Abstract
The role of the complement system in mediating human renal disease has long been recognized in immune-complex excess syndromes such as systemic lupus erythematosus and in dense deposit disease in which no immunoglobulin (Ig) is present. Over the past 15 years, mutations in complement regulatory genes have been demonstrated to predispose to thrombotic microangiopathies including atypical hemolytic uremic syndrome, C3 and C1q glomerulopathies, and preeclampsia. Excessive complement activation on an endothelial cell, due to either an auto-antibody or a regulatory protein deficiency, sets up a procoagulant state in these diseases as well as in the antiphospholipid syndrome. Knowledge of the genes involved and the functional consequences of alterations in their structure has led to therapy that blocks complement activation.
Keywords: aHUS, C3 and C1q glomerulopathy, preeclampsia, antiphospholipid syndrome, complement inhibitors
INTRODUCTION
We were taught as medical students, house officers, and fellows that renal disease in lupus is secondary to excessive proinflammatory complement-fixing immune complexes deposited in the glomerulus. Serum sickness was an informative and tractable experimental model (in those “prehistoric” days, the rabbit was the animal of choice for these investigations) of this clinical disease state. More than 50 years later, there is no reason to discard this line of reasoning to account for the pathogenesis of the membranoproliferative-glomerulonephritides (MPGN) type I.
In this review, however, we primarily focus on a different paradigm in which there is excessive complement activation in the kidney secondary to a deficiency of an inhibitor of complement activation. Pioneering studies in this regard were initially reported in the 1970s by Clark West’s group at Cincinnati Children’s Hospital. The field has been rejuvenated over the past 15 years by genetic analyses demonstrating mutations in complement inhibitors in type II MPGN (also called dense deposit disease or DDD) and thrombotic microangiopathies (TMAs). The TMAs include atypical hemolytic uremic syndrome (aHUS), preeclampsia, and the antiphospholipid syndrome (APS). A major unanswered question in this field is why the kidney is preferentially at risk in these syndromes featuring complement activation and microvascular injury.
HUMAN COMPLEMENT BIOLOGY FOR THE CLINICIAN
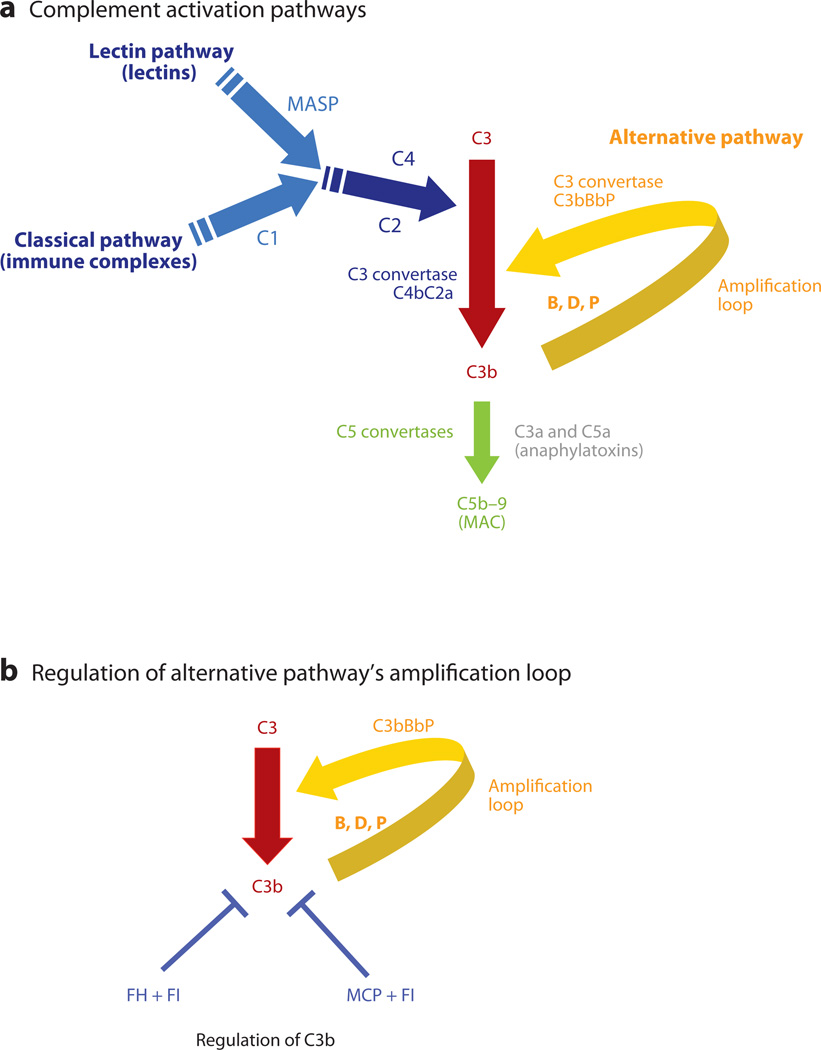
The complement system is part of innate and adaptive immunity (Figure 1a). It is an ancient host defense system of hemolymph and blood. Perhaps its most remarkable feature is that it reacts in seconds to opsonize or lyse a bacterium while simultaneously releasing pro-inflammatory and chemotactic peptides. Most other aspects of immunity require hours, days, or weeks to mount a response. One notable exception, of course, is the immune host, whose antibodies and memory T cells bind a target in seconds and, in the case of the IgM and IgG, also fix complement. The complement system is abundant in blood (the “guardian of the intravascular space”). Thus, human serum can be diluted 1/100 to 1/200 and still lyse microbes, such as many gram-negative bacteria or sensitized red blood cells in the standard whole complement (CH50) assay.
Figure 1.
Complement activation pathways. (a) Activation of the classical, lectin, and alternative pathways results in the generation of C3 convertases that cleave C3 into C3b and C3a. C1 of the classical pathway and mannose-binding associated serine proteases (MASPs) of the lectin pathway cleave C4 and C2, and the resultant larger fragments then form the classical/lectin pathway C3 convertase. C3 is cleaved by C3 convertases into C3b, which deposits on the cell surface, and C3a, an anaphylatoxin, which recruits and activates effector cells. C3b may rapidly amplify through a positive feedback loop termed the C3b alternative pathway amplification loop. C3b may also trigger activation of C5 through the generation of the C5 convertases, which are composed of C3b attached to a C3 convertase. This structure alters the substrate specificity of the convertase to favor C5. C5 convertase cleaves C5 and results in the generation of C5a (an anaphylatoxin) and assembly of the membrane attack complex (MAC) beginning with C5b (plus C6, C7, C8 and many C9s). (b) The amplification or feedback loop of the alternative pathway. Factor H (FH), factor I (FI), and membrane cofactor protein (MCP) are the key regulators of the alternative pathway and interrupt the feedback loop. Excessive activation via this loop secondary to a decrease in regulatory activity or a gain of function in a component like FB or C3 predisposes to atypical hemolytic uremic syndrome. Also see Table 1. FI is a serine protease; in conjunction with a cofactor protein (FH or MCP), it cleaves C3b to iC3b, which cannot form a C3 convertase, i.e., the feedback loop cannot be engaged. C3bBbP is the alternative-pathway C3 convertase.
