis of the polymer. To date, involvement in (1,3;1,4)-β-glucan synthesis has been demonstrated for the barley proteins CslF4, CslF6 and CslH [8], [9]. The CslF and CslH genes are members of the superfamily of cellulose synthases (CesA genes) and cellulose synthase-like (Csl) genes [10]. Further investigations of (1,3;1,4)-β-glucan synthesis and the CslH1 gene showed that the enzyme, but not the (1,3;1,4)-β-glucan, could be detected in the Golgi apparatus by antibodies. The (1,3;1,4)-β-glucan can only be detected outside the plasma membrane. The hypothesis is that a modification occurs at the plasma membrane, making polymer epitopes accessible to the antibody [7], [9], [11].
Given the emerging importance of the (1,3;1,4)-β-glucan polymer for both human health and industry, it is of interest to understand which of the Csl genes have the potential to direct (1,3;1,4)-β-glucan synthesis, where and when they mediate it, and how the polysaccharide is used by the plant during different phases of its life cycle. In this paper we use the new barley genome assembly [12] to re-examine the composition and dynamics of the HvCslF gene family from barley, and also perform an initial analysis of the gene family in wheat. Our results provide a platform for understanding the different roles HvCslF genes may play in barley growth, development and interaction with its environment.
Results
Identification and mapping of barley CslF genes including three previously undescribed genes
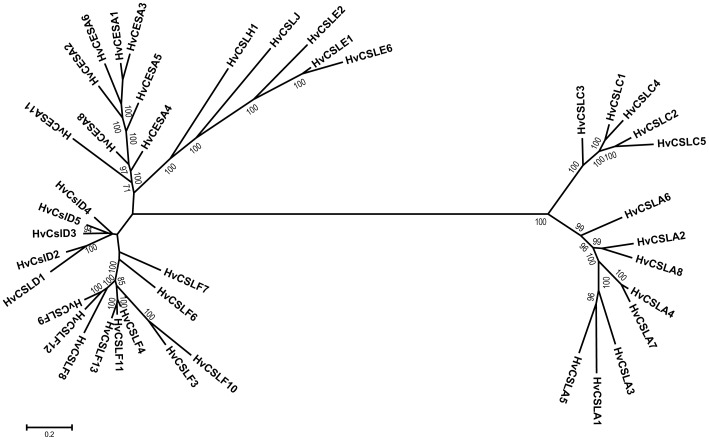
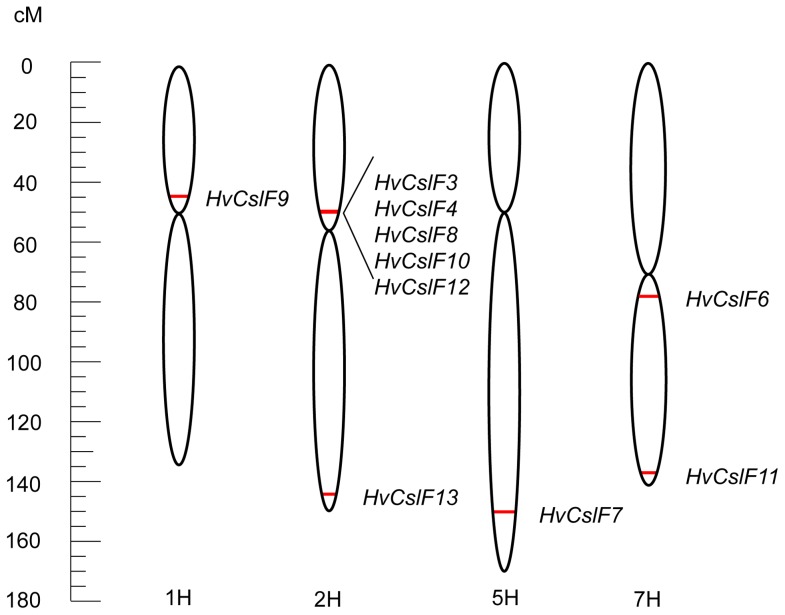
Sequences for the seven known barley CslF family genes were collected from GenBank (i.e. HvCslF3, HvCslF4, HvCslF6, HvCslF7, HvCslF8, HvCslF9, and HvCslF10). These seven genes, along with mutant versions of CslF6, are the only barley CslF sequences currently listed on the Carbohydrate-Active Enzymes database (www.cazy.org). A BLAST search of the newly available barley cv. Morex sequence assembly [12] resulted in seven sequences identical to those from GenBank together with three new sequences. The CslF family members are named after their homologs in rice, starting with HvCslF3 as there are no homologous sequences in barley to OsCslF1 and OsCslF2. OsCslF5 is thought to be a pseudogene and there are no homologs in barley. An additional CslF gene was previously found in barley compared to rice and named HvCslF10 [13]. In keeping with this scheme, the three new sequences described here were named HvCslF11, HvCslF12 and HvCslF13. A phylogenetic tree (Figure 1) clearly shows that HvCslF11 and HvCslF13 are most closely related to HvCslF4, while HvCslF12 is most closely related to HvCslF9. The genetic location of all ten barley CslF genes was determined from the barley genome assembly [12]. The HvCslF9 gene is located on the short arm of chromosome 1H, HvCslF7 is located on the long arm of chromosome 5H and HvCslF6 is located on the long arm of chromosome 7H (Figure 2). The other members, HvCslF3, HvCslF4, HvCslF8 and HvCslF10, are localized in a cluster on chromosome 2H near the centromeric region. Of the three new sequences, HvCslF12 is also located in this cluster on the short arm of chromosome 2H and HvCslF11 is on the long arm of chromosome 7H. A clear map position was initially not identified for HvCslF13 but a precise mapping position on the long arm of chromosome 2H was determined by analysis of the results from Mascher et al. [14] (Figure 2).
