Abstract
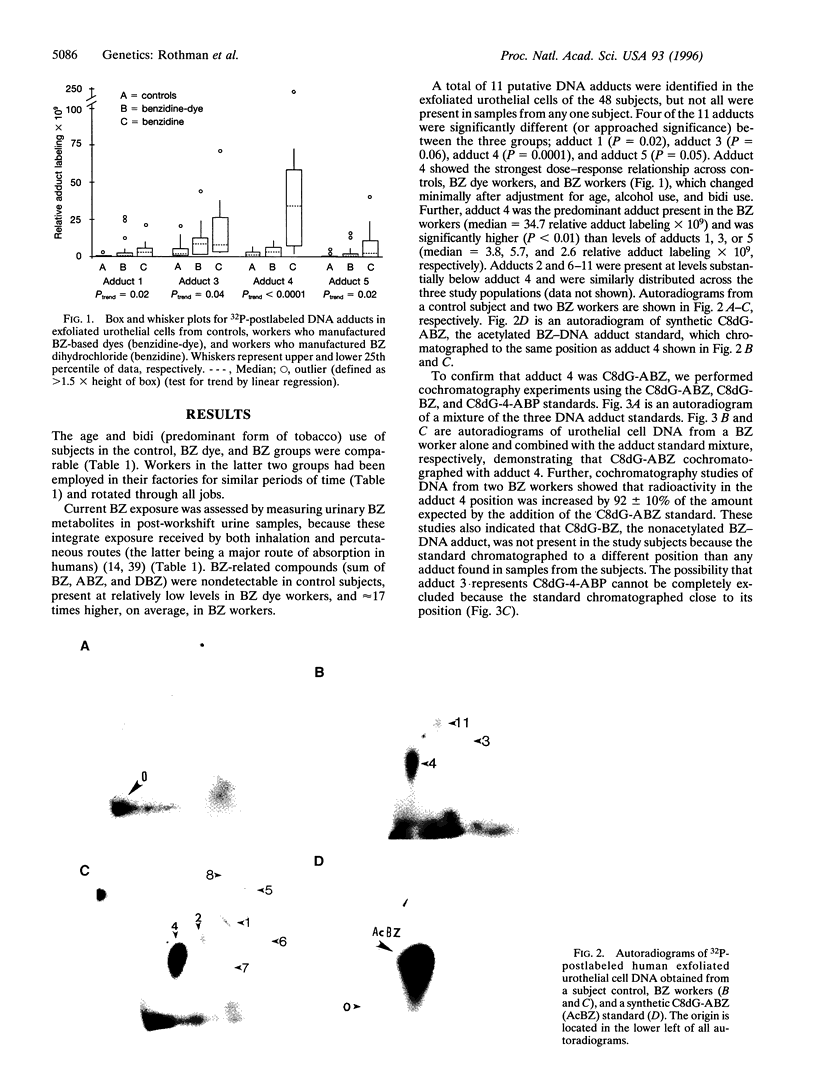
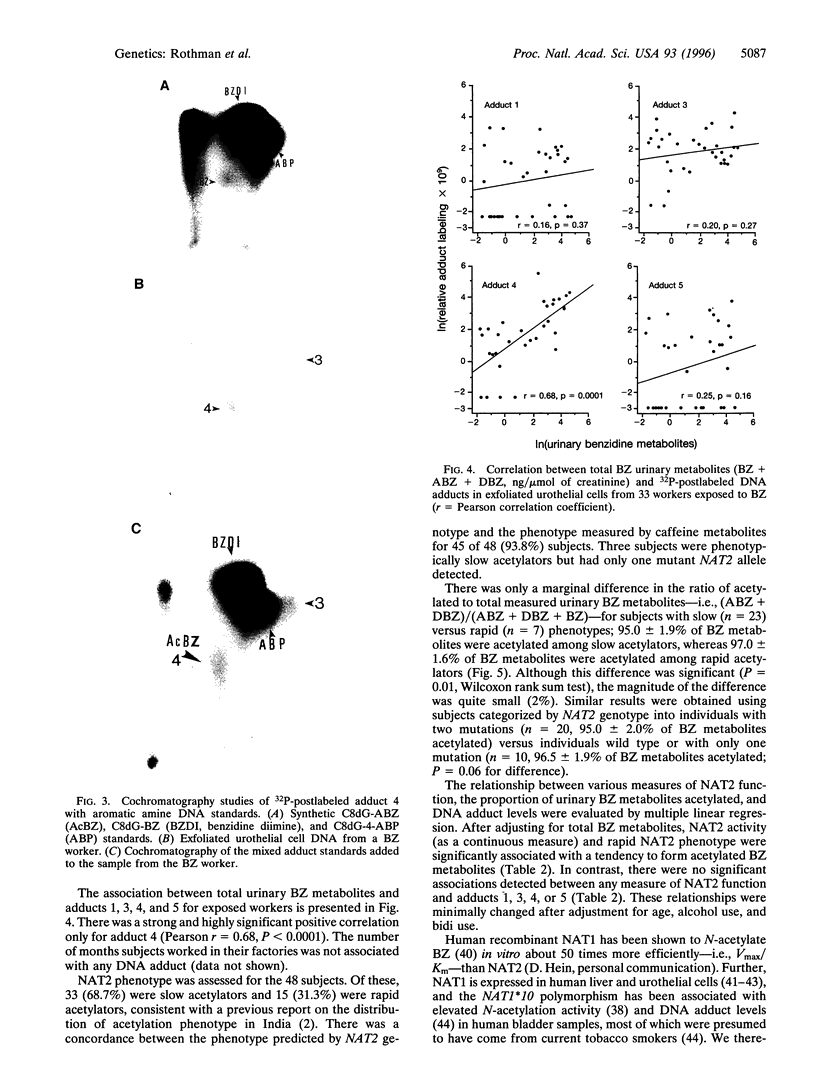
Several epidemiologic studies indicate that NAT2-related slow N-acetylation increases bladder cancer risk among workers exposed to aromatic amines, presumably because N-acetylation is important for the detoxification of these compounds. Previously, we showed that NAT2 polymorphisms did not influence bladder cancer risk among Chinese workers exposed exclusively to benzidine (BZ), suggesting that NAT2 N-acetylation is not a critical detoxifying pathway for this aromatic amine. To evaluate the biologic plausibility of this finding, we carried out a cross-sectional study of 33 workers exposed to BZ and 15 unexposed controls in Ahmedabad, India, to evaluate the presence of BZ-related DNA adducts in exfoliated urothelial cells, the excretion pattern of BZ metabolites, and the impact of NAT2 activity on these outcomes. Four DNA adducts were significantly elevated in exposed workers compared to controls; of these, the predominant adduct cochromatographed with a synthetic N-(3'- phosphodeoxyguanosin-8-yl)-N'-acetylbenzidine standard and was the only adduct that was significantly associated with total BZ urinary metabolites (r = 0.68, P < 0.0001). To our knowledge this is the first report to show that BZ forms DNA adducts in exfoliated urothelial cells of exposed humans and that the predominant adduct formed is N-acetylated, supporting the concept that monofunctional acetylation is an activation, rather than a detoxification, step for BZ. However, because almost all BZ-related metabolites measured in the urine of exposed workers were acetylated among slow, as well as rapid, acetylators (mean +/- SD 95 +/- 1.9% vs. 97 +/- 1.6%, respectively) and NAT2 activity did not affect the levels of any DNA adduct measured, it is unlikely that interindividual variation in NAT2 function is relevant for BZ-associated bladder carcinogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badawi A. F., Hirvonen A., Bell D. A., Lang N. P., Kadlubar F. F. Role of aromatic amine acetyltransferases, NAT1 and NAT2, in carcinogen-DNA adduct formation in the human urinary bladder. Cancer Res. 1995 Nov 15;55(22):5230–5237. [PubMed] [Google Scholar]
- Bell D. A., Badawi A. F., Lang N. P., Ilett K. F., Kadlubar F. F., Hirvonen A. Polymorphism in the N-acetyltransferase 1 (NAT1) polyadenylation signal: association of NAT1*10 allele with higher N-acetylation activity in bladder and colon tissue. Cancer Res. 1995 Nov 15;55(22):5226–5229. [PubMed] [Google Scholar]
- Bell D. A., Stephens E. A., Castranio T., Umbach D. M., Watson M., Deakin M., Elder J., Hendrickse C., Duncan H., Strange R. C. Polyadenylation polymorphism in the acetyltransferase 1 gene (NAT1) increases risk of colorectal cancer. Cancer Res. 1995 Aug 15;55(16):3537–3542. [PubMed] [Google Scholar]