The complement system is activated in two general ways: first, spontaneous turnover (1%–2% h−1) in the case of the alternative pathway (AP); second, antibody binding to antigen, the classical pathway (CP), or a lectin (equivalent to an antibody) binding to a sugar (equivalent to an antigen) on a microbe, the lectin pathway (LP) (Figure 1a). The LP preceded the CP in evolution. The latter arose in parallel with the adaptive humoral immune system in chordata. After the initial recognition event, the CP and LP feature specific proteases and an amplification process leading to cleavage of millions of molecules of C3 into C3a and C3b. The proteolytically generated C3b fragment binds covalently to a target and serves as the major opsonin of the system. Also, C3b, no matter how it is generated, serves as a nidus for the amplification or feedback loop of the AP to generate more C3b (Figure 1a). Subsequently, C5 cleavage produces C5b, which, via a series of protein–protein interactions (nonproteolytic) generates the membrane attack complex (MAC, C5b–9). The 10-kDa peptides released upon proteolytic cleavage of C3 and C5 are called anaphylatoxins, namely C3a and C5a. They bind to their respective receptors on epithelial, endothelial, immune, and hematopoietic cells to trigger an inflammatory response. Complement activation products contribute significantly to thrombosis by directly enhancing blood-clotting properties and by augmenting the inflammatory responses of leukocytes and endothelium, which, in turn, potentiate coagulation, a key aspect of diseases described in this review (1).
A critical feature of the AP is its amplification or feedback loop, which can be efficiently engaged once C3b becomes bound to a target (Figure 1b). Independent of the initiator of complement activation, the AP has the potential to continue to cleave C3 and generate downstream effectors, opsonins, anaphylatoxins, and the MAC. The lack of appropriate regulation of this amplification loop leads to excessive injury at sites of complement activation. The renal diseases we discuss are characterized by inappropriate overactivity of the AP amplification loop.
The three major regulators of the AP amplification loop are two plasma proteins, Factor H (FH) and Factor I (FI), and membrane cofactor protein (MCP; CD46) (Table 1). Alterations in all three have been associated with human disease. FH prevents fluid phase activation (no target), and MCP protects healthy cells (the wrong target). This division of labor, however, does not address how the host modulates activation at an acellular site or on damaged (necrotic, apoptotic, stressed) cells. FH has the remarkable ability, primarily via its COOH-terminus repeats, to attach to injured or exposed tissue (for example, an underlying basement membrane when endothelial cells are lost). FH and MCP bind to C3b and inhibit the amplification loop as this interaction allows for a serine protease (FI) to now cleave C3b to iC3b, an inactive form of C3 unable to participate in the amplification loop (Figure 1b). Heterozygous mutations in FH, MCP, or FI lead to haploinsufficiency and thereby predispose to several of the diseases we discuss because of inadequate control of complement activation. Mother Nature wants you to have 500 µg/ml of FH in serum, not 250 µg/ml; 25 µg/ml of FI in serum, not 12.5; and 400,000 copies of MCP per endothelial cell, not 200,000. Deficiencies, even 50% lower expression of these regulators, increase the risk for life-threatening renal disease, usually at a very young age.
Table 1.
Frequency and phenotypes of mutations in complement genes in atypical hemolytic uremic syndrome
| Complement protein | Distribution | Serum concentration (µg/ml) | Molecular weight (kDa) |
|---|---|---|---|
| FH | Serum | 250–750 | 150 |
| FI | Serum | 15–30 | 90 |
| MCP | Cells | NA | 60 |
| FB | Serum | 200 | 100 |
| C3 | Serum | 900–1800 | 190 |
| Thrombomodulin | Cells | NA | 75 |
FB, factor B; FH, factor H; FI, factor I; MCP, membrane cofactor protein; NA, not applicable.
HEMOLYTIC UREMIC SYNDROME
Hemolytic uremic syndrome (HUS) was discussed in an Annual Review of Medicine article in 2008 (10) and has been expertly reviewed by several authors subsequently. Herein, we summarize these findings and focus on developments over the past five years.
HUS is a TMA featuring the triad of hemolytic anemia, thrombocytopenia, and acute renal impairment. It is characterized by preferential formation of fibrin-rich platelet clots in glomerular capillaries and arterioles. Endothelial cell injury is a pathologic feature common to all subtypes of HUS.
Clinical classification is based in part on the triggers of endothelial injury:
Typical, enteropathic, or epidemic HUS is mainly caused by shiga toxin (Stx)–producing Escherichia coli. It is often preceded by bloody diarrhea and accounts for 90% of HUS cases in childhood. Typical HUS does not relapse, and renal function recovers completely in >90% of cases.
Atypical HUS (aHUS) is distinguished by the absence of diarrhea (non-enteropathic) secondary to an E. coli infection. It is associated with mutations in genes encoding proteins of the AP, and the clinical course is more severe (Figure 1b, Table 1). The heterozygous mutations in aHUS are mostly rare or unique and deleterious (Table 2). Mutations in FH, MCP, FI, C3, Factor B (FB), and thrombomodulin account for 50%–70% of cases. Another 5%–10% are related to autoantibodies to FH.
Table 2.
Complement regulatory proteins and aHUS
| Complement protein | Function | Frequency in aHUS (%) |
Clinical outcome | Risk of recurrence in renal transplant |
|---|---|---|---|---|
| FH | Binds C3b, CA | 20–30 | Poor | High |
| FI | Cleaves C3b to iC3b (requires a cofactor protein) | 5–10 | Poor | High |
| MCP | Binds C3b, CA | 10–20 | Good | Low |
| FB | Complexes with C3b to form AP convertases | <5 | Poor | Three reported cases lost grafts to recurrence |
| C3 | Pivotal component of complement system. Activation of all three pathways results in cleavage of C3 | 5–10 | Poor | High |
| Thrombomodulin | Cofactor in protein C anticoagulant system, enhances cofactor activity | 5 | Poor | One reported case lost graft to recurrence |
AP, alternative pathway; CA, cofactor activity; FB, factor B; FH, factor H; FI, factor I; MCP, membrane cofactor protein.
Genetics
Heterozygous mutations in the complement system and related genes occur in 50%–70% of cases of aHUS.