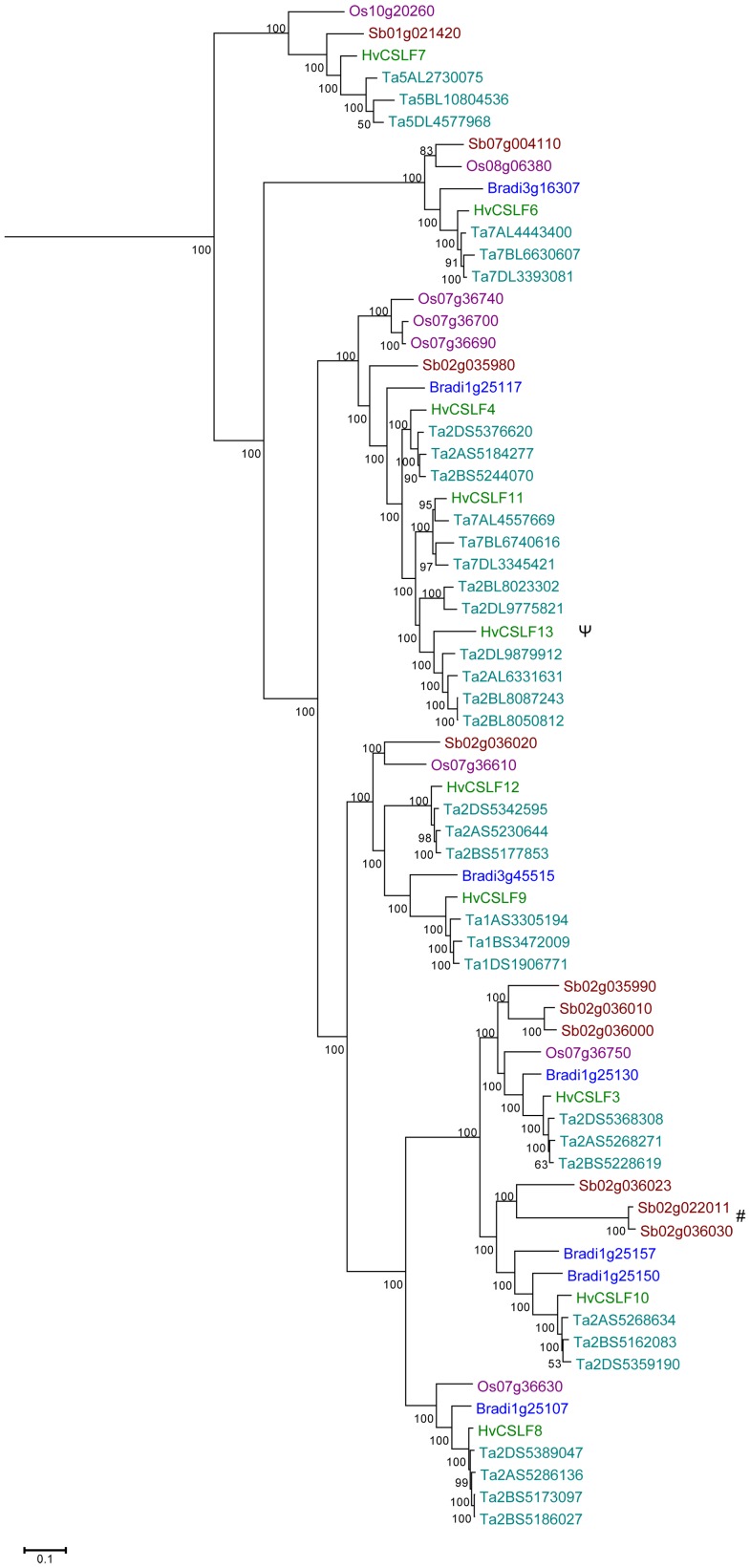
Figure 1. An unrooted phylogenetic tree of the barley Csl super family.
Phylogenetic analysis was done using MrBayes (codon position model) in TOPALi v2. The posterior probabilities have been multiplied by 100. The scale bar shows expected number of nucleotide substitutions per site.
Figure 2. The genetic location of barley HvCslF genes.
Genetic map of barley chromosomes 1H, 2H, 5H, and 7H showing the positions of barley HvCslF genes as mapped in a ‘Morex’ × ‘Barke’ population [12]. cM = centimorgan.
Predicted protein structures of the new CslF genes
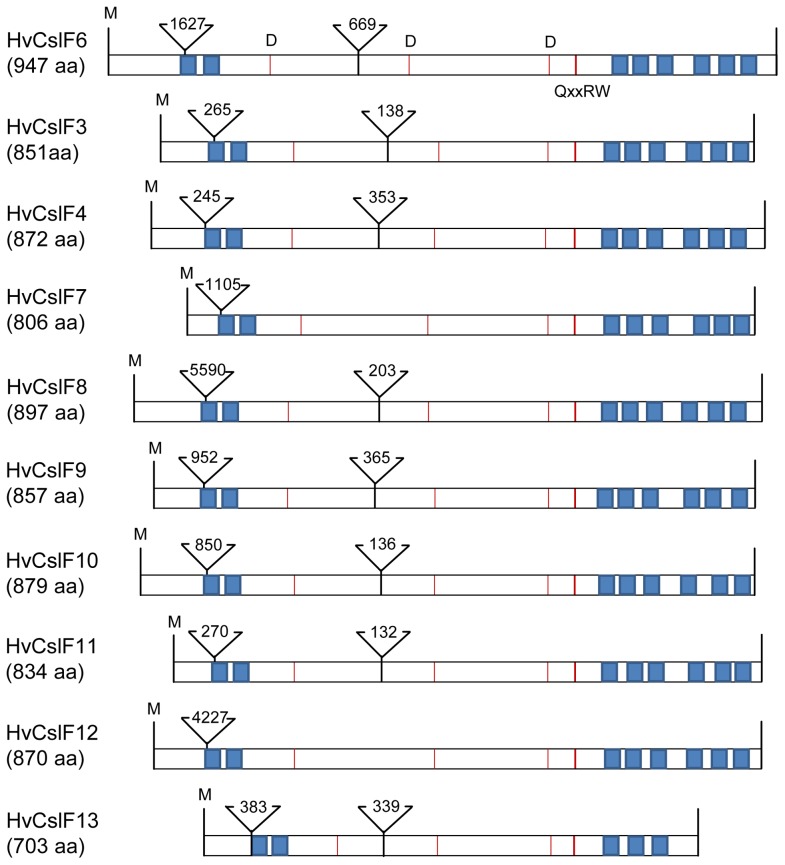
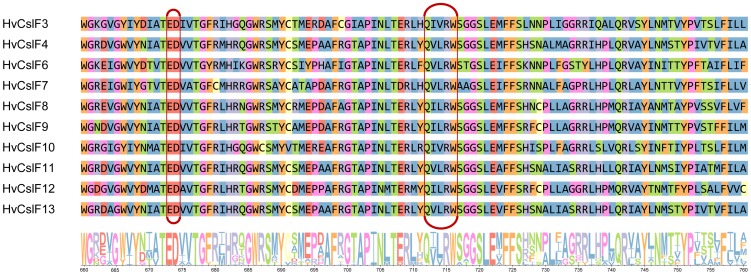
The predicted protein structures of the newly-identified HvCslF11 and HvCslF12 indicate sizes of 834 and 870 amino acids, respectively, within the range defined by other family members. The full but shorter HvCslF13 protein sequence of 703 amino acids could only be inferred by reference to the cv. Bowman [12] since the cv. Morex sequence contained premature stop codons. All three enzymes have the characteristic glycosyltransferase motif D, D, D, QxxRW and therefore belong to the GT2 family of glycosyltransferases [15]. For all HvCslF family members except HvCslF13, eight trans-membrane helices have been predicted, two near the 5’ end and six near the 3’ end, placing the 5’ end and catalytic motif putatively in the cytosol. An early stop codon in the cv. Bowman HvCslF13 gene leads to only three trans-membrane helices at the 3’ end; HvCslF13 is therefore potentially a pseudogene. With the exception of HvCslF7 and HvCslF12, which have a single intron, all other CslFs have two introns which vary from 132 to over 5500 base pairs (Figure 3). A closer look at the catalytic motif shows a strongly conserved region. The HvCslF3 and HvCslF10 enzymes have a QIVRW motif, while HvCslF8, HvCslF9 and the newly identified HvCslF12 share a QILRW motif. HvCslF4, HvCslF6, HvCslF7 and the two ‘new’ HvCslF11 and HvCslF13 enzymes have a QVLRW motif. This could be of importance because demonstration of (1,3;1,4)-β-glucan synthesis activity has so far been restricted to HvCslF4 and HvCslF6 [8], [9] i.e. the genes with the QVLRW motif. In the majority of cases, changes in amino acid residues around the motif are conservative (Figure 4). A key distinguishing feature of HvCslF6 is the presence of a 54 amino acid loop in the cytoplasmic region of the enzyme, compared to a loop of only 15–20 residues in the other HvCslF proteins [13] including the ‘new’ HvCslF11, HvCslF12 and HvCslF13.
