Abstract
Although cognitive dysfunction is a primary characteristic of schizophrenia, only recently have investigations begun to pinpoint when the dysfunction develops in the individual afflicted by the disorder. Research to date provides evidence for significant cognitive impairments prior to disorder onset. Less is known about the course of cognitive dysfunction from onset to the chronic phase of schizophrenia. Although longitudinal studies are optimal for assessing stability of cognitive deficits, practice effects often confound assessments, and large and representative subject samples have not been followed over long periods of time. We report results of a cross-sectional study of cognitive deficits early and late in the course of schizophrenia carried out at four different geographic locations to increase sample size and generalizability of findings. We examined a broad set of cognitive functions in 41 recent-onset schizophrenia patients and 106 chronic schizophrenia patients. The study included separate groups of 43 matched controls for the recent-onset sample and 105 matched controls for the chronic schizophrenia sample in order to evaluate the effects of cohort (i.e., age) and diagnosis (i.e., schizophrenia) on cognitive functions. All measures of cognitive function showed effects of diagnosis; however, select time-based measures of problem solving and fine motor dexterity exhibited interactions of diagnosis and cohort indicating that these deficits may progress beyond what is expected with normal aging. Also, worse recall of material in episodic memory was associated with greater length of illness. Nevertheless, findings indicate that nearly all cognitive deficits are comparably impaired across recent-onset and chronic schizophrenia.
Keywords: Schizophrenia, Cognition, Recent-onset, Chronic disease, Psychosis
1. Introduction
Cognitive dysfunction is increasingly considered a primary characteristic of schizophrenia. Researchers have begun to detail when the dysfunction develops in the individual afflicted by the disorder. A preponderance of evidence indicates that cognitive deficits are apparent in the adolescent with schizophrenia and at the first-episode of the disorder (Addington et al., 2005; Bilder et al., 2000; Cervellione et al., 2007; Hawkins et al., 2008; Kenny et al., 1997; Mesholam-Gately et al., 2009), as well as being relatively consistent in severity over a chronic course of the disorder (Albus et al., 2006; Hoff et al., 1999, 2005; Rund et al., 2007; Stirling et al., 2003). Impaired cognitive function is also clearly present prior to disorder onset during the premorbid period (Woodberry et al., 2008). Recent work on the period prior to frank psychosis has nonetheless provided evidence for cognitive declines prior to disorder onset (Fuller et al., 2002) from premorbid impairment to greater postmorbid impairment (Caspi et al., 2003; Seidman et al., 2006). Some studies also indicate an additional period of accelerated cognitive decline late in life in some subgroups of institutionalized schizophrenia patients (Friedman et al., 2001; Harvey et al., 1999). Thus, the first period of cognitive impairment may be a manifestation of neurodevelopmental processes that become fully expressed in late adolescence, and the latter may reflect vulnerability of the schizophrenic brain to aging and dementia late in life (Kurtz, 2005). Although there is a substantial evidence for largely comparable cognitive impairment during first-episode and chronic schizophrenia, (Heinrichs and Zakzanis, 1998), very few studies have used the same measures to directly compare two independent samples of subjects at these substantially different phases of the illness.
Although individuals with schizophrenia generally manifest stable and impaired cognitive function that is possibly bracketed by cognitive declines prior to disorder onset and preceding death in old age, the possibility that select cognitive deficits may be increasingly impaired over time has not been ruled out. For example, there is some evidence for an age-associated cognitive decline in complex information processing and executive functions in schizophrenia (Bowie et al., 2008; Fucetola et al., 2000; Granholm et al., 2000; Mesholam-Gately et al., 2009). Also, motor functions have been identified as a primary dimension of cognition in schizophrenia (Jaeger et al., 2003) and shown to be increasingly impaired over extended follow-up periods. Kurtz et al., (2005) provided evidence of slowing in speeded motor sequencing in chronic schizophrenia over a 10-year period while all other cognitive domains assessed remained the same. Gold et al. (1999) also found in the context of relatively stable or improved cognitive function over 5 years, schizophrenia patients’ performance declined on a test of simple finger tapping. Decline in motor function also appears to be associated with the onset of psychosis (Hawkins et al., 2008). Yet, other studies have demonstrated preserved simple motor functions over 1–2 years (Addington et al., 2005) and over a 10-year longitudinal period (Hoff et al., 2005). Although longitudinal studies provide a direct test for change in cognitive functions, variability in the findings from these studies may stem from reliance on small control samples, patients being lost to follow-up, short longitudinal periods of only 1–2 years, changes in motor function due to antipsychotic exposure, and variation in use of first and second generation antipsychotic medication (Weickert and Goldberg, 2005).
In order to characterize several domains of cognitive function in early and chronic phases of the disorder, and to test for evidence of progression in executive and motor dysfunction, the present study examined a broad set of cognitive and motor functions in recent-onset and chronic schizophrenia patients. Subjects were studied in four different geographic regions in the United States of America to increase generalizability of findings. The study also included separate control groups for recent-onset and chronic schizophrenia samples drawn from the four sites so effects of age (i.e., cohort) and schizophrenia (i.e., diagnosis) could be separated in analyses. We hypothesized that cognitive impairments would be comparable across the two samples, with the possible exception of impairments of motor and executive functions being larger in the chronic patients.