Mutations in the plasma complement regulators
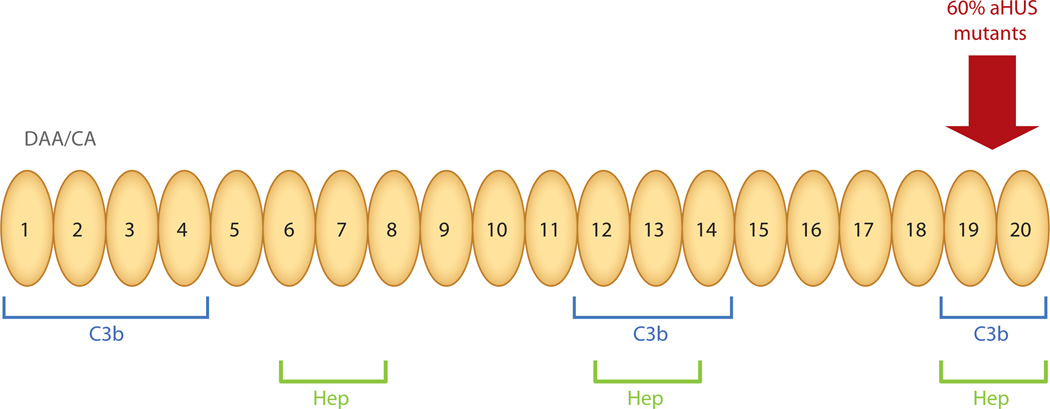
FH mutation is the most common genetic cause of aHUS, accounting for 20%–30% of cases. Atypical HUS associated with FH mutation presents early in childhood in ~70% of affected individuals and in adulthood in ~30% (2, 3). The majority of the heterozygous mutations cluster in the carboxy-terminal repeats, which carry a surface-recognition function (Figure 2). The mutant protein thus has normal cofactor activity for C3b in plasma but impaired binding to surface-bound C3b. In addition, autoantibodies to FH have been reported in 5%–10% of cases. These autoantibodies arise in the setting of a homozygous deletion of complement Factor H–related proteins 1 and 3 (FHR1, 3). The five FHR proteins (FHR1–5) are encoded by genes in the regulators of complement activation (RCA) gene cluster at 1q32. The “breaking of tolerance” that underlies the association between deletion of FHR1 and FHR3 and autoantibody formation to FH is not understood, but the functional consequences are clear. Anti-FH IgG binds to complement control protein (CCP) repeats 19 and 20 and inhibits binding of FH to C3b and cell surfaces. Further, a heterozygous FH-FHR1 hybrid allele that also alters the surface recognition motif has been detected in 3%–5% of aHUS cases (4–8).
Figure 2.
Schematic diagram of factor H. The N-terminal repeats form the regulatory domain and the C-terminal repeats 19–20 form the major surface recognition domain. The majority of mutations reported in aHUS are in the COOH− terminal repeats 19–20. Weaker C3b binding and recognition domains are also located in repeats 6–7 and 12–14. The surface-binding repeats are also known as anionic or heparin-binding sites. DAA, decay-accelerating activity; CA, cofactor activity; Hep, heparin binding. Adapted from Reference 3 with permission.
FI is the circulating serine protease that cleaves C3b into iC3b following binding of FH or MCP to C3b. Heterozygous FI mutations account for 4%–10% of aHUS cases (9–11).
Mutations in membrane-bound complement regulators
Mutations in MCP/CD46 account for 10%–20% of cases. In about 75% of cases, this widely expressed protein is not secreted; in the other 25%, the mutant protein is expressed normally but has reduced complement regulatory activity. Atypical HUS associated with MCP mutations usually presents in childhood. It tends to be less severe, often with a near complete recovery but with recurrences. Kidney transplantation in these patients, in contrast to those with mutations in FH or FI, has a nearly normal success rate because the transplanted kidney carries a normal copy number of MCP/CD46 (10, 11).
Thrombomodulin is a membrane-bound receptor for thrombin that converts it to an anticoagulant enzyme. This glycoprotein facilitates complement inactivation by FI in the presence of FH. Cells expressing the mutant protein are less efficient in degrading C3b and in generating thrombin-activatable fibrinolysis inhibitor, which cleaves C3a and C5a. The onset is in childhood in 90% of cases (9, 11, 12). A recent study has shown that ~5% of patients with aHUS carry a heterozygous mutation in thrombomodulin (12).
Mutations in complement pathway components
Gain-of-function mutations in FB enhance convertase activity by increasing the affinity of the interaction of FB with C3b. The convertase formed with C3b and mutant FB is more resistant to regulation by FH and MCP (9–11). For C3, most of the heterozygous mutations occur at the sites where C3b interacts with FH and MCP. Atypical HUS associated with C3 mutations presents in childhood in ~50% of individuals (9–11).
Pathogenesis
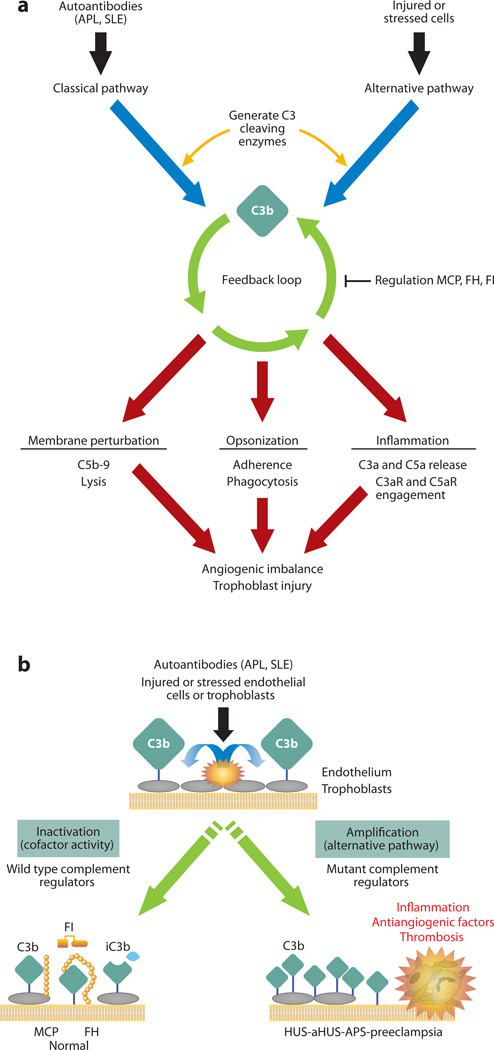
The model of aHUS most consistent with the genetic data is an excessive activation of the AP for a given degree of an environmental trigger or stressor, such as an upper respiratory tract infection or pregnancy. The functional consequence of these mutations is inefficient degradation of C3b leading to persistent activity of C3b-containing convertases that cleave C3 and C5, which in turn generate excessive amounts of complement pathway effectors (Figure 3). C5b initiates the assembly of the MAC, leading to undesirable membrane injury, while C5a recruits and activates leukocytes and upregulates vascular adhesiveness. The delicate balance between complement activation and regulation is thus disturbed on endothelial cells, resulting in a TMA (13, 14).
Figure 3.
Complement activation on injured or stressed cells. (a) Black and blue arrows: autoantibodies (such as antiphospholipid antibodies and other SLE-associated antibodies) and damaged tissues trigger the complement cascade; green arrows: deposition of C3b on a target sets in motion the powerful amplification loop of the alternative pathway; red arrows: effector activities of complement are generated by C3b deposition and C3a release and the downstream mediators C5b–9 and C5a. Reprinted from Reference 15. (b) Regulation of feedback loop of alternative pathway on injured or stressed cells. This occurs through limited proteolytic cleavage of C3b to generate iC3b. The reaction is carried out by a serine protease factor I (FI) and membrane cofactor protein (MCP) or factor H (FH). Cofactor activity terminates the feedback loop because iC3b does not bind FB, thereby shutting down the amplification loop. Because APL are autoantibodies that trigger the classical pathway, defective regulation of C4b (a component of the classical pathway C3 convertase) by MCP is also likely to influence the severity of tissue injury and risk for preeclampsia. When tissues are damaged, a delicate balance must be established to allow for repair and recovery. If regulators such as MCP, FI, or FH are dysfunctional, excessive complement activation occurs. APS, antiphospholipid syndrome; SLE, systemic lupus erythematosus. Adapted from Reference 3 with permission.