Figure 3. The protein structure of the HvCslF family members.
The shaded boxes indicate the positions of sequences encoding trans-membrane helices, which can be found in similar positions in all genes. The triangle marks the intron position with the size given in base pairs. The lines indicate the glycosyltransferase GT2-motif D,D,D,QxxRW.
Figure 4. Part of the glycosyltransferase GT2-motif with surrounding amino acid residues.
The C-terminal part of the glycosyltransferase GT2-motif, encompassing ED and QxxRW, are marked by the red border. The colour coding is as follows: Aliphatic/hydrophobic: ILVAM (blue), Aromatic: FWY (orange), Positive: KRH (purple), Negative: DE (red), Hydrophilic: STNQ (green), conformationally special: PG (pink), Cysteine: C (yellow).
Expression profiles of barley CslF genes
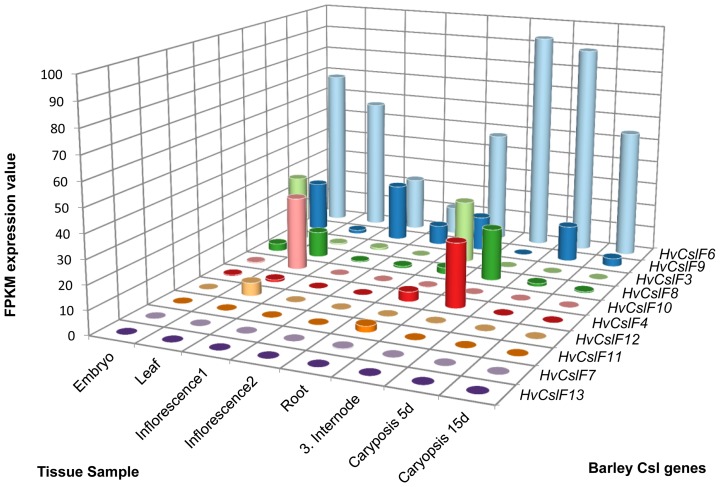
By mining the expression data published by the International Barley Genome Sequencing Consortium [12], some interesting variation between the members of this gene family is observed. In almost every tissue the highest expression is from the HvCslF6 gene (Figure 5). For HvCslF7 and HvCslF13 no expression is detectable, while HvCslF3, HvCslF8 and HvCslF10 all had distinct expression patterns. The HvCslF4 gene is transcribed in the third internode and the root. In comparison, the very closely related newly identified gene, HvCslF11, is only expressed in root tissue (Figure 5), and this result is validated by microarray analysis on the same tissue samples (Figure S1). Expression of HvCslF9 can be found in almost every tissue except for the third internode and leaf with the highest expression in the first inflorescence sample. The structurally similar HvCslF12 mRNA could only be found in the leaf (Figure 5 and Figure S1). The distinct expression patterns make the genes interesting for further analysis.
Figure 5. Expression data of the HvCslF genes based on RNA-sequence data.
The RNA-sequence data for the HvCslF genes includes three biological replicates per tissue. The results are given in FPKM expression values (fragments per kilobase of exon per million fragments mapped). Values were obtained from the International Barley Genome Sequencing Consortium [12].