2. Methods
2.1. Study participants
Subjects were recruited through the University of New Mexico in Albuquerque, University of Iowa in Iowa City, Massachusetts General Hospital and the Massachusetts Mental Health Center Public Psychiatry Division of the Beth Israel Deaconess Medical Center of the Beth Israel Deaconess Medical Center, Boston, and the University of Minnesota in Minneapolis as part of the Mental Illness and Neuroscience Discovery (MIND) Clinical Imaging Consortium (MCIC). All subjects were required to be at least age 18 and no older than 60, and to be fluent in English. Subjects were excluded if they had a history of neurological or psychiatric disease other than schizophrenia, including history of head injury resulting in prolonged loss of consciousness and/or neurological sequelae, history of skull fracture, history of epilepsy, (except for childhood febrile seizures), history of prior neurosurgical procedure, substance abuse or dependence within the past month, use of inhalants, metal in the head, metal injury to the eyes, implanted pacemaker, medication pump, vagal stimulator, deep brain stimulator, a transcutaneous electrical nerve stimulator (TENS) unit, or ventriculoperitoneal shunt, pregnancy, or estimated premorbid intelligence quotient (IQ) less than or equal to 70 (based on the Reading subtest from the WRAT-3).
Two groups of patients were studied: recent-onset and chronic. The recent-onset patients were included if they had Diagnostic and Statistical Manual-Fourth Edition (DSM-IV) diagnoses of schizophrenia, schizophreniform disorder, or schizoaffective disorder using the Structured Clinical Interview for DSM-IV (SCID). Recent-onset patients were defined as meeting any of the following criteria: (a) psychotic symptoms of <5 years with a lifetime anti-psychotic exposure of <12 weeks with no or some exposure in the last 3 weeks, or (b) psychotic symptoms of <2 years with a lifetime antipsychotic exposure of <6 months; (c) psychotic symptoms of any duration with a maximum lifetime exposure of 10 mg or less haloperidol equivalents. The chronic patients sample was limited to patients with a DSM-IV diagnosis of schizophrenia assessed via SCID who did not meet the definition of recent-onset. Table 1 presents the characteristics of subjects. Although criterion “c” above created the possibility that some individuals in the recent-onset group had illness duration of greater than 5 years, the mean length of illness of the sample was 2.6 years, while the length of illness for the chronic sample was over 11 years longer (see Table 1). Thus the contrast of recent-onset and chronic patients allowed for testing the effect of about a decade of disorder.
Table 1.
Characteristics of participants.
| Variable | Recent-onset schizophrenia patients (n = 41) | Control subjects for recent-onset sample (n = 43) | Chronic schizophrenia patients (n = 106) | Control subjects for chronic sample (n = 105) | Statistic |
|
|---|---|---|---|---|---|---|
| Cohort | Diagnosis | |||||
| Age (years) | 25.6 (5.7)b | 26.0 (6.8)b | 36.8 (10.1) | 34.7 (11.5) | F1,277 = 46.3‡ | F1,277 = .2 |
| % Female | 23.8 | 25.6 | 24.3 | 37.1a | ||
| Education (years) | 13.4 (2.2)a | 14.4 (1.9)b | 13.1 (2.6)a | 15.3 (2.2) | F1,276 = 1.4 | F1,276 = 26.8‡ |
| Subjects SES | 3.24 (.80)a,b | 2.79 (.47) | 3.63 (1.00)a | 2.57 (.63) | F1,281 = .7 | F1,281 =38.0‡ |
| Parental SES | 2.76 (.86) | 2.64 (.66) | 2.89 (1.09) | 2.67 (.85) | F1,279 = .2 | F1,276 = 2.3 |
| WRAT-3 Reading | 47.6 (6.6)a | 50.6 (4.0) | 46.8 (6.2)a | 50.8 (4.4) | F1,287 = .3 | F1,287 = 19.1‡ |
| Illness length (years) | 2.63 (3.4) | NA | 14.0 (10.2) | NA | F1,144 = 48.6‡ | NA |
| Psychosis | 5.55 (2.33) | NA | 4.63 (2.93) | NA | F1,143 = 2.4 | NA |
| Negative | 8.67 (4.67) | NA | 7.40 (3.55) | NA | F1,143 = .2 | NA |
| Disorganization | 2.35 (2.09) | NA | 1.60 (1.78) | NA | F1,143 = 1.7 | NA |
Note: Data are presented as mean (standard deviation). Cohort refers to effects evident contrasting recent-onset groups with chronic groups. Diagnosis refers to effects evident contrasting schizophrenia groups and control groups. SES = socio-economic status as measured by the modified Hollingshead scale (Hollingshead and Redlich, 1958). Psychosis = sum of global ratings for delusions and hallucinations on the Scale for the Assessment of Positive Symptoms (SAPS). Negative = sum of global ratings for alogia, affective flattening, anhedonia, and avolition on the Scale for the Assessment of Negative Symptoms (SAPS). Disorganization = global rating of formal thought disorder, bizarre behaviors, and inappropriate affect on the SAPS.
p < .0005.
Differs across diagnostic group within cohort (p < .05).
Differs across cohort within diagnostic group (p < .05).