Vascular endothelium is continuously exposed to complement activation products. C5b–9 can cause cell lysis, and sublytic amounts induce secretion of multimers of von Willebrand factor and stimulate prothrombinase and tissue factor activity, thereby activating platelets. The complex also upregulates the expression of leukocyte adhesion molecules ICAM-1 and P-selectin, leading to increased platelet localization and adhesion to generate a prothrombotic endothelial surface. The common microvascular lesions of HUS consist of vessel wall thickening with endothelial cell swelling. The widening of the subendothelial space and intraluminal platelet thrombi produce partial or complete obstruction of the vessel lumen, which in turn disrupts erythrocytes, causing hemolysis and presence of fragmented red cells (schistocytes) in the peripheral smear. Control of hypertension, which exacerbates turbulent flow, is an important therapeutic adjunct in aHUS (5, 14).
Epidemiology, Clinical Features, and Diagnosis
Onset of aHUS may be in the neonatal period, with 20% occurring under 1 year of age. Overall, it represents 5%–10% of HUS in children but the majority of HUS in adults. It is equally common in boys and girls in childhood, but there is a female preponderance in adults, partly because pregnancy is a predisposing factor for those with a genetic predisposition. Penetrance is ~50%, and thus the absence of familial history does not preclude the possibility of genetic transmission of a disease-causing variant. Several polymorphisms in complement regulatory genes have been shown to be independent or additional susceptibility factors (5, 10, 16). Thus, although a single gene mutation leading to haploinsufficiency is the most common genetic defect, in about 10%–20% of cases there is more than one polymorphic variant (17).
An infectious agent, commonly an upper respiratory tract infection, triggers onset in ~50% of patients. Pregnancy is a frequent trigger, with 20% of patients experiencing the disease prior to delivery and 80% during the postpartum period. Onset is usually sudden, although some patients may have a slowly progressive relapsing and remitting course. Young children may present with decreased appetite, vomiting, and drowsiness. Adults may have generalized fatigue, malaise, and often severe hypertension. More than 50% of patients require dialysis. Extrarenal manifestations occur in 20% of patients and may include central nervous system involvement and myocardial infarction. Irritability, drowsiness, seizures, diplopia, cortical blindness, hemiparesis, hemiplegia, stupor, and coma have been described (11, 16).
Clinical laboratory studies reveal the classic triad of anemia (hemoglobin <10 g/dl), thrombocytopenia (platelet count <150,000/mm3), and renal insufficiency (serum creatinine above normal for age and muscle mass). In addition, schistocytes on peripheral blood smear, an undetectable haptoglobin level, and a high serum lactate dehydrogenase (LDH) level (>460 U/L) confirm the intravascular origin of hemolysis. Patients suspected of having aHUS should be screened for Stx-producing E. coli. Children and adults should also undergo ADAMTS13 activity determination, and children may need to be screened for defective cobalamin metabolism. In adults, it is important to rule out chronic viral infections and autoimmune diseases (11, 16).
Serum C3 and C4 concentrations are not disease specific and are often minimally decreased or normal in aHUS. Serum concentration of FH, FI, autoantibodies to FH, and MCP expression on the surface of peripheral leukocytes should be evaluated. If reduced to ~50% of normal, the protein may be in a haploinsufficient state; however, if the protein is antigenically normal, it may be a variant that is produced but dysfunctional. Molecular genetic testing of FH, MCP, FI, C3, FB, FH/FHR1 hybrid allele, and thrombomodulin should be undertaken in all individuals in whom clinical and laboratory testing supports a diagnosis of aHUS. Identification of one mutation does not preclude the study of all genes. This becomes mandatory in planning for kidney transplantation (16).
In children, usually the age, clinical context, and symptoms on presentation help to differentiate between HUS and thrombotic thrombocytopenic purpura (TTP). However, the clinical picture in adults can be more confusing. Functional assessment of ADAMTS13 is often helpful in separating the two entities (11, 16, 18).
Differential Diagnosis
HUS can be distinguished from aHUS because the former is triggered by Stx-producing E. coli and manifests with a prodrome of diarrhea (often bloody), whereas the latter does not.
The diagnostic distinction between HUS and aHUS and disseminated intravascular coagulation (DIC) is usually based on history and laboratory studies. DIC is associated with intravascular activation and with consumption of all components of the coagulation cascade. In aHUS, patients have normal levels of coagulant components and little or no prolongation of protime or activated partial thromboplastin time (11, 16).
Atypical HUS and TTP share a common pathologic lesion (TMA) but have different clinical manifestations. In aHUS, the lesions are mainly localized in the kidney, whereas the lesions of TTP are more extensively distributed. Approximately 80% of TTP is triggered by deficient activity of ADAMTS13 secondary to autoantibodies (10, 11).
Treatment and Prognosis
Plasmatherapy has traditionally been the first line of treatment. It involves exchanging 1–1.5 plasma volumes (60–75 ml/kg) per session. Plasma exchange may remove a mutant protein or a trigger of endothelial dysfunction, and volume restitution with fresh frozen plasma may bring in the functional protein. Platelet count and serum LDH are the most sensitive markers for monitoring a response. Plasma treatment should be continued until the platelet count and LDH remain normal. About two-thirds of patients will temporarily remit. MCP, being a transmembrane protein, is not as likely to be affected by plasmatherapy. Persistence of hemolysis and/or lack of improvement of renal function after 3–5 days should lead one to consider switching the patient to eculizumab (9, 11).
Eculizumab is a recombinant, humanized, monoclonal immunoglobulin that binds to C5 and prevents its cleavage to C5a and C5b. The US Food and Drug Administration (FDA) originally approved eculizumab to treat paroxysmal nocturnal hemoglobinuria, and, in 2011, approved it to treat aHUS. It has been used in patients with aHUS and recurrence of aHUS after transplantation with encouraging results. Blockade of the complement terminal pathway increases the risk of Neisseria meningitidis infection, and “mening” vaccination is required before treatment with eculizumab. However, as no vaccine presently protects against the B serotype of the bacteria, patients and physicians have to be aware of symptoms that would necessitate urgent investigation and antibiotic therapy (11, 13).
Patients with FH mutation carry the worst prognosis, with 60%–70% dying or reaching end-stage renal disease (ESRD) within a year of disease onset. Because FH is mainly synthesized by the liver, a kidney transplant does not correct the problem. The prognosis in patients with FI mutations is similarly poor; 50%–60% die or reach ESRD within a year. Patients with MCP mutations have a better prognosis, with ~80% remaining dialysis independent. More than 50% of patients with C3 mutations reach ESRD. Correlations with FB and thrombomodulin mutations are not yet clear because of the limited number of cases. It is hoped that recent progress in genetic diagnosis and therapeutic options, including early aggressive and prolonged plasma therapy and the use of eculizumab, will improve the outcome (11).