Functional characterisation of HvCslF11 and HvCslF12 in Nicotiana benthamiana
In order to investigate the ability of the new HvCslF11 and HvCslF12 to synthesize (1,3;1,4)-β-glucan, a N. benthamiana transient expression system was used. The suspected pseudogene, HvCslF13, which gives rise to a truncated protein is not expected to have activity and was excluded from the analysis. For both genes, binary plasmid constructs were made and infiltrated into N. benthamiana leaves using Agrobacterium tumefaciens as a vector. After 6 days the leaves were harvested. Control leaves infiltrated with an ‘empty’ vector without CslF sequences showed no necrosis whereas leaves infiltrated with HvCslF11, HvCslF12 and HvCslF6 (positive control) showed unusual medium to strong necrosis (Figure 6). The leaves were analysed for (1,3;1,4)-β-glucan using a lichenase digestion which results mainly in tri-saccharide (DP3) and tetra-saccharide (DP4) hydrolysis products that can be analysed on a Dionex HPAEC column. HvCslF6 infiltrated leaves had a DP3:DP4 ratio of 1.6 but no DP3 and DP4 peaks could be detected for the HvCslF11 or HvCslF12 infiltrated leaves, despite the unusual phenotype (Table S1).
Figure 6. Transient expression of different HvCslF-constructs in Nicotiana benthamiana leaves results in necrosis.

HvCslF6 (a), HvCslF11 (b), HvCslF12 (c) and empty vector (d) constructs were transiently expressed in 4-weeks old N. benthamiana leaves using Agrobacterium tumefaciens as a vector. Photographs were taken six days after infiltration. Necrosis symptoms were observed for all three constructs.
Evolutionary analysis
Determining the relationships among the sequences of the CslF genes in different cereals can deepen our understanding of the evolutionary history of the individual genes. To put the relationship between the HvCslF genes into a wider evolutionary context, the CslF genes of barley (Hordeum vulgare), rice (Oryza sativa), sorghum (Sorghum bicolor), Brachypodium (Brachypodium distachyon) and wheat (Triticum aestivum) were compared. Our analyses identified eight members of the CslF family in rice, seven in Brachypodium, ten in sorghum and ten in barley. Searching the International Wheat Genome Sequencing Consortium (IWGSC) [16] database on the Unité de Recherche Génomique Info (URGI) [17] website revealed 34 TaCslF sequences in hexaploid wheat, although this may not be the complete gene family.
We performed Bayesian phylogenetic analysis using MrBayes from the TOPALi package [18] with the predicted coding sequences of all 69 CslF genes (plus an outgroup, not shown, of 10 CslH/CslJ genes). The resulting phylogenetic tree shows a clear division into different clades (Figure 7) and highlights several duplication events occurring in different cereals. For example, the (CslF4 (CslF11, CslF13)) clade reveals evidence of two duplication events in the rice lineage (OsCslF1 [Os07g36700], OsCslF2 [Os07g36690]), plus two duplication events barley (CslF11, CslF13) and possibly three in wheat, all independent from the rice duplication. Further duplication events in the CslF9 gene clade appear to have led to the origin of the ‘new’ HvCslF12 gene and the same duplication is also present in wheat. In the CslF3 gene clade a duplication event in sorghum can be inferred, and the nonexistence of a CslF10 gene in rice suggests a loss of this gene. Checking the coding sequences for evidence of past recombination using the NeighborNet method in the SplitsTree program [19] suggested that two sequences in sorghum (Sb02g022011 and Sb02g036030), assigned to the CslF10 clade, appear to be mosaic sequences [Schreiber M, Wright F and MacKenzie K, unpublished observation] consisting of part of a CslF10 gene and part of a CslF9 gene.
Figure 7. Phylogenetic tree of the CslF gene family including five cereals.
Phylogenetic tree of 69 members of the CslF gene family including wheat, barley, Brachypodium, rice and sorghum. The tree was constructed with TOPALi v2 on a subset of the genes using MrBayes (codon position model). The posterior probabilities have been multiplied by 100. An outgroup (not shown) comprised 10 CslH/CslJ sequences. HvCslF13 is marked as a potential pseudogene ψ. The two potential mosaic sequences Sb02g022011 and Sb02g036030 are marked by #. The scale bar shows expected number of nucleotide substitutions per site.
An analysis of natural selection among clades was performed using the branch model in the PAML package [20]. It is likely that the duplicated genes evolve in distinct ways due to exposure to different types of selection pressure that can be described with the nonsynonymous/synonymous ratio (Ka/Ks). We found significant differences in the Ka/Ks ratio among clades with the ratio varying from 0.0398 to 0.1944 among the ten clades (see Methods). The lowest Ka/Ks ratios were found in the CslF6 clade (Ka/Ks = 0.0398) and the highest in the CslF7 and CslF10 clades (Ka/Ks = 0.1953 and 0.1944 respectively). The relative Ka/Ks ratios observed after putative duplication events in the CslF12 and CslF9 clades (Ka/Ks = 0.1308 and 0.0780 respectively) suggested that these genes are not under positive selection pressure, but under purifying selection.
Discussion
High levels of (1,3;1,4)-β-glucan in some cereal grains have important positive implications for human health, while low levels are necessary for processability in the alcoholic beverages and animal feed industry. Knowing what genes are involved in (1,3;1,4)-β-glucan synthesis and breakdown, and when and where they are switched on or off is therefore important to understanding these contrasting features. The discovery of three ‘new’ HvCslF genes in barley is relevant to ongoing studies of the dynamics of cell wall synthesis in the grasses. Our data are consistent with HvCslF13 being either non-functional or a pseudogene. However, all three newly discovered HvCslFs possess the important glycosyltransferase GT2-motif, and HvCslF11 and HvCslF12 are expressed, albeit restricted to root and leaf respectively. These data suggests that HvCslF11 and HvCslF12 could play a role in (1,3;1,4)-β-glucan biosynthesis in roots or leaves but their role, if any, in grain tissues would likely be minor. Transient expression of HvCslF11 and HvCslF12 in N. benthamiana revealed no evidence for authentic (1,3;1,4)-β-glucan biosynthesis. Nevertheless, a shrivelled necrotic phenotype was observed that was not due to infiltration damage. The HPAEC profiles suggested that some new molecules were being produced, but they could not be identified. It is possible that the expressed genes are producing some unusual polysaccharide product and that this is causing the phenotype. The N. benthamiana transient system has been moderately successful for testing (1,3;1,4)-β-glucan synthase activity, producing small amounts of the polymer on expression of HvCslF4, HvCslF6 or HvCslH [Little A, Burton RA, Fincher GB, unpublished observation]. It may be that other components that are necessary for efficient (1,3;1,4)-β-glucan synthesis are missing in the dicot cells and remain to be identified. Consequently, the fact that HvCslF11 and HvCslF12 cannot synthesize (1,3;1,4)-β-glucan on their own in dicot cells does not exclude them from being involved in (1,3;1,4)-β-glucan synthesis in barley.
