Abstract
Age-related macular degeneration (AMD) is a leading cause of blindness in aged individuals. Recent advances have highlighted the essential role of immune processes in the development, progression and treatment of AMD. In this Review we discuss recent discoveries related to the immunological aspects of AMD pathogenesis. We outline the diverse immune cell types, inflammatory activators and pathways that are involved. Finally, we discuss the future of inflammation-directed therapeutics to treat AMD in the growing aged population.
Optimal vision requires a high-functioning central retina, in particular the photoreceptor-dense macula (FIG. 1), which is responsible for the fine visual acuity that is required for tasks such as reading, facial recognition and driving. Age-related macular degeneration (AMD) refers to the progressive degeneration of the macula that commonly occurs in people over 60 years of age. More than 30 million individuals worldwide suffer from visual impairment as a result of AMD, which is estimated to account for more than US$300 billion in annual economic costs1.
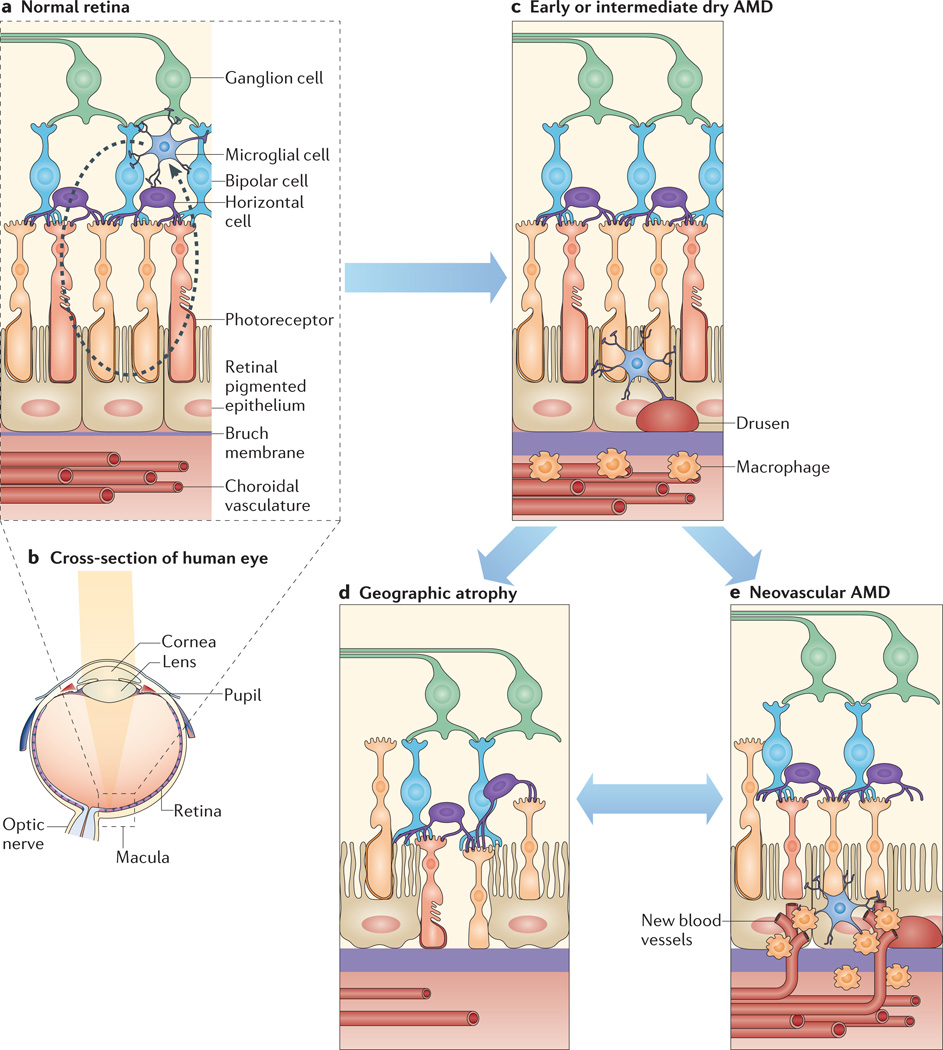
Figure 1. Retinal anatomy in health and disease.
The retinal anatomy is composed of several layers (part a); and a cross-section of the human eye (part b) shows focusing of light into the macular area, which is a dense collection of retinal photoreceptors. Normal retinal architecture (part a) is comprised of (from anterior to posterior) a ganglion cell layer, resident retinal microglia, bipolar cells, horizontal cells, the photoreceptor layer, the retinal pigmented epithelium (RPE), the Bruch membrane and a choroidal vascular network. Normal microglia migrate into and out of the subretinal space (as shown by the dashed arrow). Early or intermediate dry age-related macular degeneration (AMD) (part c) is associated with the accumulation of subretinal drusen and microglia and of choroidal macrophages and a thickened Bruch membrane. Geographic atrophy (part d) is the advanced form of dry AMD, which is characterized by confluent regions of RPE and photoreceptor degeneration as well as constriction of choroidal blood vessels. Neovascular AMD (part e) is characterized by the invasion of abnormal, leaky choroidal blood vessels and accompanying macrophages into the retina through breaks in the Bruch membrane, which leads to photoreceptor degeneration.
The study of AMD has, in many respects, been at the forefront of research into complex diseases. The discovery of the Tyr402His polymorphism of complement factor H (CFH) that confers increased statistical risk of developing AMD was the first of its kind for a complex disease2–5. Furthermore, multiple biological therapies that target vascular endothelial growth factor A (VEGFA) in neovascular AMD (also known as exudative or ‘wet’ AMD) have recently revolutionized the clinical management of this disease. However, despite advances in the understanding, diagnosis and treatment of AMD in the past decade, disease prevalence increases with the ageing human population. The next decade will see a steady increase in the incidence and economic cost of AMD1.