Transplantation in aHUS
An aHUS patient who has reached ESRD is a candidate for kidney transplantation. However, the overall risk of recurrence of disease is >50%, and the risk of graft loss after disease recurrence is 80%–90%. The risk of aHUS recurrence is 70%–90% in patients with a FH mutation, 45%–80% with a FI mutation, and 40%–70% with a C3 mutation. The three reported cases of patients with FB mutation and one with thrombomodulin mutation who were transplanted lost their grafts to recurrence. The risk of recurrence in patients with MCP mutation is low because the graft brings in the non-mutated MCP. Genotyping for complement protein genes should be performed in patients being considered for renal transplantation. Transplantation should be preceded by plasma exchange or anti-C5 therapy. Plasma should be infused intraoperatively and continued post-transplantation. It should be subsequently tapered on a case-by-case basis (10, 11, 13).
Because FH, FI, FB, and C3 are synthesized in the liver, a combined liver-kidney transplant is an option. Initially, the outcomes were poor, but recently several successful transplantations have been reported. The decision of combined transplantation to cure aHUS requires an appreciation of risks and benefits on an individual basis (10, 11).
C3 GLOMERULOPATHIES
Over the last several years, the term C3 glomerulopathy has come to be widely used. It encompasses glomerulonephritides characterized by the presence of C3 deposits in the absence of immunoglobulin or components of the CP (namely C1q and C4). These are recognizable as distinct dense deposits on electron microscopy. In contrast no electron-dense deposits are seen in aHUS. MPGN type II or DDD is now viewed as a C3 glomerulopathy. In contrast to MPGN types I and III, which are driven by chronic antigenemia and circulating immune complexes, type II is caused by dysregulation of the AP (19).
Membranoproliferative Glomerulonephritis Type II (Dense Deposit Disease)
The defining abnormality in DDD is the highly characteristic appearance of the glomerular basement membrane due to the accumulation of electron-dense deposits in the lamina densa (central part of the glomerular basement membrane). These deposits may also be seen in the mesangium and the tubular basement membrane (20).
More than 80% of patients have a C3 nephritic factor (C3NeF), which is an IgG autoantibody directed to antigenic determinants on Bb after interactions with C3b. Its binding renders the C3 convertase resistant to inactivation by FH, thereby increasing its half-life from ~2 min to hours. The uncontrolled AP convertase results in consumption of C3 and elevation of C3 activation fragments. Also, antibodies against FH (which inhibit its ability to bind to C3b), gain-of-function mutations in C3 (which render the convertase more resistant to normal regulatory mechanisms), and a mutant FH (which impairs C3 regulatory capacity in plasma but preserves normal surface-recognition capability) have been reported in DDD (2, 20, 21).
Pathogenesis
There are no characteristic light microscopic findings in DDD. The classical membranoproliferative pattern is seen in only ~25% of biopsy specimens. Immunofluorescence microscopy, though, regularly shows deposits of C3 fragments along the glomerular, tubular, and Bowman’s capsule basement membranes. Presumably, this accumulation of C3 protein modulates the structure and integrity of the filtration barrier, leading to proteinuria and the nephrotic syndrome (20–22).
Epidemiology, clinical features, and diagnosis
The incidence is two to three cases per million. It is most often diagnosed in children between five and 15 years of age (20). Patients frequently present with hypertension, hematuria, proteinuria, and the nephrotic syndrome. An acquired partial lipodystrophy may also be seen (20). Persistent depression of C3 and normal C4 in the setting of a C3NeF establish a presumptive clinical diagnosis and distinguish DDD from most other types of glomerulonephritis. However, a definitive diagnosis requires a renal biopsy. Once a diagnosis of DDD is made, FH and FB should be measured. Further, a search for mutations in FH and C3 as well as for autoantibodies to FH should be considered (19–21, 23).
Treatment and prognosis
The treatment goals are to control blood pressure, reduce proteinuria, and correct electrolyte imbalances and other abnormalities resulting from declining renal function. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor blockers (ARBs) are commonly prescribed. Although immunosuppression with steroids has been advocated for >30 years for all types of MPGNs, there is no evidence for its benefit in type II (DDD). There are no disease-specific treatments for DDD, although plasma infusion and exchange to provide intact FH and remove C3NeF have been used. Successful treatment with eculizumab has recently been reported (20, 21, 24).
Approximately 50% of DDD patients progress to ESRD within 10 years of diagnosis. ESRD is more likely if the disease onset is in childhood. Unfortunately, ~50% of transplant recipients lose their allografts within five years, making recurrence of the disease and graft failure frequent issues (20, 21).
C3 Nephropathy
Servais et al. (25) described 19 patients with what they referred to as C3 glomerulonephritis. Thirteen patients had a membranoproliferative pattern; the others had mesangial deposits but without mesangial proliferation or capillary wall thickening. Interestingly, the incidence of C3 dysregulation (evidenced by low plasma C3 levels and presence of C3NeF) was higher in the MPGN group whereas the incidence of FH mutations was higher in the group without MPGN. Specifically, electron microscopy showed evidence of mesangial and epimembranous C3 deposits; however, there were no intramembranous deposits. Thus, C3 nephropathy appears distinct from DDD. This syndrome affects younger patients who often present with hematuria and proteinuria but less commonly have the nephrotic syndrome or progress to ESRD. The disease usually recurs in renal allografts (19, 24, 25).
Complement Factor H–Related Protein 5 Nephropathy
Complement factor H–related protein 5 nephropathy, a recently recognized form of C3 glomerulopathy, is endemic in Cyprus and accounts for a significant proportion of ESRD on the island (26).
Pathogenesis
In humans, there are a number of proteins closely related to complement FH. These include five complement FH-related proteins called FHR1–5. Their genes are arranged head-to-toe on chromosome 1 at q32. FHR5 is produced in the liver and circulates in the blood, although at an ~100-fold lower concentration than FH. It is known to inhibit C3 convertases and acts as a cofactor for cleavage of C3b. FHR5 was first identified as a component of the glomerular immune complexes, being colocalized with C3 and C5b–9 in glomerular deposits. The mutation associated with FHR5 nephropathy is detectable in the circulation of patients and has reduced affinity for complement-coated surfaces. Interestingly, the mutant protein also has enhanced cofactor activity. Therefore, it is unclear if the nephropathy results from a gain-of-function or a loss-of-function mutation. However, the normal circulating C3 levels in patients with the disease suggest that the complement dysregulation is at the glomerular surface and is not a systemic activation process. FHR5 nephropathy is inherited in an autosomal dominant pattern with >90% penetrance (2, 4, 26).
Epidemiology, clinical features, and diagnosis
The mutation is present in approximately 1 in 6,500 Cypriots, and >100 patients with this disease have been identified. The age at presentation ranges from 30 to 70. Patients with FHR5 nephropathy typically present with hematuria, both microscopic and macroscopic, and the disease is almost always preceded by a respiratory or other type of infection. These episodes are often associated with an acute deterioration in kidney function. Proteinuria is mild (<1 g/24 h) and is a late manifestation. Kidney biopsy shows a MPGN-type pattern, and electron microscopy reveals dense deposits in subendothelium and mesangium. There is positive glomerular staining for C3, C5, and C9 but no Ig deposition (26).
Treatment and prognosis
There is no treatment of proven efficacy. Disease progression correlates with infectious episodes, and tonsillectomy has proven beneficial in one case. Response to conventional chemotherapy has been inconsistent. However, plasma exchange carried out during an episode of acute renal failure had a good short-term outcome. It is not known if eculizumab is beneficial. In >80% of male patients, there is progressive stepwise deterioration in renal function leading to ESRD. Although outcomes after renal transplantation have been good, the disease has recurred following transplant from an unrelated donor, suggesting that a circulating abnormality is present (26).