The first connection between grain (1,3;1,4)-β-glucan content and the CslF genes emerged from a genetic study on QTL (Quantitative Trait Loci) that affect grain (1,3;1,4)-β-glucan content [21]. While this study identified four QTL, Burton et al. [7] used conservation of synteny around the major QTL on chromosome 2H to identify the orthologous cluster of CslF genes on rice chromosome Os07. They then showed using stable expression that two genes in the cluster, OsCslF2 and OsCslF4, were able to synthesise (1,3;1,4)-β-glucan in Arabidopsis. An intriguing observation about these QTL studies is that the genetic analyses of [21] used a population derived from a cross between the barley cultivars Steptoe and Morex. The expression studies we report here used RNA-seq. data from the cv. Morex. Importantly in our datasets, none of the 2H CslF cluster genes are expressed in the developing grain suggesting they may not be involved in synthesising grain (1,3;1,4)-β-glucan. This conclusion is supported by observations that when CslF6 on chromosome 7H is mutated, no detectable (1,3;1,4)-β-glucan is found in the leaf or in the grain [22], indicating CslF6 as a major gene responsible for grain (1,3;1,4)-β-glucan biosynthesis. However, this apparent absence of (1,3;1,4)-β-glucan in the HvCslF6 mutant must be reconciled with our observations (Figure 5) and those of Burton et al. [13], that transcripts of both HvCslF9 and HvCslF8 are present in developing grain, albeit at lower levels than HvCslF6 transcripts. The HvCslF8 gene is a member of the cluster on chromosome 2H. Furthermore, our transcript profiles here are limited to 15 days post pollination, and we have found in other studies that HvCslF gene transcription can be initiated as late as 30-35 days post pollination [Wong SC, Mather DE, Burton RA, Fincher GB, unpublished observation]. At this stage, the level of involvement of the HvCslF genes in the chromosome 2H cluster in (1,3;1,4)-β-glucan synthesis remains unclear. Similarly, the final levels of (1,3;1,4)-β-glucan in mature barley grain may also be controlled by (1,3;1,4)-β-glucan endohydrolases for which high levels of gene transcripts are sometimes detectable in developing grain [13].
The solubility of (1,3;1,4)-β-glucan is believed to be affected by changes in the DP3:DP4 ratio which could influence other (1,3;1,4)-β-glucan properties. HvCslF6 and HvCslF4 have been shown to alter the DP3:DP4 ratio when overexpressed in barley [8]. HvCslF11 is very closely related to HvCslF4 and therefore could have an influence on (1,3;1,4)-β-glucan characteristics of roots, where HvCslF11 is specifically expressed. There has been no study so far to our knowledge that has examined the importance of (1,3;1,4)-β-glucan in the roots.
Using synteny (Figure S2) between several members of the grass family, and combining this information with phylogenetic analyses between the CslF genes allowed us to better understand the evolution of this gene family. Originating from a common ancestor, sorghum was the first to diverge, followed by rice and then Brachypodium [23]. Sorghum has SbCslF7 (Sb01g021420) on chromosome Sb01, SbCslF6 (Sb07g004110) on chromosome Sb07 and a cluster of CslFs on chromosome Sb02. These genes exhibit conserved synteny with their likely orthologs in rice, but different duplication events characterise the cluster. The sequence from SbCslF3 (Sb02g035990) is duplicated twice (Sb02g036010, Sb02g036000) and there is a recombination event between two sequences (Sb02g022011, Sb02g036030) which are assigned to the SbCslF10 clade. One of these sequences (Sb02g022011) appears to have been translocated to a more distal position, further from the centromere, on the chromosome Sb02 (Figure 7). Rice has one cluster of CslFs on chromosome Os07, and two outliers with OsCslF6 (Os08g06380) on chromosome Os08 and OsCslF7 (Os10g20260) on chromosome Os10. Based on homology one would expect to find HvCslF9, HvCslF11 and HvCslF13 as part of the cluster of genes located on barley chromosome 2H, instead of chromosomes 1H, 7H and 2H respectively. The HvCslF11 and HvCslF13 genes appear to have resulted from duplication followed by translocation. The wheat homologs of HvCslF9, HvCslF11 and HvCslF13 are present in all three genomes on chromosomes 1ABD, 7ABD and 2ABD, but they are not found in Brachypodium. The duplication and translocation must therefore have happened after the separation from Brachypodium. Wheat also appears to have duplicated a gene closely related to CslF13 and CslF11 after the separation from barley (Figure 7). Brachypodium shows a different pattern with the cluster on chromosome Bd01, and BdCslF6 (Bradi3g16307) on chromosome Bd03, while BdCslF7 is lost. BdCslF9 (Bradi3g45515) in Brachypodium is an outlier as in barley, but synteny is not conserved with either rice or barley. The different composition of the CslF genes, their locations in the different grass species and patterns of gene expression could help us understand how they evolved and how they influence (1,3;1,4)-β-glucan content and function at different times and in different tissues in related species. Why, for example does rice have little or no (1,3;1,4)-β-glucan in the grain, whereas the (1,3;1,4)-β-glucan content in Brachypodium grain is up to 40% w/w and barley shows a moderate amount of (1,3;1,4)-β-glucan with 4-10% w/w [5], [24]? Is this due to selection for starchy grains during domestication? It is clear that the (1,3;1,4)-β-glucan in the grain of Brachypodium largely replaces starch as the primary storage carbohydrate, consistent with suggestions that (1,3;1,4)-β-glucan acts as an alternative source of metabolizable glucose in leaves of young barley seedlings [25]. Thus, (1,3;1,4)-β-glucans in the Poaceae may play several functional roles in cell walls and in plant energy biology.