- Bell D. A., Taylor J. A., Butler M. A., Stephens E. A., Wiest J., Brubaker L. H., Kadlubar F. F., Lucier G. W. Genotype/phenotype discordance for human arylamine N-acetyltransferase (NAT2) reveals a new slow-acetylator allele common in African-Americans. Carcinogenesis. 1993 Aug;14(8):1689–1692. doi: 10.1093/carcin/14.8.1689. [DOI] [PubMed] [Google Scholar]
- Bi W., Hayes R. B., Feng P., Qi Y., You X., Zhen J., Zhang M., Qu B., Fu Z., Chen M. Mortality and incidence of bladder cancer in benzidine-exposed workers in China. Am J Ind Med. 1992;21(4):481–489. doi: 10.1002/ajim.4700210404. [DOI] [PubMed] [Google Scholar]
- Blum M., Demierre A., Grant D. M., Heim M., Meyer U. A. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5237–5241. doi: 10.1073/pnas.88.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. A., Lang N. P., Young J. F., Caporaso N. E., Vineis P., Hayes R. B., Teitel C. H., Massengill J. P., Lawsen M. F., Kadlubar F. F. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992 Jun;2(3):116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Cartwright R. A., Glashan R. W., Rogers H. J., Ahmad R. A., Barham-Hall D., Higgins E., Kahn M. A. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982 Oct 16;2(8303):842–845. doi: 10.1016/s0140-6736(82)90810-8. [DOI] [PubMed] [Google Scholar]
- Dewan A., Jani J. P., Patel J. S., Gandhi D. N., Variya M. R., Ghodasara N. B. Benzidine and its acetylated metabolites in the urine of workers exposed to Direct Black 38. Arch Environ Health. 1988 Jul-Aug;43(4):269–272. doi: 10.1080/00039896.1988.10545948. [DOI] [PubMed] [Google Scholar]
- Flammang T. J., Yamazoe Y., Benson R. W., Roberts D. W., Potter D. W., Chu D. Z., Lang N. P., Kadlubar F. F. Arachidonic acid-dependent peroxidative activation of carcinogenic arylamines by extrahepatic human tissue microsomes. Cancer Res. 1989 Apr 15;49(8):1977–1982. [PubMed] [Google Scholar]
- Frederick C. B., Weis C. C., Flammang T. J., Martin C. N., Kadlubar F. F. Hepatic N-oxidation, acetyl-transfer and DNA-binding of the acetylated metabolites of the carcinogen, benzidine. Carcinogenesis. 1985 Jul;6(7):959–965. doi: 10.1093/carcin/6.7.959. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Blum M., Beer M., Meyer U. A. Monomorphic and polymorphic human arylamine N-acetyltransferases: a comparison of liver isozymes and expressed products of two cloned genes. Mol Pharmacol. 1991 Feb;39(2):184–191. [PubMed] [Google Scholar]
- Grant D. M., Josephy P. D., Lord H. L., Morrison L. D. Salmonella typhimurium strains expressing human arylamine N-acetyltransferases: metabolism and mutagenic activation of aromatic amines. Cancer Res. 1992 Jul 15;52(14):3961–3964. [PubMed] [Google Scholar]