C1q GLOMERULOPATHY
Jennette & Hipp in 1985 evaluated 800 renal biopsy specimens for immunostaining for C1q and found 36% to be positive. Among this group, 15 patients had mesangial C1q immune deposits but no clinical or serologic evidence of systemic lupus erythematosus (SLE). In addition, the dominant staining was for C1q, and corresponding electron-dense deposits by electron microscopy were identified. These patients were thus designated as having C1q nephropathy (27).
C1q is a complex complement protein with multiple binding sites for immune complexes containing IgG or IgM. In concert with C1r and C1s, it is responsible for activation of the CP. Glomerular deposition of C1q has been reported in cases of MPGN, SLE, membranous nephropathy, and focal segmental glomerulosclerosis (28).
Epidemiology, Clinical Features, and Diagnosis
C1q glomerulopathy affects young adults, predominantly women. Patients present with nephrotic-range proteinuria, microscopic or gross hematuria, hypertension, and renal insufficiency. Light microscopic findings are nonspecific, ranging from no glomerular abnormalities to mesangial proliferation or focal segmental glomerulosclerosis. The typical feature on immunofluorescence is extensive and dominant mesangial C1q deposition. By electron microscopy, mainly immune complex–type electron-dense deposits are appreciated (28).
Treatment and Prognosis
No randomized trials have evaluated the treatment of C1q nephropathy. Current therapy involves treatment of the predominant light microscopic lesion. Patients who have C1q nephropathy and focal segmental glomerulosclerosis may be more likely to progress to ESRD (28).
RELATED CONDITIONS WITH RENAL INVOLVEMENT AND THROMBOTIC MICROANGIOPATHIES
Antiphospholipid Syndrome
APS is an autoimmune multisystemic disorder characterized by recurrent arterial and venous thrombosis and/or pregnancy complications (miscarriage and fetal death, preeclampsia, placental insufficiency, and fetal growth restriction) in association with antiphospholipid (APL) antibodies, including anticardiolipin antibodies, anti-β2-glycoprotein I antibodies, and the lupus anticoagulant (29).
Clinical features
The kidney represents a major target organ of APS, and thrombosis may occur at any location within the renal vasculature. TMA is the most characteristic lesion of APS nephropathy and manifests with hypertension, proteinuria (mild to nephrotic range), and acute and/or chronic renal failure (30, 31). The pathologic changes resemble those of other conditions associated with endothelial injury, including HUS, TTP, preeclampsia, and renal transplant rejection.
Catastrophic antiphospholipid syndrome (CAPS) is an accelerated, severe form of APS with acute multiple-organ involvement and small-vessel thrombosis (in kidney, lung, brain, heart, skin, and/or gastrointestinal tract) resulting in multi-organ failure, most commonly affecting kidneys (31). Catastrophic episodes are typically preceded by a triggering event such as infection, trauma, or surgery, reminiscent of aHUS. Although <1%of patients with APS develop CAPS, the very high morbidity and mortality in this subgroup pose a serious clinical challenge (32).
Pathogenesis
APL antibodies induce a proinflammatory, proadhesive, and procoagulant phenotype in the vasculature (Figure 3). Complement activation may initiate and amplify the cellular features characteristic of APS: endothelial cell activation, monocyte tissue factor expression and platelet aggregation, and the histological hallmark of CAPS, TMA. C5a-C5aR interactions produce lesions such as those seen in TMA in mouse models, and blockade of terminal complement activation at C5 prevents complications of APS. In mouse models of surgically induced thrombus formation, complement activation plays an important role in the increased thrombosis and adhesion of leukocytes to endothelial cells caused by treatment with APL antibodies. Mice deficient in complement components C3, C5, C6, or C5a receptors are resistant to APL antibody–induced enhanced thrombophilia and endothelial cell activation (33, 34). The importance of complement regulation in APS-associated organ damage is underscored by a series of reports showing effectiveness of anti-C5 monoclonal antibody in the treatment and prevention of recurrent renal TMA and CAPS (35–37).
Pregnancy complications in APS are triggered by inflammation at the maternal-fetal interface, and complement activation plays an essential role. Mouse models of APL antibody–induced pregnancy loss and growth restriction show that C4, factor B, C3, C5, and C5aR are required for placental injury (38). These results indicate activation of the CP followed by amplification of C3b deposition via the AP’s amplification loop. Complement deposition is present in human placenta from patients with APS (39). The patients with APL antibodies, with or without clinical manifestation of APS, show elevated circulating levels of Bb and C3a fragments (Figure 1). This suggests dysregulation of the alternative pathway, which may decrease the threshold for autoantibody-mediated injury (40) (Figure 3).
Preeclampsia
Preeclampsia is a pregnancy-specific condition typically diagnosed with the onset of hypertension and proteinuria after 20 weeks of gestation. It is a major public health problem and complicates 4%– 6%of all first pregnancies worldwide, causing ~76,000 maternal deaths and ~500,000 neonatal deaths globally (41).
Clinical features
Disease manifestations range from mild blood pressure elevations to severe hypertension, the HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), and eclampsia (seizures). Although typically diagnosed after 20 weeks’ gestation, the syndrome begins much earlier in pregnancy with abnormal placental development. Uterine spiral arteries fail to remodel into dilated, flaccid vessels, which results in under perfusion of the intervillous space and placental hypoxia (42). Clinical manifestations of widespread endothelial cell dysfunction represent the maternal response to an excess of antiangiogenic factors (43) released by the underperfused placenta. These vasculopathic factors include soluble fms-like tyrosine kinase 1 (sFlt-1), a potent antagonist of vascular endothelial growth factor (VEGF) that sequesters circulating VEGF and placental growth factor and prevents their interaction with endogenous receptors, and soluble endoglin, an inhibitor of transforming growth factor (TGF)–β signaling (43).
Pathogenesis
We and others have suggested a relationship between activation of the complement system and angiogenic factor imbalance linked to placental dysfunction (44–46). In normal pregnancies, excessive complement activation is prevented by complement regulatory proteins that are highly expressed on trophoblast membranes (MCP, decay-accelerating factor [DAF (CD55)], and CD59) and circulating complement regulatory proteins (FH, FI, and C4 binding protein). In preeclampsia, complement activation products, notably the terminal complement complex (C5b–9), have been demonstrated on villous trophoblasts and are associated with injury (47). In mouse models, complement deposition and neutrophil infiltrates in placenta are associated with abnormal placental development, miscarriage, and glomerular fibrin deposition. In vitro studies show that C5a–C5aR interactions initiate the release of antiangiogenic factors from leukocytes (44). Inhibiting complement at the maternal-fetal interface rescues pregnancies, restores angiogenic balance, and prevents preeclampsia in mice, suggesting that complement triggers a feed-forward cycle of placental damage, antiangiogenic factor production, and maternal vascular damage (38) and supporting a role for complement inhibition therapies in the prevention or treatment of preeclampsia (Figure 3).