Despite the fact that AMD is presumed to have a multifaceted aetiology, immune dysfunction is a recurring theme in its pathogenesis. In this Review, we discuss the fundamental concepts and current ideas of AMD pathogenesis, with a particular focus on several recent advances in the immune aspects of the disease. We begin by describing the normal structure of the retina and the characteristic features of AMD. Next, we focus on the major immunological processes that have important roles in AMD development and we discuss the inflammatory component of neovascular AMD. We then present an integrated model of the immune modulation of AMD pathogenesis. Finally, we summarize the possibility of using immune-based therapeutics to prevent and treat AMD development. The aim of this Review is to present an up-to-date discussion of recent immunological findings in AMD. Consequently, we have condensed the discussion of the complement pathway, which is the most thoroughly studied immune pathway in AMD; for a more comprehensive summary of complement biology in AMD, the reader is referred to other reviews6–9.
Structure of the retina
The sensory retina is organized into alternating layers of neuronal extensions and cell nuclei. The conversion of light into chemical signals occurs in the outer segments of the cone and rod photoreceptors in the posterior of the neural retina (FIG. 1). Encoded visual information is integrated through retinal networks and ultimately travels to the optic nerve. Blood is supplied to the neural retina by retinal blood vessels that originate from the central retinal artery. Transport across retinal blood vessels is controlled by endothelial tight junctions, which constitute the inner blood–retinal barrier.
Next to the photoreceptor layer is a highly organized monolayer of retinal pigmented epithelium (RPE), which has several roles in supporting the metabolically active photoreceptor layer. These include recycling biochemical by-products of photoreception through the phagocytosis of photoreceptor outer segments, supplying trophic factors such as VEGFA10 and maintaining the integrity of the outer blood–retinal barrier through tight junctions. Thus, the integrity of the RPE is essential for homeostasis in the retina. Posterior to the RPE is the Bruch membrane — a thick, elasto-collagenous extracellular matrix. Together, the RPE and the Bruch membrane form the outer blood–retinal barrier, which prevents the entrance of macromolecules and immune cells from the underlying choroid into the photoreceptor layer. Located behind the Bruch membrane, the choroid contains a dense network of blood vessels (choriocapillaris), which supplies oxygen and nutrients to the RPE, outer retina and optic nerve. The choroid endothelium is fenestrated, which enables the transport of molecules to the metabolically demanding RPE11. The choroid also contains tissue-resident melanocytes, fibroblasts, macrophages, mast cells and dendritic cells.
A brief description of AMD
The first clinical feature of early ‘dry’ AMD is the presence of drusen (BOX 1), which are extracellular deposits below the RPE that comprise lipid- and protein-rich debris12. Small, isolated drusen do not affect visual function but their expansion and coalescence are hallmarks of AMD progression. The two main advanced forms of AMD are: neovascular AMD, which is characterized by the invasion of abnormal choroidal (or occasionally retinal) blood vessels and fluid leakage into the retina; and geographic atrophy (also known as end-stage dry AMD or atrophic AMD).
Box 1 | The pro-inflammatory components of drusen.
Drusen are extracellular deposits that accumulate between the retinal pigmented epithelium (RPE) and the Bruch membrane, or sometimes between the RPE and the photoreceptors117. The RPE is a major source of drusen components, and extracellular and serum-derived factors are also highly abundant12. RPE monocultures can be made to produce ‘drusen-like’ deposits and to release factors that are abundant in drusen, such as vitronectin, clusterin, serum amyloid P, complement proteins and apolipoprotein E (APOE)118. Local drusen biogenesis probably occurs as a result of a failure of lysosome- and autophagy-mediated digestion of cellular components119. The accumulation of waste material is eventually extruded in exosomes and might function as nucleating sites for the deposition of other extracellular factors51. Data on the genetic influence of drusen formation have not conclusively determined whether complement dysregulation has a role in this process22,120,121. Furthermore, drusen also contain components of dendritic cell processes, which supports a role for local immunity in their biogenesis122.
Drusen consist of aggregated intracellular, extracellular and secreted proteins, and lipids and cellular components. Proteomic, histological and biochemical analyses show that the major components of human drusen include albumin, APOE, complement factors and related proteins (including complement components C1q, C3, C5 and C5b-9, and vitronectin), immunogloblulins and amyloid-β123–125. Interestingly, the protein content of drusen from the eyes of individuals who do not have age-related macular degeneration (AMD) is similar to that from the eyes of individuals with AMD123. The proteins in drusen commonly contain carboxyethylpyrrole modifications123. Drusen also contain double-stranded RNAs60.
Drusen extracts can induce inflammasome activation58, and individual components of drusen can experimentally induce profound cellular effects: for example, C1q induces inflammasome activation58 and amyloid-β induces retinal degeneration43. However, because drusen are solid, insoluble deposits, it is difficult to infer the in situ activity of drusen constituents — which may have abnormal conformation and low biological availability — from cell culture treatments of soluble factors.
Thus, the relationship between drusen formation and AMD is entirely correlative — increased numbers and size of drusen correlate with increased risk of developing advanced AMD. Whether drusen are a cause or a symptom of AMD or some combination of the two is unknown. Although AMD almost never occurs in the absence of prior drusen formation, the transition from intermediate AMD to geographic atrophy is associated with drusen regression126. Indeed, drusen formation could be an adaptive process to prevent the widespread dispersal of activated complement and other toxic constituents in the retina. One might speculate that the spontaneous release of drusen material might be an immediate cause of RPE loss in geographic atrophy.