Conclusions
We have characterised here three newly identified CslF genes in barley that do not appear in rice, Brachypodium or sorghum, but are present in wheat. While their involvement in (1,3;1,4)-β-glucan synthesis has yet to be proven, at least two are expressed specifically in leaf and root tissues. Emerging genomic data for barley and related grass species is a powerful resource for characterising the evolution and dynamics of the complex CslF gene family. This will, in the longer term, contribute to a more thorough understanding of the mechanisms and processes regulating complex carbohydrate metabolism in grass cell walls.
Methods
Sequence data, databases and preprocessing
The available HvCslF gene sequences were collected from the National Center for Biotechnology Information (NCBI) [HvCslF3, GenBank: EU267179; HvCslF4, GenBank: EU267180; HvCslF6, GenBank: EU267181; HvCslF7, GenBank: EU267182; HvCslF8, GenBank: EU267183; HvCslF9, GenBank: EU267184; and HvCslF10, GenBank: EU267185], (http://www.ncbi.nlm.nih.gov/). These sequences were used for a BLAST [26] search on the Barley WGS Morex Assembly version3 [International Barley Genome Sequencing 12]. The deep-sequencing dataset is available for download from: http://mips.helmholtz-muenchen.de/plant/barley/index.jsp or is available for a BLAST search on: http://webblast.ipk-gatersleben.de/barley/. The accession numbers for the CslF gene sequences are included (HvCslF3: MLOC_59289, HvCslF4: MLOC_74149, HvCslF6: MLOC_57200, HvCslF7: MLOC_51212, HvCslF8: MLOC_52692, HvCslF9: MLOC_59327, HvCslF10: MLOC_13463, HvCslF11: MLOC_19594, HvCslF12: MLOC_7825). Additionally, the MSU Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/) was used to obtain the CslF genes from rice and a BLAST search was conducted on the Barley WGS Morex Assembly to search for further sequences. These sequences from rice and barley were used to conduct a BLAST search for the CslF genes from sorghum (http://mips.helmholtz-muenchen.de/plant/sorghum/) [27], Brachypodium (http://mips.helmholtz-muenchen.de/plant/brachypodium/) [28] and wheat (IWGSC, URGI) [16], [17]. The default settings of the respective websites were used to conduct the BLAST search. The alignment was then checked by eye and the sequences were validated by a reciprocal BLAST search to the rice genome. The identified coding sequences (excluding wheat due to pre-publication access) are given in Dataset S1.
Multiple alignment and Phylogenetic/evolutionary analyses
The above-mentioned 79 protein sequences were aligned using MUSCLE within MEGA5 [29] and from this a 4323bp-long codon alignment was created by replacing the amino acids with codons and single amino acid gaps with codon-sized gaps. Unreliable alignment positions were then removed using the BMGE method [30] resulting in an 1845bp alignment. The subsequent model selection and phylogenetic analysis took into account codon structure by having a nucleotide substitution model for each codon position: this “codon position” model thus consists of three models. The choice of model at each codon position was optimised using the TOPALi v2 [18] model selection method and the models chosen were GTR+I+G for the first and third positions and GTR+G for the second position. This model was then used to estimate a Bayesian phylogenetic tree using MrBayes v3.1.1 [31] launched from TOPALi v2. The Bayesian analysis settings were 2 runs of 625,000 generations, a 25% burn-in with trees sampled every 10 generations, resulting in 100,000 trees from two independent runs. The potential scale reduction factor (PRSF) values of all parameters were less than 1.06 (95% had values < 1.03) suggesting good convergence (i.e. less than a PRSF threshold of 1.2 as suggested by Gelman et al. [32]) of the two runs. The tree was rooted with ten sequences from the CslH and CslJ clusters (not shown in Figure 7). The posterior probabilities that show support for each cluster have been converted into percentages by MEGA5 during the production of the tree diagram shown in Figure 7.
Visual checking for evidence of recombination was done using the default analysis, NeighborNet, in the SPLITSTREE package [19]. When the NeighborNet phylogenetic network suggested that certain sequences were mosaic sequences, the analysis was rerun excluding them to see if the recombination signal was still present. By running NeighborNet interactively excluding alignment regions, the putative origin of regions in mosaic sequences was investigated. Phylogenetic trees were also estimated, using PhyML within TOPALi, from regions on each side of a putative recombination breakpoint to assist in determining the likely origin of regions in the mosaic sequences.
The PAML package [20] was used to investigate the variation in Ka/Ks ratios among clades using a likelihood ratio test for variation in selective pressure among branches in a gene tree based on the Yang and Bielawski protocol [33]. We found significant differences in the Ka/Ks ratio among clades by testing a null hypothesis, H0, that Ka/Ks was the same in all clades (Ka/Ks equal to 0.1329) versus the alternative hypothesis, H1, that Ka/Ks varied among the clades (Log likelihoods of -46465.1 and -46669.5, respectively for H0 and H1, were used to produce a Likelihood Ratio test statistic of 408.4 which was significant at p<0.001). The Ka/Ks ratio varied from 0.0398 to 0.1944 among the ten clades with most clades in the range (0.075, 0.150).
Genetic location and RNA-sequence experiments
Information on the genetic location of the genes and expression data is provided by [12]. For the genetic location over 3.90 gigabases of sequence contigs were anchored to a consensus genetic map based on the analysis and integration of maps from a number of populations, the largest contributor being a recombinant inbred line population derived from a cross between the cultivars Morex and Barke. Eight tissues from cultivar Morex were subjected to RNA sequencing with three replications per tissue [12]. These eight tissues were: germinated embryo (four days after germination), young leaf tissue (from a 10 cm high plant), young root tissue (from a 10 cm high plant), developing inflorescence (5 mm-long inflorescence and 10–15 mm-long inflorescence), the third internode (42-day-old plants) and two time points for the developing caryopsis (five days after anthesis and 15 days after anthesis). The data are presented in FPKM expression values (fragments per kilobase of exon per million fragments mapped).