- Gupta R. C., Earley K., Sharma S. Use of human peripheral blood lymphocytes to measure DNA binding capacity of chemical carcinogens. Proc Natl Acad Sci U S A. 1988 May;85(10):3513–3517. doi: 10.1073/pnas.85.10.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke J., Krajewska B. Acetylation phenotypes and bladder cancer. J Occup Med. 1990 Sep;32(9):917–918. doi: 10.1097/00043764-199009000-00032. [DOI] [PubMed] [Google Scholar]
- Hayes R. B., Bi W., Rothman N., Broly F., Caporaso N., Feng P., You X., Yin S., Woosley R. L., Meyer U. A. N-acetylation phenotype and genotype and risk of bladder cancer in benzidine-exposed workers. Carcinogenesis. 1993 Apr;14(4):675–678. doi: 10.1093/carcin/14.4.675. [DOI] [PubMed] [Google Scholar]
- Hein D. W. Acetylator genotype and arylamine-induced carcinogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):37–66. doi: 10.1016/0304-419x(88)90004-2. [DOI] [PubMed] [Google Scholar]
- Hsu F. F., Lakshmi V., Rothman N., Bhatnager V. K., Hayes R. B., Kashyap R., Parikh D. J., Kashyap S. K., Turk J., Zenser T. Determination of benzidine, N-acetylbenzidine, and N,N'-diacetylbenzidine in human urine by capillary gas chromatography/negative ion chemical ionization mass spectrometry. Anal Biochem. 1996 Feb 15;234(2):183–189. doi: 10.1006/abio.1996.0070. [DOI] [PubMed] [Google Scholar]
- Josephy P. D. Benzidine: mechanisms of oxidative activation and mutagenesis. Fed Proc. 1986 Sep;45(10):2465–2470. [PubMed] [Google Scholar]
- Kennelly J. C., Beland F. A., Kadlubar F. F., Martin C. N. Binding of N-acetylbenzidine and N,N'-diacetylbenzidine to hepatic DNA of rat and hamster in vivo and in vitro. Carcinogenesis. 1984 Mar;5(3):407–412. doi: 10.1093/carcin/5.3.407. [DOI] [PubMed] [Google Scholar]
- Kirlin W. G., Trinidad A., Yerokun T., Ogolla F., Ferguson R. J., Andrews A. F., Brady P. K., Hein D. W. Polymorphic expression of acetyl coenzyme A-dependent arylamine N-acetyltransferase and acetyl coenzyme A-dependent O-acetyltransferase-mediated activation of N-hydroxyarylamines by human bladder cytosol. Cancer Res. 1989 May 1;49(9):2448–2454. [PubMed] [Google Scholar]
- Kloth M. T., Gee R. L., Messing E. M., Swaminathan S. Expression of N-acetyltransferase (NAT) in cultured human uroepithelial cells. Carcinogenesis. 1994 Dec;15(12):2781–2787. doi: 10.1093/carcin/15.12.2781. [DOI] [PubMed] [Google Scholar]
- Lakshmi V. M., Bell D. A., Watson M. A., Zenser T. V., Davis B. B. N-acetylbenzidine and N,N'-diacetylbenzidine formation by rat and human liver slices exposed to benzidine. Carcinogenesis. 1995 Jul;16(7):1565–1571. doi: 10.1093/carcin/16.7.1565. [DOI] [PubMed] [Google Scholar]