The healthy volunteer subjects were recruited from the community through newspaper advertising. They were screened using the nonpatient version of the Structured Clinical Interview for DSM-IV (SCID) to rule out psychiatric illnesses, including substance use disorders and assessed to rule out any medical or neurological illnesses. Healthy normal volunteers who had not been diagnosed with any psychiatric disorders but had been medicated with antidepressants, antianxiety medication or medication for sleep disturbance could be included in the study, provided that the duration of the medication did not exceed 2 months of continuous use at any time and they had not been used at least 6 months prior to the study.
2.2. Neuropsychological assessment
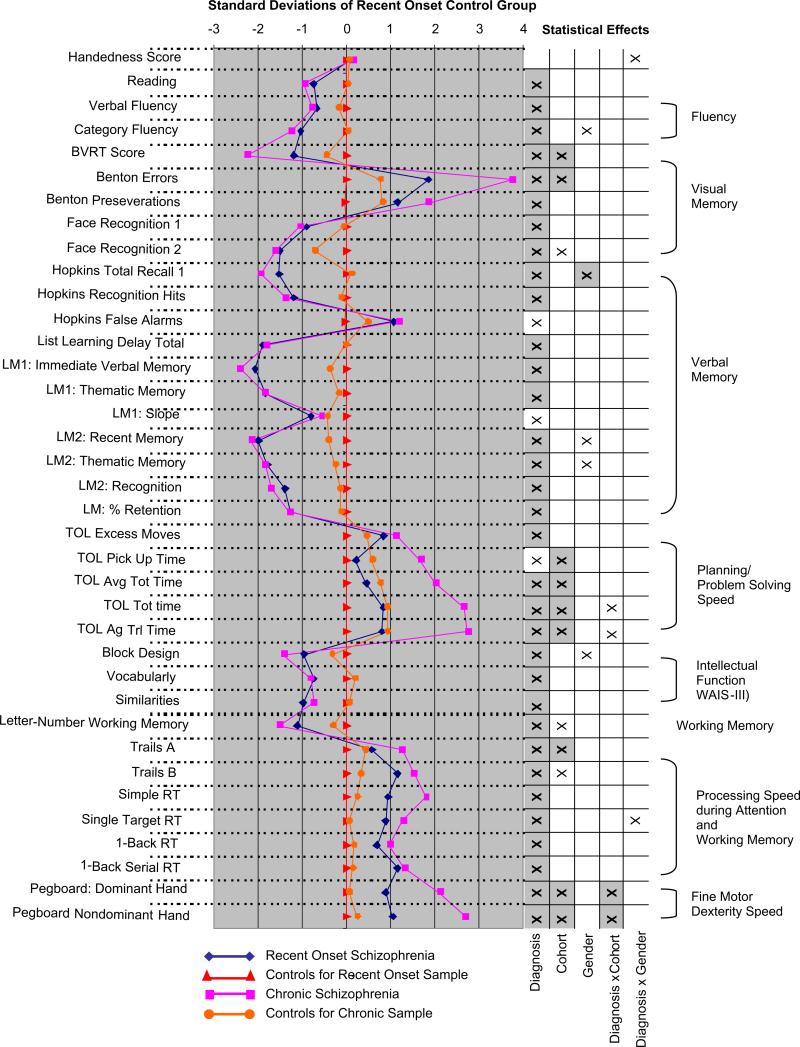
The cognitive assessments were conducted by psychometrists and were supervised by experienced neuropsychologists who had participated in an in person standardization training for the four sites. The instruments were chosen to sample a wide range of functions. General cognitive abilities and achievement were assessed using Block Design, Vocabulary, and Similarities subtests of the Wechsler Adult Intelligence Scale – Third Edition (Wechsler, 1997a) (WAIS-III) and the Reading subtest of the Wide Range Achievement Test – Third Edition (Wilkinson, 1993) (WRAT-3). Fluency was measured using verbal and category fluency tests of the Delis–Kaplan Executive Function System (Delis et al., 2001) (D-KEFS). We assessed visual memory using the Benton Visual Retention Test (Benton, 1962) (BVRT) and the Face Recognition subtest of the Wechsler Memory Scale-III (Wechsler, 1997b) (WMS-III), and characterized verbal memory and learning using the Logical Memory Test of the WMS-III and the Hopkins Verbal Learning Test-Revised (Brandt, 1991). The Letter-Number Sequencing subtest of the WAIS-III was used to assess working memory. Processing speed during attention and working memory was measured using parts A and B of the Trail Making Test (Reitan, 1958) (TMT) and the California Computerized Assessment Package (Miller, 1990) (CalCap) computerized reaction time test. We used a computerized version of the Tower of London test (Shallice, 1982) (TOL) to assess planning and problem solving. The Grooved Pegboard Test (Ruff and Parker, 1993) (Lafayette Instruments, Lafayette, IN) was used to characterize fine motor dexterity and speed and the Annett Scale of Hand Preference (Annett, 1970) yielded a quantitative measure of handedness. See Fig. 1 regarding specific indices that were subject to analyses. Because of computer malfunctions at the University of New Mexico, CalCap data were not gathered on 36 subjects and TOL data were not gathered for 27 subjects. Subjects with missing data were exclusively from the chronic schizophrenia patients and their control sample.
Fig. 1.