Protein prediction
The intron prediction was conducted using Softberry (FGENESH, HMM-based gene structure prediction, http://linux1.softberry.com/berry.phtml). The result was then confirmed using the RNA-seq. data, if available. Transmembrane helices were predicted using the following websites http://topcons.cbr.su.se/ and http://www.cbs.dtu.dk/services/TMHMM-2.0/ and taking a consensus of both predictions.
Transient Nicotiana benthamiana expression system
Full length HvCslF11 and HvCslF12 cDNAs were amplified from Morex, root and young leaf tissue (10 days old), respectively. The following primer pairs were used: HvCslF11_F - AGCCACGGTTTACAGTACGA; HvCslF11_R - ACTACGTACGTGTCTATCCAGA; HvCslF12_F - GAAGAGCCAATGGTTTCGC; HvCslF12_R - CCAGAGAAACGGCATCATCC. The genes were cloned into the Gateway entry vector pCR8/GW/TOPO (Invitrogen, Carlsbad, CA, USA) and sequenced on an ABI 3700 (Applied Biosystems Inc., Foster City, CA, USA) at the Australian Genome Research Facility, Adelaide, Australia, to eliminate constructs with errors. In a LR recombination reaction the inserts were transferred into the Gateway destination vector pEAQ-HT-Dest1 under the control of a CaMW 35S promoter [34]. As a positive control HvCslF6 was included and as a negative control the vector pEAQ-HT-Dest1 without the chloramphenicol resistance gene and ccdB gene was used. The constructs were transformed into the Agrobacterium tumefaciens strain AGL1 and left to grow overnight at 28°C in LB medium containing rifampicin and kanamycin. 2 mL of the overnight culture were spread on a LB plate and grown for 2 days at 28°C. 10 mL of an infiltration buffer (10 mM MgCl2, 10 mM MES (2-(N-morpholino) ethanesulfonic acid)) were added per plate and cells scraped off the surface. OD600 was measured and adjusted to an infiltration OD of 1. 1 µL of 100 mM Acetosyringone was added per mL and left for 3 hours at room temperature. Nicotiana benthamiana seedlings were grown under glasshouse conditions, 22°C with natural light, in the Plant Accelerator (University of Adelaide). Whole leaves of 4 week old Nictotiana benthamiana plants were infiltrated from the underside using a 10 mL syringe without a needle. Leaves were harvested after 6 days, freeze-dried and ground using a ball bearing mill. Analysis of (1,3;1,4)-β-D-glucan was performed using 20 mg of ground tissue following the commercially available reagents (Megazyme International Ireland Ltd, Bray, Ireland) and a protocol based on [35]. Method modifications include two washes of 50% ethanol and two washes of 100% ethanol for 10 minutes at 97°C, followed by a 20 minute extraction at 90°C in 1 ml 20 mM sodium phosphate buffer (pH 6.5) and a 1.5 hour incubation at 50°C with 40 µL U/ml Lichenase. Total beta glucan levels within the samples were analysed using the glucose oxidase-peroxidase reagent supplied with the kit. DP3:DP4 levels were analysed using HPAEC according to [8] with samples collected following Lichenase digestion.
Supporting Information
Microarray validation of RNA seq expression pattern of HvCslF11 and HvCslF12. Microarray processing was performed on aliquots of identical RNA samples used for the RNAseq (IBGSC, 2012 [11]), using a custom-designed barley Agilent microarray (A-MEXP-2357; www.ebi.ac.uk/arrayexpress). The barley microarray contains c. 61,000 barley 60-mer probes derived from predicted barley transcripts and full-length cDNAs (IBGSC, 2012 [11]). Processing was performed according to the ‘One-Color Microarray-Based Gene Expression Analysis’ protocol (v. 6.5; Agilent Technologies). Data were extracted using Feature Extraction (FE) software (v. 10.7.3.1; Agilent Technologies) with default settings, and subsequently analysed using GeneSpring GX (v. 7.3; Agilent Technologies) software. Data were normalised using default Agilent FE one-colour settings in GeneSpring.
(TIF)
Chromosome position of the CslF family members highlights synteny between sorghum, rice, Brachypodium and barley. The Figure was created using Strudel (see Bayer M, Milne I, Stephen G, Shaw P, Cardle L, et al. (2011) Comparative visualization of genetic and physical maps with Strudel. Bioinformatics 27: 1307-1308.).
(TIF)
β-glucan content and DP3:DP4 ratio of CslF gene constructs in the N. benthamiana transient expression system. MLG = mixed linkage glucan; nd = not detected.
(DOCX)
Coding sequences of the identified CslF genes.
(DOCX)
Acknowledgments
We thank Linda Milne and Jenny Morris for help with experiments and data analysis. We thank “The International Wheat Genome Sequencing Consortium” for pre-publication access to wheat genome sequence data (http://www.wheatgenome.org/Projects/IWGSC-Bread-Wheat-Projects/Sequencing/Whole-Chromosome-Survey-Sequencing).