Neovascular AMD is the leading cause of blindness among the elderly in industrialized nations, affecting more than 1 million individuals over the age of 40 in the United States alone13. Although the fundamental aetiology of neovascular AMD remains unclear, therapies that target VEGFA, which is a potent stimulator of angiogenesis and vasopermeability, have been successful in substantially improving central vision in approximately 30% of patients and in arresting vision loss in 94% of patients (compared with 62% of sham-treated patients)14,15.
Geographic atrophy is characterized by large, confluent regions of atrophied RPE in the macular area. There are no approved therapies for geographic atrophy, which is mainly due to the lack of suitable molecular targets. Unlike VEGFA in neovascular AMD, a single crucial factor has not been identified as promoting RPE degeneration in geographic atrophy. As geographic atrophy is considered to be the default end point of dry AMD16, further investigation into the initiating factors that promote the early development of this disease should be useful in the search for new therapies.
Importantly, these advanced stages of AMD are not mutually exclusive — neovascular lesions are often present in the periphery of eyes with geographic atrophy17. In addition, long-term treatment of neovascular AMD with VEGFA-targeted therapy is associated with the development of geographic atrophy18, possibly as a result of atrophy of the choriocapillaris19 and loss of the neurotrophic activity of VEGFA in the retina10. Genetic polymorphisms that are associated with AMD — including those in the genes encoding CFH20–23 and high-temperature requirement A serine peptidase 1 (HTRA1)24,25 — confer similar statistical risk of developing both forms of AMD, which indicates that there are many shared underlying pathological mechanisms. The effect of polymorphisms in the HTRA1 or age-related maculopathy susceptibility 2 (ARMS2) gene locus on gene activity or expression, as well as the functional consequences for AMD, are unclear.
AMD is associated with marked changes in normal retinal anatomy. Early AMD is associated with thickening of the Bruch membrane, deposition of drusen, RPE hypertrophy and pigment extrusion26. Advanced AMD is associated with photoreceptor degeneration following either invasion of choroidal blood vessels through the Bruch membrane into the retina in neovascular AMD or degeneration of the choroidal vasculature and RPE cells in geographic atrophy27.
Immune surveillance in maintaining retinal health
Maintenance of the blood–retinal barrier confers a degree of immune privilege to the mammalian eye. This immune privilege is characterized by tolerance of foreign antigens owing to the expression of endogenous immunosuppressive factors and the absence of functional intraocular lymphatics28–30. However, the introduction of specific foreign or endogenous inflammatory signals in the retina evokes innate immune responses.
The resident inflammatory cells of the retina are the microglia, which are similar to tissue macrophages and central nervous system microglia. The retinal microglia normally reside in proximity to retinal blood vessels in the inner layers of the neural retina. The number of resident retinal microglial cells increases with age in mice31. Aged microglia have a branched morphology, which is indicative of a ‘resting’ phenotype, and they show decreased responsiveness to tissue injury31.
Evidence indicates that the subretinal migration of microglia is necessary to eliminate visual by-products and to maintain vision31,32. The impairment of microglial migration into or out of the subretinal space promotes the death of photoreceptor cells33,34. In AMD, microglia accumulate in the subretinal space35; this is probably both a symptom of inflammatory damage and a beneficial response to injury, and the impairment of this accumulation in the subretinal space exacerbates retinal degeneration. However, infiltration of microglia and macrophages to sites of retinal injury can also promote the growth of neovascular lesions32,36,37. Macrophages, together with RPE cells, are a major source of pro-angiogenic factors such as VEGFA38. Macrophages and dendritic cells are not normally present in the retina but reside in the underlying choroid. In cases of breakdown of the blood–retinal barrier, these cells are recruited from the underlying choroid or from the systemic circulation into the retina where they modulate disease.
The use of aged animals in models of impaired immune cell trafficking, such as those with CC-chemokine ligand 2 (CCL2), CC-chemokine receptor 2 (CCR2) and/or CX3C-chemokine receptor 1 (CX3CR1) deficiency, results in animals that have AMD-like features, including changes to the Bruch membrane, RPE swelling and retinal thinning39–41. Aged mice that are deficient in CCL2 or its receptor CCR2 develop spontaneous hypopigmented subretinal lesions that are visible using fundus photography33,39,42, as well as developing photoreceptor and RPE damage33,39; they also infrequently develop spontaneous choroidal neovascular lesions that are similar to those in neovascular AMD. Subretinal lesions of CCL2- or CCR2-deficient mice are comprised of bloated outer segment-laden macrophages42 (which are similar to foam cells in atherosclerosis), and CCL2- or CCR2-deficient macrophages in the periphery show impaired chemotaxis and phagocytosis33. Importantly, CCL2–CCR2-dependent immune cell function prevents photoreceptor apoptosis following amyloid-β treatment43, which adds further support to the idea that immune cell trafficking to sites of local tissue damage is an essential component of retinal health. Similarly, aged mice that are deficient in CX3CR1 show subretinal microglial accumulation and retinal degeneration, which have both been attributed to impaired trafficking of immune cells in the retina40. As in the CCR2- and CCL2-deficient mouse models that accumulate subretinal macrophages, CX3CR1-deficient mice acquire large bloated subretinal microglia44. Genetic studies also support a role for impaired repair of tissue damage in AMD, as polymorphisms in CX3CR1 that cause macrophage migratory defects38,43 are associated with AMD45.