Means for recent-onset and chronic schizophrenia patients and their respective control groups for cognitive indices as z-scores derived using the recent-onset control means and standard deviations. Significant statistical effects (p < .05) identified through ANOVA's are indicated by an ‘x’ in the right-hand columns (effects that survived Bonferroni correction for multiple comparisons are indicated by grayed squares). Positive z-scores for time-based (TOL time indices, Trails A, Trails B, RT's, and Pegboard) and error-based (Benton Errors, Benton Preseverations, Hopkins False Alarms, TOL Excess Moves) indices indicate worse performance. Indices in the graph were derived from the Annett Scale of Hand Preference (Handedness), the WRAT-3 (Reading), the Delis–Kaplan Executive Function System (Verbal and Category Fluency), the Benton Visual Retention Test (Benton Visual Memory, Errors, and Preseverations), WMS-III (Face Recognition 1, Face Recognition 2 [delay], Logical Memory [LM]1: Immediate Verbal memory, LM1: Thematic Memory, LM1: Slope, LM2: Recent Memory [delayed], LM2 Thematic Memory [delayed], LM2: Thematic Memory [delayed], LM2: Recognition [delayed], LM: % Retention), Hopkins Verbal Learning Test-Revised (Hopkins Total Recall 1, Hopkins Recognition Hits, Hopkins False Alarms, List Learning Delay Total), Tower of London (TOL) Test, the WAIS-III (Block Design, Vocabulary, Similarities, Letter-Number Sequencing), the Tail Making Test (Trails A and B), the California Computerized Assessment Package (CalCap) (Simple Reaction Time [RT], Single Target RT, 1-Back RT, 1-Back Serial RT), and the Grooved Pegboard Test (Pegboard: Dominant Hand; Pegboard Nondominant Hand).
2.3. Statistical analyses
To test effects across all measured cognitive functions, an omnibus repeated measures analysis of variance (ANOVA) was performed with between-subjects factors of diagnosis (schizophrenia, control), cohort (recent-onset, chronic), and gender (male, female), and a within subjects factor of index (i.e., indices from all cognitive tests served as dependent variables1). As a follow-up to significant effects, an ANOVA was performed for each index to determine diagnostic, cohort, and gender effects on specific cognitive measures. A significant interaction of diagnosis and cohort was interpreted as indicating differential cognitive deficits in recent-onset and chronic schizophrenia. Effects that survived Bonferroni correction (p < .05) for multiple comparisons are noted in Fig. 1. To further examine whether duration of illness was related to cognitive functions in schizophrenia partial correlations were computed between length of illness and cognitive variables with age as a covariate (to address effects of normal aging) disregarding the assignment of patients to either recent-onset or chronic groups.
We performed ANOVA's for demographic variables presented in Table 1. For the schizophrenia groups, we carried out ANOVA's (without diagnosis as a factor) to test for differences between recent-onset and chronic schizophrenia patients on dimensions of symptomology. To determine whether cognitive deficits were associated with the type of antipsychotic medication prescribed or history of alcohol or drug use we carried out separate omnibus ANOVAs of all cognitive indices for antipsychotic medication (atypical, typical), and history of alcohol dependence, cannabis use, and hallucinogen use. Significant effects were followed-up with t-tests for individual cognitive indices. We computed Pearson correlations to determine whether antipsychotic dosage and dimensions of psychotic symptomatology were associated with cognitive functions in the schizophrenia samples. To test for effects of data collection site we also carried out ANOVAs with between-subjects factors of diagnosis (schizophrenia, control), cohort (recent-onset, chronic), and site (Albuquerque, Iowa City, Boston, and Minneapolis) for each cognitive, clinical, and demographic variable.2
3. Results
On matching variables, all groups had similar parental socioeconomic status, and the recent-onset and chronic schizophrenia samples were of similar age to their respective control groups (see Table 1). The controls for chronic schizophrenia patients had a higher percentage of females, thus gender was specified as a factor in subsequent analyses. Both schizophrenia groups had significantly lower reading achievement (regarded as an estimate of premorbid intelligence), suggesting that schizophrenia patients may have had some cognitive impairment prior to illness onset.
Chronic schizophrenia patients had fewer years of education than their control subjects, and both schizophrenia groups had lower socio-economic status than their respective control groups. There were interactions of diagnosis and cohort for socio-economic status, F1,281 = 6.35, p = .01, and years of education, F1,276 = 4.30, p = .04, indicating that socio-economic and educational disadvantages were greater in chronic schizophrenia than at recent-onset. ANOVA's failed to reveal any differences in symptom dimensions between recent-onset and chronic schizophrenia samples. An interaction of gender and cohort, F1,143 = 4.33, p = .04, for negative symptomology resulted from recent-onset male patients exhibiting more negative symptoms than male chronic patients, while female patients showed the opposite pattern across recent-onset and chronic cohorts.