Funding Statement
This work was funded by The Scottish Government's Rural and Environment Science and Analytical Services Division (RESAS) through a Strategic Partnership for Food and Drink Science (http://www.scotland.gov.uk) and by the Australian Research Council (http://www.arc.gov.au/) Centre of Excellence in Plant Cell Wall Biology. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Food and Drug Administration (2006) Food labeling: health claims; soluble dietary fiber from certain foods and coronary heart disease. Final rule. Fed Regist 71: 29248–29250. [PubMed] [Google Scholar]
- 2. Food and Drug Administration (2008) Food labeling: health claims; soluble fiber from certain foods and risk of coronary heart disease. Final rule. Fed Regist 73: 47828–47829. [PubMed] [Google Scholar]
- 3. Brennan CS, Cleary LJ (2005) The potential use of cereal (1→3,1→4)-β-D-glucans as functional food ingredients. J Cereal Science 42: 1–13. [Google Scholar]
- 4. Annison G (1993) The Role of Wheat Nonstarch Polysaccharides in Broiler Nutrition. Aust J Agr Res 44: 405–422. [Google Scholar]
- 5. Burton RA, Fincher GB (2012) Current challenges in cell wall biology in the cereals and grasses. Front Plant Sci 3: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega-Sanchez ME, Verhertbruggen Y, Scheller HV, Ronald PC (2013) Abundance of mixed linkage glucan in mature tissues and secondary cell walls of grasses. Plant Signal Behav 8. [DOI] [PMC free article] [PubMed]
- 7. Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, et al. (2006) Cellulose Synthase-Like CslF Genes Mediate the Synthesis of Cell Wall (1,3;1,4)-ß-D-Glucans Science. 311: 1940–1942. [DOI] [PubMed] [Google Scholar]
- 8. Burton RA, Collins HM, Kibble NAJ, Smith JA, Shirley NJ, et al. (2011) Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-D-glucans and alters their fine structure. Plant Biotechnol J 9: 117–135. [DOI] [PubMed] [Google Scholar]
- 9. Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, et al. (2009) A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-D-glucan synthesis in transgenic Arabidopsis . Proc Natl Acad Sci U S A 106: 5996–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doblin MS, Pettolino F, Bacic A (2010) Plant cell walls: the skeleton of the plant world. Funct Plant Biology 37: 357–381. [Google Scholar]
- 11. Wilson SM, Burton RA, Doblin MS, Stone BA, Newbigin EJ, et al. (2006) Temporal and spatial appearance of wall polysaccharides during cellularization of barley (Hordeum vulgare) endosperm. Planta 224: 655–667. [DOI] [PubMed] [Google Scholar]
- 12. IBGSC (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491: 711–716. [DOI] [PubMed] [Google Scholar]
- 13. Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, et al. (2008) The Genetics and Transcriptional Profiles of the Cellulose Synthase-Like HvCslF Gene Family in Barley. Plant Physiol 146: 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, et al.. (2013) Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ). Plant J. [DOI] [PMC free article] [PubMed]
- 15. Coutinho PM, Deleury E, Davies GJ, Henrissat B (2003) An Evolving Hierarchical Family Classification for Glycosyltransferases. J Mol Biol 328: 307–317. [DOI] [PubMed] [Google Scholar]
- 16.International Wheat Genome Sequencing Consortium. Available: http://www.wheatgenome.org/.
- 17.Unité de Recherche Génomique Info. Available: http://wheat-urgi.versailles.inra.fr/Seq-Repository] (IWGSC).
- 18. Milne I, Lindner D, Bayer M, Husmeier D, McGuire G, et al. (2009) TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 25: 126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huson DH, Bryant D (2006) Application of Phylogenetic Networks in Evolutionary Studies. Mol Biol Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 20. Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 21. Han F, UIlrich SE, Chirat S, Menteur S, Jestin L, et al. (1995) Mapping of β-glucan content and β-glucanase activity loci in barley grain and malt. Theor Appl Genet 91: 921–927. [DOI] [PubMed] [Google Scholar]
- 22. Taketa S, Yuo T, Tonooka T, Tsumuraya Y, Inagaki Y, et al. (2012) Functional characterization of barley betaglucanless mutants demonstrates a unique role for CslF6 in (1,3;1,4)-β-D-glucan biosynthesis. J Exp Bot 63: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doust A (2007) Architectural Evolution and its Implications for Domestication in Grasses. Ann Bot 100: 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guillon F, Larre C, Petipas F, Berger A, Moussawi J, et al. (2012) A comprehensive overview of grain development in Brachypodium distachyon variety Bd21. J Exp Bot 63: 739–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roulin S, Buchala AJ, Fincher GB (2002) Induction of (1→3,1→4)-β-D-glucan hydrolases in leaves of dark-incubated barley seedlings. Planta 215: 51–59. [DOI] [PubMed] [Google Scholar]
- 26. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic Local Alignment Search Tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 27. Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556. [DOI] [PubMed] [Google Scholar]
- 28. International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463: 763–768. [DOI] [PubMed] [Google Scholar]
- 29. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Criscuolo A, Gribaldo S (2010) BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol 10: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 32.Gelman A, Carlin J, Stern H, Rubin D (1995) Bayesian data analysis. London, UK: Chapman & Hall.
- 33. Yang Z, Bielawski JP (2000) Statistical methods for detecting molecular adaptation. Trends Ecol Evol 15: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sainsbury F, Thuenemann EC, Lomonossoff GP (2009) pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7: 682–693. [DOI] [PubMed] [Google Scholar]
- 35. McCleary BV, Codd R (1991) Measurement of (1-3),(1-4)-Beta-D-Glucan in Barley and Oats - a Streamlined Enzymatic Procedure. Journal of the Science of Food and Agriculture 55: 303–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microarray validation of RNA seq expression pattern of HvCslF11 and HvCslF12. Microarray processing was performed on aliquots of identical RNA samples used for the RNAseq (IBGSC, 2012 [11]), using a custom-designed barley Agilent microarray (A-MEXP-2357; www.ebi.ac.uk/arrayexpress). The barley microarray contains c. 61,000 barley 60-mer probes derived from predicted barley transcripts and full-length cDNAs (IBGSC, 2012 [11]). Processing was performed according to the ‘One-Color Microarray-Based Gene Expression Analysis’ protocol (v. 6.5; Agilent Technologies). Data were extracted using Feature Extraction (FE) software (v. 10.7.3.1; Agilent Technologies) with default settings, and subsequently analysed using GeneSpring GX (v. 7.3; Agilent Technologies) software. Data were normalised using default Agilent FE one-colour settings in GeneSpring.
(TIF)
Chromosome position of the CslF family members highlights synteny between sorghum, rice, Brachypodium and barley. The Figure was created using Strudel (see Bayer M, Milne I, Stephen G, Shaw P, Cardle L, et al. (2011) Comparative visualization of genetic and physical maps with Strudel. Bioinformatics 27: 1307-1308.).
(TIF)
β-glucan content and DP3:DP4 ratio of CslF gene constructs in the N. benthamiana transient expression system. MLG = mixed linkage glucan; nd = not detected.
(DOCX)
Coding sequences of the identified CslF genes.
(DOCX)