- Lakshmi V. M., Zenser T. V., Goldman H. D., Spencer G. G., Gupta R. C., Hsu F. F., Davis B. B. The role of acetylation in benzidine metabolism and DNA adduct formation in dog and rat liver. Chem Res Toxicol. 1995 Jul-Aug;8(5):711–720. doi: 10.1021/tx00047a011. [DOI] [PubMed] [Google Scholar]
- Lin H. J., Han C. Y., Lin B. K., Hardy S. Slow acetylator mutations in the human polymorphic N-acetyltransferase gene in 786 Asians, blacks, Hispanics, and whites: application to metabolic epidemiology. Am J Hum Genet. 1993 Apr;52(4):827–834. [PMC free article] [PubMed] [Google Scholar]
- Lower G. M., Jr, Nilsson T., Nelson C. E., Wolf H., Gamsky T. E., Bryan G. T. N-acetyltransferase phenotype and risk in urinary bladder cancer: approaches in molecular epidemiology. Preliminary results in Sweden and Denmark. Environ Health Perspect. 1979 Apr;29:71–79. doi: 10.1289/ehp.792971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso T. F., el-Attar A. A. Cohort study of workers exposed to betanaphthylamine and benzidine. J Occup Med. 1967 Jun;9(6):277–285. [PubMed] [Google Scholar]
- Martin C. N., Beland F. A., Roth R. W., Kadlubar F. F. Covalent binding of benzidine and N-acetylbenzidine to DNA at the C-8 atom of deoxyguanosine in vivo and in vitro. Cancer Res. 1982 Jul;42(7):2678–2686. [PubMed] [Google Scholar]
- Meigs J. W., Marrett L. D., Ulrich F. U., Flannery J. T. Bladder tumor incidence among workers exposed to benzidine: a thirty-year follow-up. J Natl Cancer Inst. 1986 Jan;76(1):1–8. [PubMed] [Google Scholar]
- Morinaga K., Yutani S., Hara I. [Cancer mortality of male workers exposed to benzidine and/or beta-naphthylamine]. Nihon Eiseigaku Zasshi. 1990 Oct;45(4):909–918. doi: 10.1265/jjh.45.909. [DOI] [PubMed] [Google Scholar]
- Morton K. C., Wang C. Y., Garner C. D., Shirai T. Carcinogenicity of benzidine, N,N'-diacetylbenzidine, and N-hydroxy-N,N'-diacetylbenzidine for female CD rats. Carcinogenesis. 1981;2(8):747–752. doi: 10.1093/carcin/2.8.747. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Gordon G. R., Lin E., Green C. E., Tyson C. A. Polymorphic N-acetylation of sulfamethazine and benzidine by human liver: implication for cancer risk? Anticancer Res. 1990 Jan-Feb;10(1):225–229. [PubMed] [Google Scholar]
- Radomski J. L. The primary aromatic amines: their biological properties and structure-activity relationships. Annu Rev Pharmacol Toxicol. 1979;19:129–157. doi: 10.1146/annurev.pa.19.040179.001021. [DOI] [PubMed] [Google Scholar]
- Risch A., Wallace D. M., Bathers S., Sim E. Slow N-acetylation genotype is a susceptibility factor in occupational and smoking related bladder cancer. Hum Mol Genet. 1995 Feb;4(2):231–236. doi: 10.1093/hmg/4.2.231. [DOI] [PubMed] [Google Scholar]