Whether increased or impaired CCL2–CCR2 signalling is a cause or a symptom of AMD pathogenesis remains to be shown. CCL2 levels are increased in the RPE and the choroid of aged mice46, as well as in the serum of elderly individuals47; it is possible that this is related to the increased burden of tissue damage in the aged retina. The presence of increased systemic chemokine levels in patients with AMD indicates that systemic inflammation manifests itself locally in the retina. In addition, CCL2 expression is upregulated early in laser-induced choroidal neovascularization48, and inhibition of CCR2 decreases neovascular growth49. At first glance, these observations of increased CCL2–CCR2 signalling in AMD are not consistent with the idea that deficiencies in CCL2–CCR2-mediated immune cell trafficking contribute to AMD. However, the distinction between local and systemic chemokine signalling is important; local signalling probably has a protective role in the immune-mediated repair of damaged retinal tissue in early AMD, whereas systemic signalling contributes to the recruitment of pro-angiogenic immune cells to sites of neovascularization in late-stage AMD. It is also important to consider the distinction between immune responses to acute events and those to long-term chronic para-inflammation or to persistent low-level inflammation (for example, involving complement activation and cytokine expression) owing to sustained tissue stress in the ageing retina35. This distinction probably accounts for some of the discrepancies that have been observed between age-related animal models of retinal degeneration, which rarely involve choroidal neovascularization, and acute injury models (such as light-induced retinal damage), in which retinal degeneration occurs within days. CCL2–CCR2 signalling might modulate AMD development in different directions depending on the disease context; this would be in line with current models of immune cell involvement in AMD development that suggest that immune cells have dual, opposing roles in preventing and promoting disease.
Dysregulated immune activation in AMD
Although a functioning retinal immune system is crucial for visual homeostasis, a substantial amount of evidence also indicates that the overactivation of specific immune processes is important in AMD pathogenesis. Over a lifetime, the retina and RPE are in contact with a large number of innate immune activators that could potentially contribute to vision loss in AMD. Data from a decade of research strongly indicate that improper immune activation is involved in inducing and advancing AMD pathology. Among these immune activation pathways, the complement pathway is the most well-established and widely accepted as contributing to AMD. Genetic evidence from genome-wide association studies (GWASs) and rare variant analyses50 strongly indicates that the alternative pathway of complement activation is overactive in AMD (BOX 2). This evidence has been discussed in several excellent reviews6–9 and, therefore, these data are only summarized in this Review in relation to the general idea that excessive inflammation contributes to AMD pathogenesis.
Box 2 | Genetics of complement in AMD.
The risk of developing age-related macular degeneration (AMD) is influenced not only by the common complement factor H (CFH) polymorphic variant 402His (the minor allele) but also by multiple other variants (common, uncommon and rare)6–9,50. The complement factor H-like protein (CFHL1) is generated by alternative splicing of the CFH gene127. The complement factor H-related proteins 1–5 (CFHR1–5) arose by gene duplication events and are encoded just downstream of CFH on the long arm of chromosome 1. Their function is poorly understood. A simple concept is that CFHR1 competes with CFH for binding to substrates and thereby reduces the effectiveness of CFH. This hypothesis is consistent with a deletion of CFHR1 and CFHR3 being protective against AMD128.
These studies of CFH and related proteins, combined with the identification of risk and protective variants in alternative pathway complement activators and other regulators, provide powerful evidence implicating overactivation of the alternative complement pathway in AMD. A finding that is consistent with this idea is that the protective variants result in less alternative pathway activity, whereas risk variants result in more activity129. In summary, common and rare variants in multiple members of a pro-inflammatory pathway of innate immunity are associated with the same disease; those that decrease the function of the pathway are protective and those that increase the function create risk.
Complement
In 2005, GWASs showed that approximately 50% of the heritability of AMD could be accounted for by a single nucleotide polymorphism (SNP) in an exon encoding CFH2–5. However, it should be noted that the complement system had been implicated in AMD pathogenesis before this discovery12,51,52. The risk variant of CFH (402His) does not regulate the alternative pathway of complement activation as efficiently as the main allele (Tyr402) does. The 402His variant binds with lower affinity to numerous constituents of the damaged retina6,53–56; therefore, the inhibitory effect of CFH on the complement pathway is decreased, which results in a greater degree of complement activation following retinal injury.
Thus, one hypothesis for the aetiopathogenesis of AMD is that in individuals carrying a ‘complement hyperinflammatory phenotype’ the complement pathway overreacts to cellular damage and debris in the retina6–9, 160. In addition to common alleles of CFH that confer greater risk of, or protection from, AMD as well as rare variants of CFH with high penetrance50, similar findings have been shown for other members of the alternative complement pathway, including activating components C3 and factor B (gain-of-function mutations associated with AMD) and the regulator factor I (loss-of-function mutations associated with AMD). Carrying the 402His CFH variant or these other risk factors enables the alternative pathway to generate undesirable quantities of complement components C3b and C3a, as well as of the downstream effectors C5a and C5b-C9.
Inflammasome activation
The inflammasome is a protein complex that is activated by foreign or endogenous danger signals, which culminate in caspase 1-mediated maturation of the cytokines interleukin-1β (IL-1β) and IL-18 and, ultimately, in pyroptosis or apoptosis. The inflammasome complex is comprised of three protein constituents: a NOD-like receptor family member (the best studied of which is NLRP3 (NOD-, LRR- and pyrin domain-containing 3)), the adaptor protein ASC and the protease caspase 1.