3.1. Cognitive function in recent-onset and chronic schizophrenia
The omnibus analysis revealed a main effect of diagnosis, F1,279 = 75.9, p < .0005, and interactions of cognitive index and diagnosis, Greenhouse-Geisser (G-G) F7.34,2049.0 = 34.6, p < .0005, and index and cohort, G-G F7.34,2040.0 = 4.37, p < .0005. The absence of an interaction between diagnosis and cohort, or any effect involving gender, indicated that across the broad set of cognitive measures, recent-onset schizophrenia patients exhibited similar deficits to chronic schizophrenia patients and that these deficits were independent of gender. When an estimate of premorbid intellectual function was entered as a covariate the main effect of diagnosis persisted, F1,278 = 51.11, p < .0005, suggesting that cognitive dysfunction in the schizophrenia samples extended beyond what might be seen in the premorbid period. Results of follow-up analyses for each cognitive index are depicted in Fig. 1 along with the mean z-scores for each group determined by the mean and standard deviation of the control subjects for recent-onset patients. Diagnosis effects were evident for every index except a measure of handedness. Diagnosis and cohort interactions were only present for two measures of timed planning-problem solving (TOL) and measures of fine motor dexterity (Grooved Pegboard). Thus, cognitive deficits were of similar magnitude in recent-onset and chronic schizophrenia with the exception of a limited set of time-based measures involving fine motor movements and problem solving, which were worse in chronic patients. Main effects of cohort indicated that visual memory, planning and problem solving times, working memory, processing speed, and speed of fine motor movements showed decrements in the older samples regardless of diagnosis. Gender, and interactions of gender and diagnosis were largely absent across cognitive indices.
3.2. Associations with length of illness
Examination of the association of duration of illness with cognitive functions across all 147 schizophrenia patients revealed several significant but small associations. Length of illness, after removing variance related to age, was associated with several measures of episodic memory. Longer duration of disorder was associated with worse scores (r = –.21, p < .01) and more errors (r = .21, p < .01) on the Benton Visual Retention Test, worse retention of verbal material on Logical Memory (r = –.26, p < .01), and lower total recall of words on the Hopkins Verbal Learning Test in immediate (r = –.20, p < .05) and delayed (r = –.23, p < .01) conditions. Greater length of illness was also associated with slower completion of the grooved pegboard test of fine motor movements with the dominant (r = .28, p < .01) and nondominant (r = .26, p < .01) hands, worse performance on indices of executive functioning (Letter-Number Sequencing [r = –.19, p < .05], excess moves on the Tower of London test [r = .20, p < .05]), and worse performance on the Vocabulary subtest of the WAIS-III (r = –.21, p < .01). The associations with length of illness are consistent with the greater motor and problem solving deficits noted in chronic patients as compared to recent-onset patients, but also point to the possibility of slight progression of episodic memory deficits in schizophrenia.3
3.3. Effects of past substance use and medications on cognitive functions in schizophrenia
Omnibus ANOVA's assessing the effects of past substance use on cognitive measures failed to reveal any effects of alcohol dependence, hallucinogen abuse, and cannabis abuse. The 20 schizophrenia patients who had a history of cannabis dependence tended to have worse memory for semantic elements of a story (thematic memory on the Logical Memory Test), t22.2 = 2.07, p = .05, worse recognition of verbal material (recognition on the Logical Memory Test), t145 = 2.36, p = .02, more errors on recall of visual material (BVRT), t147 = 2.58, p = .01, and slower simple reaction times on the CalCap, t122 = 2.36, p = .02.
An omnibus ANOVA for the effect of antipsychotic medications across cognitive indices revealed an interaction of antipsychotic medication status and index, F6.2,828 = 2.13, p = .04, which derived from individuals on antipsychotic medications tending to do better on cognitive tasks than individuals not on antipsychotic medication. An analysis of the differential effects of typical and atypical antipsychotics on cognitive functions revealed that chronic schizophrenia patients on typical antipsychotics tended to have worse reading (WRAT-3) t87 = –2.30, p = .02, working memory (Letter-Number Sequencing, t87 = –2.78, p = .007, and 1-back reaction times for the CalCap, t71 = 3.09, p = .003 [match], t70 = 2.39, p = .02 [sequential]), planning (excess moves on the TOL) t43.1 = 3.32, p = .002, verbal intellectual functions (Vocabulary, t88 = 2.43, p = .02, and Similarities, t88 = 2.02, p = .05), and fine motor control (time to completion on Grooved Pegboard for dominant, t86 = 2.83, p = .006, and nondominant hands, t85 = 3.45, p = .001) than chronic patients on atypicals. No recent-onset patients were taking typical antipsychotics.
Greater antipsychotic dosage as measured by chlorpromazine equivalents was associated with more errors during visual memory (BVRT) r = .27, p = .02, worse recall of verbal material (total delayed recall on Logical Memory) r = –.25, p = .04, and working memory (Letter-Number Sequencing) r = –.30, p = .01, worse visual spatial analysis (Block Design) r = –.41, p < .0005, and slowed processing (Trails A and B, r = .31, p = .009 and r = .32, p = .007, respectively; CalCap reaction times, [choice RT] r = .37, p = .004, [1-back match] r = .34, p = .007, [1-back sequential] r = .27, p = .03) and fine motor dexterity (time to completion for Grooved Pegboard [dominant] r = .43, p < .0005, [nondominant] r = .33, p = .006). Although days on antipsychotics correlated with a number of cognitive indices, when age was partialed out it was only predictive of slowed fine motor dexterity (Grooved Pegboard [dominant] r = .37, p < .001). There were also 18 patients on antiparkinsonian agents who, compared to patients not on the medications, tended to have worse fine motor dexterity (Grooved Pegboard [dominant] t145 = 4.47, p < .0005, [nondominanat] t144 = –2.97, p = .004) and visual spatial analysis (Block Design t147 = 2.59, p = .01). In summary, we found evidence for antipsychotic and antiparkinsonian medications being associated with worse motor function and impaired performance on select cognitive functions.