- Rothman N., Hayes R. B., Bi W., Caporaso N., Broly F., Woosley R. L., Yin S., Feng P., You X., Meyer U. A. Correlation between N-acetyltransferase activity and NAT2 genotype in Chinese males. Pharmacogenetics. 1993 Oct;3(5):250–255. doi: 10.1097/00008571-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Talaska G., Au W. W., Ward J. B., Jr, Randerath K., Legator M. S. The correlation between DNA adducts and chromosomal aberrations in the target organ of benzidine exposed, partially-hepatectomized mice. Carcinogenesis. 1987 Dec;8(12):1899–1905. doi: 10.1093/carcin/8.12.1899. [DOI] [PubMed] [Google Scholar]
- Talaska G., Dooley K. L., Kadlubar F. F. Detection and characterization of carcinogen-DNA adducts in exfoliated urothelial cells from 4-aminobiphenyl-treated dogs by 32P-postlabelling and subsequent thin-layer and high-pressure liquid chromatography. Carcinogenesis. 1990 Apr;11(4):639–646. doi: 10.1093/carcin/11.4.639. [DOI] [PubMed] [Google Scholar]
- Talaska G., Schamer M., Skipper P., Tannenbaum S., Caporaso N., Unruh L., Kadlubar F. F., Bartsch H., Malaveille C., Vineis P. Detection of carcinogen-DNA adducts in exfoliated urothelial cells of cigarette smokers: association with smoking, hemoglobin adducts, and urinary mutagenicity. Cancer Epidemiol Biomarkers Prev. 1991 Nov-Dec;1(1):61–66. [PubMed] [Google Scholar]
- Talaska G., al-Juburi A. Z., Kadlubar F. F. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsis K. P., Martell K. J., Weber W. W. Diverse point mutations in the human gene for polymorphic N-acetyltransferase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6333–6337. doi: 10.1073/pnas.88.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsis K. P., Weber W. W., Bell D. A., Dupret J. M., Evans D. A., Grant D. M., Hein D. W., Lin H. J., Meyer U. A., Relling M. V. Nomenclature for N-acetyltransferases. Pharmacogenetics. 1995 Feb;5(1):1–17. doi: 10.1097/00008571-199502000-00001. [DOI] [PubMed] [Google Scholar]
- Wise R. W., Zenser T. V., Davis B. B. Characterization of benzidinediimine: a product of peroxidase metabolism of benzidine. Carcinogenesis. 1984 Nov;5(11):1499–1503. doi: 10.1093/carcin/5.11.1499. [DOI] [PubMed] [Google Scholar]
- You X. Y., Chen J. G., Hu Y. N. Studies on the relation between bladder cancer and benzidine or its derived dyes in Shanghai. Br J Ind Med. 1990 Aug;47(8):544–552. doi: 10.1136/oem.47.8.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavon M. R., Hoegg U., Bingham E. Benzidine exposure as a cause of bladder tumors. Arch Environ Health. 1973 Jul;27(1):1–7. doi: 10.1080/00039896.1973.10666297. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Mattammal M. B., Wise R. W., Rice J. R., Davis B. B. Prostaglandin H synthase-catalyzed activation of benzidine: a model to assess pharmacologic intervention of the initiation of chemical carcinogenesis. J Pharmacol Exp Ther. 1983 Dec;227(3):545–550. [PubMed] [Google Scholar]