Inflammasome activation has recently been implicated in AMD pathogenesis. One group found that carboxyethylpyrrole-adducted proteins, which accumulate in the retina with age57, prime the NLRP3 inflammasome58. They also found that drusen extracts that had been isolated from AMD donor tissue, as well as the complement component C1q (a component of human drusen), activate the NLRP3 inflammasome in macrophages.
In a separate study, we identified another endogenous activator of the inflammasome that is present in the RPE of patients with geographic atrophy59. Repetitive element-derived Alu RNA transcripts, which are non-canonical targets of DICER1-mediated enzymatic degradation, accumulate in human geographic atrophy following the loss of DICER1 expression (which might result from oxidative stress in the RPE)60. These transcripts function as both priming and activating signals to stimulate NLRP3 inflammasome signalling. Importantly, we found evidence of inflammasome activation in the eyes of patients with AMD; this included increased levels of NLRP3, IL-18 and activated caspase 1 in the RPE. Two recent studies have confirmed evidence of inflammasome activation in the eyes of patients with AMD61 (C. Chan, personal communication).
An important inflammasome effector is IL-1β. Human drusen extracts induce NLRP3-dependent IL-1β secretion from lipopolysaccharide-primed peripheral blood mononuclear cells58. In our study we did not detect significant release of mature IL-1β from RPE cells in response to Alu RNA59, although inflammasome-mediated IL-1β release from RPE cells has been more recently found in response to 4-hydroxynonenal62, amyloid-β63, A2E (N-retinyl-N-retinylidene ethanolamine) (D. Shima, personal communication) and lysosomal destabilization61, which indicates that RPE cells are capable of inflammasome-mediated IL-1β release. In our study we also found increased levels of IL1B mRNA in the RPE of donor eyes from individuals with geographic atrophy59, which indicates that IL-1β might be involved in the development of AMD.
Taken together, these studies show that the retina can respond to diverse ‘danger signals’ that are abundant in AMD through the activation of the NLRP3 inflammasome. However, the consequences of inflammasome activation in AMD pathogenesis are poorly understood. Both IL-1β and IL-18 signal through the adaptor protein myeloid differentiation primary-response protein 88 (MYD88) to induce inflammatory and apoptotic effects. However, although we have found potent cytotoxic effects of IL-18 and IL-1β on the RPE59, in another study, beneficial effects of inflammasome-mediated IL-18 release were reported to occur through the inhibition of neovascularization in an acute laser-induced injury model of neovascular AMD58. These contrasting findings might imply that a single factor (IL-18) or pathway (NLRP3 inflammasome activation) can be simultaneously anti-angiogenic and destructive to the RPE. This model mirrors our findings that Toll-like receptor 3 (TLR3) activation is beneficial in terms of decreasing choroidal neovascularization64, but that it also promotes RPE degeneration65 (see below). In contrast to the reported anti-angiogenic effects of IL-18 on laser-induced choroidal neovascularization58, IL-1β promotes neovascularization66.
In summary, the extent, the underlying mechanisms and the cumulative effect of inflammasome activation and the secretion of IL-1β and IL-18 in human AMD remain uncertain. Therefore, we caution against pursuing IL-1β- or IL-18-based therapeutic strategies for neovascular AMD.
TLR signalling
Activation of pathogen- or danger-associated molecular pattern-mediated signalling in the retina often results in rapid and vigorous inflammatory responses. Retinal cells express multiple TLR family members, and the RPE in particular expresses most TLRs67. The contribution of TLR signalling to AMD is still under intense investigation. Initial reports of the association of polymorphisms in TLR3 (REF. 68) and TLR4 (REF. 69) with AMD susceptibility were controversial70. TLR activation in the retina by synthetic or endogenous ligands can have positive or negative effects in AMD pathogenesis and this probably depends on context- and ligand-specific factors. Activation of TLR3 signalling by administration of double-stranded RNAs (dsRNAs) of 21 nucleotides or longer inhibits the development of choroidal neovascularization after acute laser-induced injury64. However, the benefits of TLR3 signalling in terms of preventing neovascular lesions are counteracted by RPE cytotoxicity65. Genetic ablation of TLR3 prevents disease development in a mouse model of retinal injury that is induced by impairment of all-trans-retinal clearance71, which indicates that endogenous TLR3 agonists promote retinal degeneration. Our investigations identified strong immunoreactivity with a dsRNA-specific antibody in human drusen, which supports the idea that the TLR3 signalling pathway is potentially involved in the progression of geographic atrophy60. The findings of TLR3-induced RPE degeneration probably limit the use of nonspecific TLR3 agonist-based therapies for neovascular AMD.
Unlike experimental TLR3 activation that suppresses the development of choroidal neovascularization, TLR2 ligands (carboxyethylpyrrole-adducted proteins and Chlamydia pneumoniae antigen) promote experimental choroidal neovascularization72,73. We speculate that TLR activation broadly contributes to AMD pathologies; however, the full range of agonists and outcomes is unknown.
Adaptive immunity
Although AMD research has mostly focused on innate immune processes, a growing body of evidence supports the idea that there is some contribution of adaptive immunity to AMD. The mammalian eye lacks functional intraocular lymphatic vessels, and B cells or T cells have not been widely reported to be present in proximity to either neovascular or geographic atrophy lesions. As B cells and T cells do not have a directly defined role in mediating angiogenesis or tissue damage in human AMD, any contribution of adaptive immunity to AMD development probably involves retinal antigen presentation and indirect autoantibody-mediated retinal degeneration.
Increased titres of autoantibodies recognizing components that are enriched in the retina have been measured in the serum of patients with AMD74–76 and have led to the hypothesis that AMD might be an autoimmune disorder. Indeed, the Ccl2−/−Cx3cr1−/− mouse model of retinal degeneration involves retina-specific autoantibodies41, which indicates that there might be at least a circumstantial relationship between AMD and adaptive immune processes.