Preeclampsia has been classified as a TMA based in part on the renal pathologic findings of endothelial cell swelling, expansion of the subendothelial zone, duplication of the glomerular basement membrane, mesangial cell interposition, and glomerular or microvascular thrombi. Clinical features of preeclampsia syndromes, notably microangiopathic hemolytic anemia, thrombocytopenia, microthrombi, and glomerular endothelial cell injury with refractory hypertension, resemble aHUS and CAPS. That CAPS and aHUS may present in pregnancy suggests that pregnancy triggers complement activation, which is inadequately controlled in vulnerable hosts (48). Indeed, pregnancy precipitated microthrombotic angiopathy in the form of atypical HELLP syndrome with severe renal involvement in four patients with genetic defects in complement regulatory protein function (49, 50). The importance of AP activation in this syndrome is suggested by the findings of elevated levels of the complement activation fragments C3a and Bb in blood during the first 20 weeks of pregnancy in patients who develop preeclampsia later in pregnancy (45, 51). This is underscored by the recent finding that mutations in the same AP genes involved in aHUS are present in 5%–15% of preeclampsia cases (52) and in a higher percentage of HELLP syndrome cases. Mutations in MCP, FH, and FI have been associated with preeclampsia in patients with SLE and/or APL antibodies and with the HELLP syndrome in nonautoimmune patients (50).
Ischemia-Reperfusion Injury as Related to the Complement System
The transient interruption of the blood supply (ischemia) and its subsequent restoration (reperfusion) paradoxically exacerbate tissue damage by initiating a cascade of inflammatory events including the release of proinflammatory cytokines and chemokines, recruitment of leukocytes, and activation of the complement system. Ischemia-reperfusion injury remains a major problem in renal transplantation. Numerous studies have shown that renal ischemia-reperfusion injury profoundly impacts short and long-term graft survival and is associated with delayed graft function and increased clinical morbidity and mortality. Renal ischemia is accompanied by reduction of membrane-bound complement regulators on proximal tubular epithelial cells, rendering these cells more susceptible to complement activation following reperfusion (53). The result is C3 and C5b–9 deposition along the tubular basement membrane. Binding of FH to tubular epithelial cells limits interstitial complement activation and attenuates ischemic injury (54).
One of the greatest obstacles to effective treatment of ischemic renal failure is our inability to recognize the disease early in its clinical course. Serum markers such as blood urea nitrogen and creatinine may not be elevated until >24 h after the insult. Complement inhibition (for example, by preventing C5 activation) may have a role in mitigating inflammation and cell death and thereby protecting against renal injury if given early enough (55–59).
SUMMARY POINTS.
APS, aHUS, and preeclampsia are TMAs. Mutations leading to a gain of AP function and complement-fixing autoantibodies to phospholipids expressed on endothelial cells predispose to aHUS and APS, respectively. In preeclampsia, the pathologic role of the complement system is not as clear, but mutations in complement regulators have been identified.
Complement regulation is essential for the integrity of kidney cells and particularly the glomerular basement membrane. A delicate balance exists between activation and inhibition of the complement system. Improperly controlled complement activation is detrimental. Inhibitors in plasma synthesized by the liver and those expressed by host cells prevent excessive complement activation leading to tissue injury.
Dysfunction of these complement regulatory proteins teaches us the importance of maintaining homeostasis with the expression levels nature uses as a “set point.” In the example of aHUS, haploinsufficiency leads to an assault on the integrity of the endothelium that is mediated by both the complement and coagulation systems. The incomplete penetrance of the disease points out that other genetic and environmental factors play an important role in pathogenesis.
Most human glomerulonephritides are related to autoimmune diseases featuring immune complexes. However, dysfunction of the alternative complement pathway is key to the pathogenesis of DDD, with an autoantibody to the AP convertase (a C3 nephritic factor) being the most common. Gain-of-function mutations inC3, complete FH deficiency due to mutations in the FH gene, and an anti-FH antibody have also been recently identified.
Heterozygous mutations in complement regulatory proteins that predispose to aHUS have also been reported in C3 nephropathy. It is unclear why some individuals develop aHUS and some have MPGN while others remain free of any apparent disease.
The role of FHR proteins in the development of disease is poorly understood. FH autoantibodies are associated with homozygous deletion of FHR1 and 3, and mutations in FHR5 lead to a nephropathy.
The progress in the understanding of the various complement-mediated diseases has opened the way to new therapies. Eculizumab represents the most promising new approach, which may prevent evolution to ESRD and allow successful kidney transplantation. It is approved to treat aHUS. A future challenge will be to define the best choice of a complement inhibitor for each individual according to the identified complementopathy.
Glossary
- MPGN
membranoproliferative-glomerulonephritides
- DDD
dense deposit disease
- TMA
thrombotic microangiopathy
- aHUS
atypical hemolytic uremic syndrome
- APS
antiphospholipid syndrome
- AP
alternative pathway
- CP
classical pathway
- MAC
membrane attack complex
- MCP
membrane cofactor protein
- Stx
shiga toxin
- TTP
thrombotic thrombocytopenic purpura
- APL
antiphospholipid
Footnotes
DISCLOSURE STATEMENT
J.A. and J.S. have consulted for Alexion Pharmaceuticals, Inc. J.S. has consulted for Quidel Corporation.
Contributor Information
Anuja Java, Email: AJAVA@dom.wustl.edu.
John Atkinson, Email: jatkinso@dom.wustl.edu.
Jane Salmon, Email: SalmonJ@HSS.EDU.
LITERATURE CITED
- 1.Markiewski MM, Nilsson B, Ekdahl KN, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin. Exp. Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory states: the examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv. Immunol. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. Recent, comprehensive, and authoritative reviews on aHUS.
- 4.Abarrategui-Garrido C, Martinez-Barricarte R, Lopez-Trascasa M, et al. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations ofCFHR1 associated with atypical hemolytic uremic syndrome. Blood. 2009;114:4261–4271. doi: 10.1182/blood-2009-05-223834. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson JP, Goodship TH. Complement factor H and the hemolytic uremic syndrome. J. Exp. Med. 2007;204:1245–1248. doi: 10.1084/jem.20070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dragon-Durey MA, Blanc C, Garnier A, et al. Anti-factor H autoantibody-associated hemolytic uremic syndrome: review of literature of the autoimmune form of HUS. Semin. Thromb. Hemost. 2010;36:633–640. doi: 10.1055/s-0030-1262885. [DOI] [PubMed] [Google Scholar]
- 7.Pechtl IC, Kavanagh D, McIntosh N, et al. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J. Biol. Chem. 2011;286:11082–11090. doi: 10.1074/jbc.M110.211839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zipfel PF, Skerka C, Caprioli J, et al. Complement factor H and hemolytic uremic syndrome. Int. Immunopharmacol. 2001;1:461–468. doi: 10.1016/s1567-5769(00)00047-3. [DOI] [PubMed] [Google Scholar]
- 9. Kavanagh D, Goodship TH. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematol. Am. Soc. Hematol. Educ. Program. 2011;2011:15–20. doi: 10.1182/asheducation-2011.1.15. Recent, comprehensive, and authoritative reviews on aHUS.
- 10. Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu. Rev. Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. Recent, comprehensive, and authoritative reviews on aHUS.
- 11. Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J. Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. Recent, comprehensive, and authoritative reviews on aHUS.