4. Discussion
Contrasts of cognitive functions in recent-onset and chronic schizophrenia patients from four geographic regions with separate matched control samples yielded evidence for similar cognitive deficits from shortly after onset to chronic phases of the disorder. Two measures for the amount of time taken to plan and problem solve showed diagnosis and cohort interactions suggesting progressive impairment from recent-onset to chronic schizophrenia, but these effects failed to survive correction for multiple comparisons. Effect size analysis revealed a .76 (Cohen's d) effect for the chronic schizophrenia patients on the index of average time for planning and problem solving, which was only marginally larger than the effect of .68 for first-episode patients. The twofold larger samples of chronic patients and controls compared to recent-onset samples likely provided better statistical power for detecting effects and thus contributed to the diagnosis-by-cohort interaction. An alternative analytic strategy of examining years of illness across both groups of schizophrenia patients showed small but significant associations of longer illness duration with worse performance on several indices of episodic memory, but the most variance accounted for by any index was 6.7%. Fine motor dexterity was also worse in chronic than recent-onset schizophrenia and worsened with longer duration of illness, but higher antipsychotic dosages and typical antipsychotic medications exclusively taken by the chronic patients were associated with worse dexterity on the task. Thus, chronic schizophrenia patients’ larger deficit in fine motor dexterity may be a result of medications. Although the analysis fails to provide direct evidence that lifetime antipsychotic exposure leads progressive motor dysfunction, the pattern of results is consistent with chronic motor dysfunction being exacerbated by conventional pharmacological treatments. Chronic schizophrenia patients prescribed typical antipsychotics tended to have worse performance on several tests of intellectual function, achievement, and planning, in addition to motor dexterity than chronic patients on other medications. Symptom dimensions were sparsely and weakly associated with cognitive functions with correlations in the predicted direction accounting for no more than 4.8% of the variance in any cognitive function (see Supplemental material).
Overall, results are consistent with cognitive deficits being comparable from early in the course of schizophrenia to the chronic phase of the disorder, and not progressing beyond what is expected with normal aging. Considering the present findings in concert with existing scientific evidence, cognitive impairment is typically present before formal diagnosis of schizophrenia (Woodberry et al., 2008) but additional cognitive declines appear to occur during the progression toward psychosis, and then deficits remain mostly stable over the course of the disorder. Given that estimated premorbid intelligence failed to account for cognitive deficits observed in the schizophrenia patients, findings in the present study are consistent with such a course of cognitive dysfunction in schizophrenia. An exception to a general pattern of stable cognitive deficits across recent-onset and chronic schizophrenia may be slowed planning and problem solving functions that appear to become marginally more pronounced when an individual progresses into a chronic course of schizophrenia. This is consistent with one other study showing greater executive function deficits in older schizophrenia patients (Fucetola et al., 2000). If deficits in executive functions increase as the disorder persists, greater planning difficulties evident in chronic schizophrenia may be a contributor to the marked impairment in community and vocational functioning often noted in the chronic phase of the disorder. Therefore, functional declines may not only be due to the social effects of the mental disorder but also difficulties with efficiently solving problems of every day living.
Normal developmental changes in cognitive function and brain tissue pose a challenge in describing the presence of cognitive deficits at various stages of schizophrenia. Although longitudinal designs are the most direct means by which to characterize the course of cognitive deficits, such studies involve significant challenges of ensuring minimal dropout of schizophrenia patients, retaining a matched control group over long periods of time, controlling practice effects from repeated exposures to tests (Goldberg et al., 2007), and addressing treatment effects over the period of follow-up (Keefe et al., 2004, 2006). A recent meta-analysis by Szoke et al. (2008) of longitudinal studies of cognition in schizophrenia revealed that cognitive deficits remain static or decrease over the course of the disorder. Similar degrees of cognitive dys-function across first-episode and chronic schizophrenia also need to be reconciled with neuroimaging findings of changes in brain structure over the course of the disorder (van Haren et al., 2008). Hulshoff Pol and Kahn (2008) reviewed eleven longitudinal studies of brain structures in schizophrenia and found evidence of progressive reductions in tissue over the first 20 years of the disorder, with schizophrenia subjects showing over twice the rate of tissue loss as compared to controls. The decrements were noted as greatest in frontal and temporal lobes along with an increase in lateral ventricle size. Interestingly, there were minimal associations between brain structures and measures of cognition suggesting that tissue volume is unlikely to be the primary limitation on cognitive functions. Similarly, a recent study testing whether relationships between cognitive functions and brain structures were more evident at advanced stages of schizophrenia found few differences between the association of cognitive deficits and brain volumes across first-episode and chronic schizophrenia (Premkumar et al., 2008). One exception was that chronic schizophrenia patients had a stronger association between diminished delayed episodic memory and prefrontal cortical volume than did first-episode patients. Supportive evidence of an association between prefrontal volumes and episodic memory in schizophrenia also comes from Zipparo et al. (2008). They found that over the first several years of schizophrenia, there were few cognitive changes but reductions in prefrontal gray matter volume were more evident in individuals who showed decreases in verbal episode memory (Zipparo et al., 2008). The present study revealed length of illness in schizophrenia to be associated with worse performance on several measures of episodic memory raising the possibility that progression of frontal lobe pathology may underlie modest progression of problem solving and memory deficits in the disorder. Thus, despite what appears to be largely stable cognitive deficits, progressive declines in select functions may occur and be related to specific changes in brain structure and function over the course of schizophrenia.