Immunization of mice with carboxyethylpyrrole-modified albumin induces retinal degeneration that has features similar to those of AMD, including drusen-like deposits, sub-RPE complement deposition and photoreceptor dysfunction57, and that requires B cells and T cells. Importantly, the authors of this study observed macrophage infiltration into retinal lesions, which indicates that antibody-mediated complement activation can induce a ‘primary’ retinal injury to which macrophages respond and potentially further modulate the disease. The mechanisms involved in antigen presentation and antibody-mediated RPE degeneration (presumably by activation of the classical complement cascade) have not been resolved; they may provide important and interesting insights into AMD pathogenesis. The lack of functional lymphatic vessels and immune cells in the normal mammalian retina indicates that antigen presentation involves either a direct breakdown of the blood–retinal barrier and could be mediated by local dendritic cells, or the leakage of retinal antigens into the circulation. Importantly, some retinal antigens such as docosahexaenoic acid-derived carboxyethylpyrrole protein modifications are not strictly unique to the retina. Thus, the antigen need not be derived from the retina for a damaging antibody-mediated immune response to be mounted against the retina. However, whether lymphocytes have a causal role in AMD pathogenesis is uncertain.
Other immune stimulators
In addition to the pathways highlighted above, several other factors that are linked to immune activation in AMD merit discussion. One of the most important of these factors is A2E, which is a principal component of retinal lipofuscin. Accumulation of A2E owing to mutations in the retinal-specific ATP-binding cassette A4 transporter (ABCA4) causes a form of Stargardt’s disease, which is an early-onset, inherited disease that results in retinal degeneration and that has many aetiological similarities to AMD. A2E has multiple damaging effects on RPE cells, including complement activation77, inflammasome activation (D. Shima, personal communication; J.A. and N. Kerur unpublished observations), cytokine expression78,79 and RPE degeneration80,81. ABCA4-deficient mice also have increased complement activation82, and subretinal injection of A2E exacerbates experimental neovascularization83.
Other important immune activators in the retina that might have a role in AMD are lipid oxidation byproducts, which are formed from the oxidation of polyunsaturated fatty acids. The retina is prone to lipid peroxidation because it has an abundance of lipid material and a high metabolic demand84. The major by-products of these reactions are malondialdehyde (MDA), carboxyethylpyrrole and 4-hydroxynonenal (4-HNE) protein modifications. Lipid oxidation products have potent degenerative and inflammatory effects: both MDA and 4-HNE induce lysosomal dysregulation85 and lipofuscin generation86, which prevent the photoreceptor maintenance function of the RPE; MDA-modified proteins induce VEGFA expression in RPE cells and pro-inflammatory gene expression in macrophages87, which is prevented by the binding of CFH to MDA; 4-HNE induces inflammasome activation62 and RPE apoptosis88; and carboxyethylpyrrole (as discussed above) directly activates TLR2 signalling72 and might function as an antigen for retina-specific autoantibody production57.
Amyloid-β is also abundant in drusen89; it promotes RPE and photoreceptor damage90 as well as retinal inflammation63,70,91. Other environmental factors, such as excessive light exposure, hypercholesterolaemia and smoking, damage the RPE and the retina by inducing pro-inflammatory effects that are associated with AMD (reviewed in REF. 92).
In summary, clear evidence supports a role for a range of pro-inflammatory factors that are abundant in the ageing retina as driving forces in AMD pathogenesis. We discuss below the special case of neovascular AMD, in which the breakdown of the blood–retinal barrier allows systemic immune cells unusual access to the retina. In this way, immune regulation of neovascular AMD represents an ‘outside-in’ phenomenon, in contrast to early dry AMD and geographic atrophy, in which immune activation is generally self-contained and self-inflicted.
Immune cell recruitment in neovascular AMD
Clear evidence from animal models supports dual roles for resident and invading macrophages/microglia in preventing and promoting neovascular AMD. These paradoxical effects have been attributed to two different functions of innate immune cells. First, as discussed above, the surveillance and phagocytosis of damaged cellular components is a necessary function of resident microglia for the maintenance of retinal health. Second, invading macrophages provide potent pro-angiogenic signals that exacerbate choroidal vessel invasion and pathogenesis. Importantly, whereas ample evidence supports the involvement of immune cells in both human neovascular AMD93–96 and experimental choroidal neovascularization36,37,97, the evidence for immune cell involvement in early and intermediate dry AMD and geographic atrophy is less impressive. Histological evidence showing relatively few immune cells in the atrophic area of geographic atrophy98 indicates that local immune cells may not have a substantial role in this form of AMD. However, this does not rule out immune-mediated tissue damage being involved in geographic atrophy, as emerging evidence highlights RPE-specific inflammasome-mediated degeneration as a driving force for RPE atrophy59,61,62 (D. Shima and C. Chen, personal communication). In this respect, the role of innate immunity in neovascular AMD compared with geographic atrophy pathogenesis probably diverges at the local tissue level, and might represent both a consequence and a cause of the ultimate pathogenic outcome.