- 12.Delvaeye M, Noris M, DeVriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkins KS, Bobrowski AE, Lane JC, et al. Clinical grand rounds: atypical hemolytic uremic syndrome. Am. J. Nephrol. 2012;35:394–400. doi: 10.1159/000337954. [DOI] [PubMed] [Google Scholar]
- 14.Richards A, Kavanagh D. Pathogenesis of thrombotic microangiopathy: insights from animal models. Nephron. Exp. Nephrol. 2009;113:e97–e103. doi: 10.1159/000235253. [DOI] [PubMed] [Google Scholar]
- 15.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLOS Med. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. Recent, comprehensive, and authoritative reviews on aHUS.
- 17.Rodriguez de Cordoba S. aHUS: a disorder with many risk factors. Blood. 2010;115:158–160. doi: 10.1182/blood-2009-11-252627. [DOI] [PubMed] [Google Scholar]
- 18.Abarrategui-Garrido C, Melgosa M, Pena-Carrion A, et al. Mutations in proteins of the alternative pathway of complement and the pathogenesis of atypical hemolytic uremic syndrome. Am. J. Kidney Dis. 2008;52:171–180. doi: 10.1053/j.ajkd.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Couser WG. Basic and translational concepts of immune-mediated glomerular diseases. J. Am. Soc. Nephrol. 2012;23:381–399. doi: 10.1681/ASN.2011030304. [DOI] [PubMed] [Google Scholar]
- 20. Smith RJ, Harris CL, Pickering MC. Dense deposit disease. Mol. Immunol. 2011;48:1604–1610. doi: 10.1016/j.molimm.2011.04.005. Excellent reviews on the pathogenesis of complement-mediated MPGN. Supplement describes case reports and renal biopsy findings that give further insight into the disease.
- 21. Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N. Engl. J. Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. Excellent reviews on the pathogenesis of complement-mediated MPGN. Supplement describes case reports and renal biopsy findings that give further insight into the disease.
- 22.Benz K, Amann K. Pathological aspects of membranoproliferative glomerulonephritis (MPGN) and haemolytic uraemic syndrome (HUS)/thrombocytic thrombopenic purpura (TTP) Thromb. Haemost. 2009;101:265–270. [PubMed] [Google Scholar]
- 23.Licht C, Fremeaux-Bacchi V. Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis. Thromb. Haemost. 2009;101:271–278. [PubMed] [Google Scholar]
- 24.Bomback AS, Smith RJ, Barile GR, et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012;7:748–756. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Servais A, Fremeaux-Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J. Med. Genet. 2007;44:193–199. doi: 10.1136/jmg.2006.045328. C3 Glomerulopathy and aHUS are distinct entities that share common genetic risk factors.
- 26.Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennette JC, Hipp CG. C1q nephropathy: a distinct pathologic entity usually causing nephrotic syndrome. Am. J. Kidney Dis. 1985;6:103–110. doi: 10.1016/s0272-6386(85)80150-5. [DOI] [PubMed] [Google Scholar]
- 28. Vizjak A, Ferluga D, Rozic M, et al. Pathology, clinical presentations, and outcomes of C1q nephropathy. J. Am. Soc. Nephrol. 2008;19:2237–2244. doi: 10.1681/ASN.2007080929. One of the few reviews that gives a comprehensive description of the rare C1q glomerulopathy.
- 29.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 30.Tektonidou MG, Sotsiou F, Nakopoulou L, et al. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 2004;50:2569–2579. doi: 10.1002/art.20433. [DOI] [PubMed] [Google Scholar]
- 31.Cervera R, Tektonidou MG, Espinosa G, et al. Task Force on Catastrophic Antiphospholipid Syndrome (APS) and Non-criteria APS Manifestations (I): catastrophic APS, APS nephropathy and heart valve lesions. Lupus. 2011;20:165–173. doi: 10.1177/0961203310395051. [DOI] [PubMed] [Google Scholar]
- 32.Cervera R, Asherson RA, Font J. Catastrophic antiphospholipid syndrome. Rheum. Dis. Clin. North Am. 2006;32:575–590. doi: 10.1016/j.rdc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Pierangeli SS, Girardi G, Vega-Ostertag M, et al. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52:2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 34. Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Semin. Thromb. Hemost. 2012;38:305–321. doi: 10.1055/s-0032-1311827. Complement activation by antiphospholipid antibodies has many similarities to antinuclear antibodies and complement in SLE.
- 35.Shapira I, Andrade D, Allen SL, et al. Induction of durable remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719–2723. doi: 10.1002/art.34440. [DOI] [PubMed] [Google Scholar]
- 36.Hadaya K, Ferrari-Lacraz S, Fumeaux D, et al. Eculizumab in acute recurrence of thrombotic microangiopathy after renal transplantation. Am. J. Transplant. 2011;11:2523–2527. doi: 10.1111/j.1600-6143.2011.03696.x. [DOI] [PubMed] [Google Scholar]
- 37.Lonze BE, Singer AL, Montgomery RA. Eculizumab and renal transplantation in a patient with CAPS. N. Engl. J. Med. 2010;362:1744–1745. doi: 10.1056/NEJMc0910965. [DOI] [PubMed] [Google Scholar]
- 38.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shamonki JM, Salmon JE, Hyjek E, et al. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am. J. Obstet. Gynecol. 2007;196:167, e1–e5. doi: 10.1016/j.ajog.2006.10.879. Complement activation by antiphospholipid antibodies has many similarities to antinuclear antibodies and complement in SLE.
- 40. Breen KA, Seed P, Parmar K, et al. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb. Haemost. 2012;107:423–429. doi: 10.1160/TH11-08-0554. Complement activation by antiphospholipid antibodies has many similarities to antinuclear antibodies and complement in SLE.
- 41.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia—a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod. Sci. 2007;14:508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 43.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 44.Girardi G, Yarilin D, Thurman JM, et al. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am. J. Obstet. Gynecol. 2008;198:385, e1–e9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch AM, Salmon JE. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta. 2010;31:561–567. doi: 10.1016/j.placenta.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rampersad R, Barton A, Sadovsky Y, et al. The C5b–9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fakhouri F, Jablonski M, Lepercq J, et al. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008;112:4542–4545. doi: 10.1182/blood-2008-03-144691. [DOI] [PubMed] [Google Scholar]
- 50.Fang CJ, Richards A, Liszewski MK, et al. Advances in understanding of pathogenesis of aHUS and HELLP. Br. J. Haematol. 2008;143:336–348. doi: 10.1111/j.1365-2141.2008.07324.x. [DOI] [PubMed] [Google Scholar]
- 51.Lynch AM, Gibbs RS, Murphy JR, et al. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet. Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurman JM, Ljubanovic D, Royer PA, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J. Clin. Invest. 2006;116:357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renner B, Ferreira VP, Cortes C, et al. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int. 2011;80:165–173. doi: 10.1038/ki.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Vries B, Matthijsen RA, Wolfs TG, et al. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- 56.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin. Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Stein JH, Osgood RW, Barnes JL, et al. The role of complement in the pathogenesis of postischemic acute renal failure. Miner. Electrolyte Metab. 1985;11:256–261. [PubMed] [Google Scholar]
- 58.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin. Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, Farrar CA, Abe K, et al. Predominant role for C5b–9 in renal ischemia/reperfusion injury. J. Clin. Invest. 2000;105:1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]