Finally, additional evidence points to the importance of episodic memory in the early stages of psychosis (Cirillo and Seidman, 2003). Hurlemann et al. (2008) found late prodromal stage subjects performed worse on the delayed recall of verbal material than early prodromal subjects. The late prodromal individuals also had reduced hippocampal volumes compared to the early prodromal subjects. Recent reviews have highlighted how cognitive functions dependent on the prefrontal cortex may worsen in at-risk individuals as they proceed toward psychosis (Lawrie et al., 2008) and that abnormalities in prefrontal structures may be predictive of later psychosis (Wood et al., 2008).
There are several limitations to the present study. Because the study was not longitudinal, it did not allow for direct examination of the course of cognitive deficits in schizophrenia; however, the inclusion of two control groups matched to the recent-onset and chronic schizophrenia groups allowed differentiation of age and diagnosis effects. The study also included individuals who were beyond their first-episode, but with minimal pharmacological treatment, as recent-onset patients. Nevertheless, recent-onset and chronic schizophrenia groups differed by more than 11 years of disorder on average. The present study provided evidence consistent with comparable cognitive deficits across recent-onset and chronic stages of schizophrenia. We found marginal evidence of progressive declines in the efficiency of problem solving, fine motor dexterity, and episodic memory in advanced phases of the disorder. For problem solving and memory indices greater deficits with longer disorder duration appear unrelated to medication status, symptomatology, and clinical status, and may reflect changes in prefrontal cortex over the course of the disorder. Thus, this study of a substantial number of subjects representing people from across the United States provides important information about cognitive function in early and chronic schizophrenia, a finding which will be important to consider in interpreting findings of anomalous brain structure and function in the disorder.
Supplementary Material
Acknowledgements
We thank the psychometrists and clinical raters of the MCIC sites (Albuquerque, Iowa City, Boston, and Minneapolis) within the MIND research network.
Role of funding source
Funding for this study was provided by Department of Energy (DE-FG03-99ER62764) and the work was carried out as part of the MIND Research Network. Commonwealth Research Center, Massachusetts Department of Mental Health (L.J.S., R.M.G.) provided support at one of the sites. The Department of Energy had no further role in the study design, in the collection of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Presented in part at the 14th biennial Winter Workshop on Schizophrenia and Bipolar Disorders, Montreux, Switzerland.
Contributors
Dr. Sponheim assisted in design of the cognitive assessment battery, reviewed data, conducted data analyses, and wrote the manuscript for publication. Dr. Jung oversaw and designed the administration of the cognitive test battery across sites and directed the collection of cognitive data. Drs. Seidman, Mesholam-Gately, Manoach, and O'Leary designed the cognitive assessment battery and assisted in site-specific data collection and preparation of the manuscript. Drs. Ho, Andreasen, and Lauriello designed and oversaw administration of the clinical protocol. Dr. Schulz oversaw administration of the clinical protocol, advised in the analysis of cognitive data, and assisted in writing the manuscript for publication. All authors contributed to and have approved the final manuscript.
CalCap and Tower of London variables were not included in the omnibus ANOVA due to missing data on a subset of subjects.
Effects involving site indicated that the Boston site tended to use older subjects for their chronic sample and its control group, and that their chronic sample had fewer years of education and parents of lower SES. Patients from Iowa had worse negative and disorganization symptoms. Patients from Minnesota tended to have higher levels of social functioning than the other sites. For cognitive variables there were main effects of site for category fluency, Face Recognition 1, Hopkins Total Recall 1, Logical Memory 2 thematic memory, the four Tower of London timed indices, and grooved-pegboard time for the dominant hand. There were interactions of site and diagnosis for Logical Memory 1 and 2 thematic memory, and WAIS-III subtests Vocabulary, Similarities, and Letter-Number sequencing. There were interactions of cohort and site for Benton Visual Retention Test errors and perseverations, Logical Memory 2 recognition, Trails B time, and simple reaction times from the CalCap. Finally, there were three-way interactions between diagnosis, cohort, and site for CalCap simple reaction time, and nondominant hand grooved pegboard speed.
When antipsychotic dosage and age were entered as covariates, no associations with length of illness showed a change in statistical significance and the values or the Pearson correlations were essentially unchanged.
Conflict of interest
Because the study was only funded through the Department of Energy and the report is focused on cognitive indices all authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jpsychires.2009.09.010.