The development of neovascular AMD is accompanied by the recruitment of inflammatory cells to the site of vascularization, the consequence of which is the expression of numerous cytokines and chemokines that induce angiogenesis. Human choroidal neovascular lesions contain inflammatory cell infiltrates93–96,99, and experimental depletion of macrophages decreases choroidal neovascularization in laser-injured mice36,37. Subsequent analyses have also shown that macrophages can have antiangiogenic properties in the context of experimental choroidal neovascularization; indeed, intraocular injection of macrophages before injury decreases laser-induced choroidal neovascularization100. Furthermore, mice that are deficient in the anti-inflammatory cytokine IL-10 have both an increased accumulation of macrophages in neovascular lesions and decreased choroidal neovascularization100. Indeed, our initial finding of spontaneous choroidal neovascularization in Ccl2- or Ccr2-deficient mice39 can be generally interpreted as a systemic impairment of macrophage chemotaxis enhancing the development of choroidal neovascularization.
The contradictory contributions of macrophages that have been observed in choroidal neovascularization might be explained by differential macrophage polarization with respect to M1 macrophages (pro-inflammatory) and M2 macrophages (pro-angiogenic). So far, the role of macrophage polarization in AMD development has not been well characterized. One report identified an increased expression of the M1 marker CCL22 compared with an M2 marker, CXC-chemokine ligand 11 (CXCL11), in macrophages from individuals with AMD compared with those from the eyes of unaffected individuals101, which indicates that inflammatory macrophage polarization might contribute to or be symptomatic of AMD development. Furthermore, choroidal M1 (inducible nitric oxide synthase (iNOS)+) macrophages have been measured in proximity to subclinical neovascular AMD lesions98. Whether M1 macrophages contribute to pathological angiogenesis and whether increased numbers of M2 polarized macrophages are also present in choroidal or retinal neovascular membranes are not yet known but further studies will probably provide insights into the role of macrophage polarization in neovascular AMD. In mice, IL-10 was found to be a crucial factor driving M2 polarization, and inhibition of IL-10 decreased angiogenesis100. Although the role of IL-10 remains unclear (two studies show that IL-10 suppresses choroidal angiogenesis in other inflammatory contexts102,103), a follow-up study found that both IL-10 levels and M2 polarization are increased in the retinal macrophage population of aged mice104. Macrophage polarization as a directing factor in AMD is an intriguing possibility that merits further study.
In addition to macrophages, other analyses have found invading neutrophils to make variable contributions to promoting choroidal neovascularization in animal models. With respect to the number of invading cells, neutrophils constitute a large fraction of the inflammatory cell infiltrate in experimental neovascular lesions of the retina, although their contribution to laser-induced choroidal neovascularization is minimal when macrophage infiltration is impaired97. Other studies have found that antibody-mediated depletion of neutrophils prevents laser-induced choroidal neovascularization97,105, and that they produce VEGF105 as well as matrix metalloproteinase 9 (MMP9), which promote the loss of RPE barrier integrity106. However, neutrophils are not a major constituent of human AMD lesions107. The role of neutrophils in animal models of AMD probably represents an acute injury response and may not reflect the mechanisms that occur in human AMD, which is a progressive disease that typically spontaneously develops over decades.
In addition to the roles of CCL2, CCR2 and CX3CR1 in immune cell migration and retinal neovascularization, several other cytokines and chemokines have potentially important roles in the development of neovascular AMD (BOX 3).
Box 3 | Cytokine and chemokine signalling in neovascular AMD.
In addition to the main molecules and pathways involved in neovascular age-related macular degeneration (AMD), several important secreted factors might also affect disease processes and could be possible therapeutic targets.
Eotaxins
Eotaxins (CC-chemokine ligand 11 (CCL11), CCL24 and CCL26) and their receptor CC-chemokine receptor 3 (CCR3) are highly expressed in neovascular lesions before the retinal invasion by new blood vessels130. CCL11 levels are increased in retinal specimens from aged humans and in choroidal endothelial cells from patients with AMD131, and both CCL11 and CCL24 levels are increased in the circulation of patients with AMD132,133. CCR3 antagonism prevents laser-induced choroidal neovascularization, and CCR3 expression may be a useful biomarker of subclinical AMD130,134. Studies of eotaxin signalling might provide therapeutic insights that enable neovascular AMD to be diagnosed and treated at early stages.
IL-6
Increased interleukin-6 (IL-6) levels are found in ocular fluids of patients with neovascular AMD and they predict AMD progression135. The pro-angiogenic effects of IL-6 are well-described in the context of tumour angiogenesis and involve the upregulation of vascular endothelial growth factor A136. Genetic ablation of IL-6 or its receptor decreases laser-induced choroidal neovascularization137. IL-6 signalling also promotes degeneration of the retinal pigmented epithelium (RPE) following lipopolysaccharide stimulation138. Thus, IL-6 might have dual pathogenic functions by promoting both angiogenesis and RPE degeneration in advanced AMD.
CXCL10
CXC-chemokine ligand 10 (CXCL10), which is a potent anti-angiogenic chemokine, is highly expressed in the retina of patients with AMD132 and laser-injured mice139. Choroidal endothelial cells express CXC-chemokine receptor 3 (CXCR3) — the CXCL10 receptor — and genetic ablation of CXCR3 exacerbates laser-induced choroidal neovascularization in mice139. Thus, CXCL10 might be an important endogenous negative regulator of neovascular AMD.
TNF
Tumour necrosis factor (TNF) is abundant in human choroidal neovascular membranes95, and genome-wide association studies have identified two polymorphisms in the TNF receptor TNFRSF10 (which encodes the protein TAILR1) that are associated with neovascular AMD140,141. Inhibition of TNF by antibodies142,143 or recombinant receptor fragments144 decreases experimental choroidal neovascularization in laser-injured rodents. However, TNF-targeted therapy was not well tolerated in human trials of neovascular AMD145.