References
- Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophrenia Research. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Albus M, Hubmann W, Mohr F, Hecht S, Hinterberger-Weber P, Seitz NN, et al. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:442–51. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970:303–21. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Benton AL. The visual retention test as a constructional praxis task. Confina Neurologica. 1962;22:141–55. doi: 10.1159/000104348. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. American Journal of Psychiatry. 2000;157:549–59. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, McClure MM, Leung WL, Harvey PD. Age-associated differences in cognitive performance in older community dwelling schizophrenia patients: differential sensitivity of clinical neuropsychological and experimental information processing tests. Schizophrenia Research. 2008;106:50–8. doi: 10.1016/j.schres.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–42. [Google Scholar]
- Caspi A, Reichenberg A, Weiser M, Rabinowitz J, Kaplan Z, Knobler H, et al. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophrenia Research. 2003;65:87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- Cervellione KL, Burdick KE, Cottone JG, Rhinewine JP, Kumra S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:867–78. doi: 10.1097/chi.0b013e318054678d. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychology Review. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer J. Delis–Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer's disease and normal aging. American Journal of Psychiatry. 2001;158:1441–8. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM, Tsuang MT. Age and neuropsychologic function in schizophrenia: a decline in executive abilities beyond that observed in healthy volunteers. Biological Psychiatry. 2000;48:137–46. doi: 10.1016/s0006-3223(00)00240-7. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. American Journal of Psychiatry. 2002;159:1183–9. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Gold S, Arndt S, Nopoulos P, O'Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. American Journal of Psychiatry. 1999;156:1342–8. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Archives of General Psychiatry. 2007;64:1115–22. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris S, Asarnow RF, Chock D, Jeste DV. Accelerated age-related decline in processing resources in schizophrenia: evidence from pupillary responses recorded during the span of apprehension task. Journal of the International Neuropsychological Society. 2000;6:30–43. doi: 10.1017/s1355617700611049. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, et al. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biological Psychiatry. 1999;45:32–40. doi: 10.1016/s0006-3223(98)00273-x. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Keefe RS, Christensen BK, Addington J, Woods SW, Callahan J, et al. Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment Study. Schizophrenia Research. 2008;105:1–9. doi: 10.1016/j.schres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. American Journal of Psychiatry. 1999;156:1336–41. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten-year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophrenia Research. 2005;78:27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: a community study. John Wiley and Sons Inc; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophrenia Bulletin. 2008;34:354–66. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Jessen F, Wagner M, Frommann I, Ruhrmann S, Brockhaus A, et al. Interrelated neuropsychological and anatomical evidence of hippocampal pathology in the at-risk mental state. Psychological Medicine. 2008:1–9. doi: 10.1017/S0033291708003279. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Czobor P, Berns SM. Basic neuropsychological dimensions in schizophrenia. Schizophrenia Research. 2003;65:105–16. doi: 10.1016/s0920-9964(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. American Journal of Psychiatry. 2004;161:985–95. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biological Psychiatry. 2006;59:97–105. doi: 10.1016/j.biopsych.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kenny JT, Friedman L, Findling RL, Swales TP, Strauss ME, Jesberger JA, et al. Cognitive impairment in adolescents with schizophrenia. American Journal of Psychiatry. 1997;154:1613–5. doi: 10.1176/ajp.154.11.1613. [DOI] [PubMed] [Google Scholar]
- Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophrenia Research. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Seltzer JC, Ferrand JL, Wexler BE. Neurocognitive function in schizophrenia at a 10-year follow-up: a preliminary investigation. CNS Spectrums. 2005;10:277–80. doi: 10.1017/s1092852900022598. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophrenia Bulletin. 2008;34:330–40. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately R, Giuliano A, Faraone S, Goff K, Seidman L. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Miller EN. California Computerized Assessment Battery (CalCAP) Norland Software; Los Angeles: 1990. [Google Scholar]
- Premkumar P, Kumari V, Corr PJ, Fannon D, Sharma T. Neuropsychological function-brain structure relationships and stage of illness: an investigation into chronic and first-episode schizophrenia. Psychiatry Research. 2008;162:195–204. doi: 10.1016/j.pscychresns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–6. [Google Scholar]
- Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the finger tapping and grooved pegboard tests. Perceptual and Motor Skills. 1993;76:1219–30. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Rund BR, Melle I, Friis S, Johannessen JO, Larsen TK, Midboe LJ, et al. The course of neurocognitive functioning in first-episode psychosis and its relation to premorbid adjustment, duration of untreated psychosis, and relapse. Schizophrenia Research. 2007;91:132–40. doi: 10.1016/j.schres.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology. 2006;28:225–42. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society. 1982:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Stirling J, White C, Lewis S, Hopkins R, Tantam D, Huddy A, et al. Neurocognitive function and outcome in first-episode schizophrenia: a 10-year follow-up of an epidemiological cohort. Schizophrenia Research. 2003;65:75–86. doi: 10.1016/s0920-9964(03)00014-8. [DOI] [PubMed] [Google Scholar]
- Szoke A, Trandafir A, Dupont ME, Meary A, Schurhoff F, Leboyer M. Longitudinal studies of cognition in schizophrenia: meta-analysis. British Journal of Psychiatry. 2008;192:248–57. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Pol HE, Schnack HG, Cahn W, Brans R, Carati I, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biological Psychiatry. 2008;63:106–13. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd ed. Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Weickert TW, Goldberg TE. First- and second-generation antipsychotic medication and cognitive processing in schizophrenia. Current Psychiatry Reports. 2005;7:304–10. doi: 10.1007/s11920-005-0085-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test. 3rd ed. Wide Range Inc.; Wilmington, DE: 1993. [Google Scholar]
- Wood SJ, Pantelis C, Velakoulis D, Yucel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophrenia Bulletin. 2008;34:322–9. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry. 2008;165:579–87. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Zipparo L, Whitford TJ, Redoblado Hodge MA, Lucas S, Farrow TF, Brennan J, et al. Investigating the neuropsychological and neuroanatomical changes that occur over the first 2–3 years of illness in patients with first-episode schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:531–8. doi: 10.1016/j.pnpbp.2007.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.