CXCL12
CXCL12 is a potent chemoattractant. It drives the recruitment of endothelial progenitor cells and haematopoietic stem cells to regions of neovascularization146; these cells constitute as much as 50% of the neovascular lesions in laser injury models. In laser-induced choroidal neovascularization, CXCL12 expression is increased, and inhibition of its receptor CXCR4 decreases angiogenesis146,147. Conversely, expression of CXCL12 is variable in neovascular human AMD (two of ten148 and zero of four149 neovascular lesions stained positive for CXCL12 expression). Thus, the contribution of CXCL12 signalling in human AMD has not been firmly established.
An integrated model of immunity in AMD
The metabolic and immunological demands of the macular retina are unlike any other tissue in the body. Recent findings have brought diverse immunological aspects of AMD pathogenesis into sharper focus. We propose below a generalized model of AMD progression on the basis of these observations (FIG. 2).
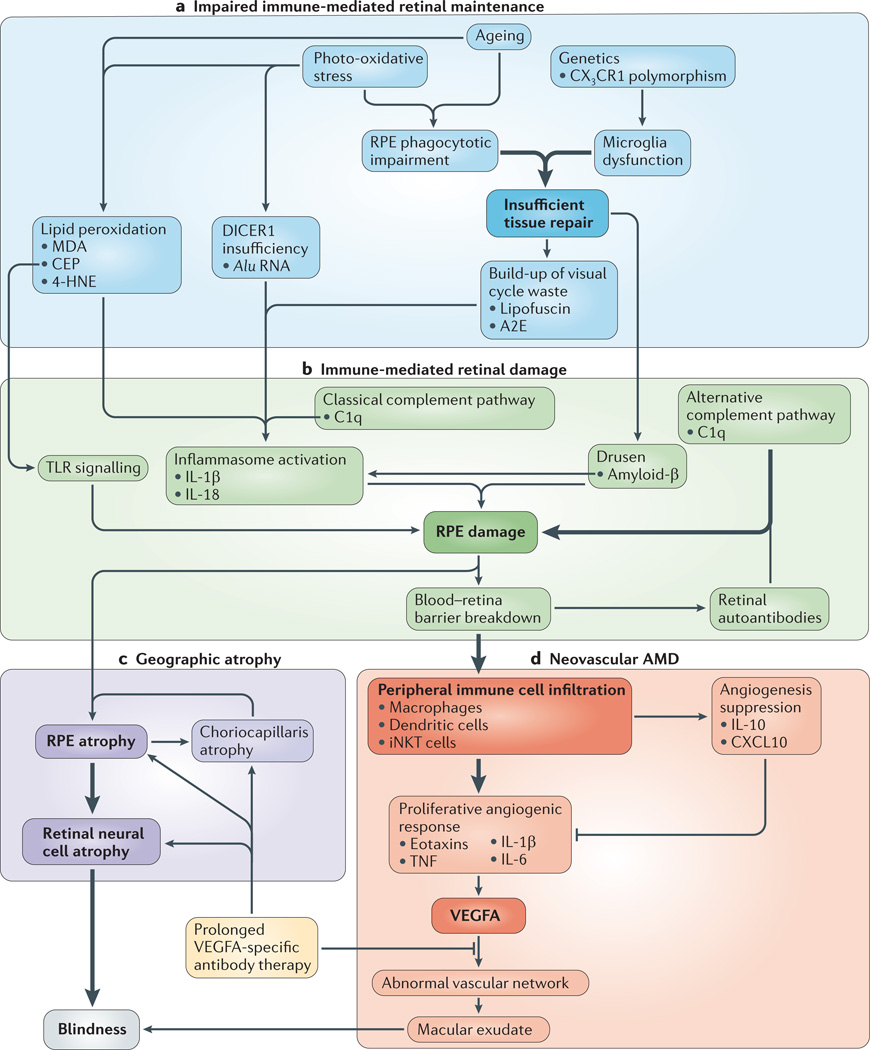
Figure 2. An integrated model of AMD pathogenesis.
The cell types, environmental factors and immune pathways that contribute to age-related macular degeneration (AMD) are categorized as either impaired immune-mediated retinal maintenance (part a) or immune-mediated retinal damage (part b). The combination of these factors leads to either geographic atrophy (part c) or neovascular AMD (part d). The most important pathways are highlighted in bold. The earliest known steps in AMD pathogenesis (part a) reflect a reduced capacity to manage the metabolic demands of the retina. The convergence of genetic, environmental and metabolic factors leads to a state in which the accumulation of toxic elements (such as lipid peroxidation by-products, lipofuscin and Alu RNAs) ultimately tips the balance towards immune activation. Once it is replete with unwanted waste material (part b), the retina is the target of inappropriate immune activation. The toxic contents of the retina induce inappropriate activation of diverse immune pathways, including classical and alternative complement pathways, the inflammasome and Toll-like receptor (TLR) signalling. Ultimately, the sustained activation of these pro-inflammatory and damaging pathways leads to advanced AMD. In the case of geographic atrophy (part c), sustained damage to the retinal pigmented epithelium (RPE) leads to the development of degeneration of the RPE, the choriocapillaris and, finally, neural retinal cells. In neovascular AMD (part d), breakdown of the blood–retinal barrier results in immune cell trafficking into the retina, which drives vascular endothelial growth factor A (VEGFA)-dependent neovascularization, causing blindness. 4-HNE, 4-hydroxynonenal; A2E, N-retinyl-N-retinylidene ethanolamine; C1q, complement component 1q; CEP, carboxyethylpyrrole; CX3CR1, CX3C-chemokine receptor 1; CXCL10, CXC-chemokine ligand 10; IL, interleukin; iNKT, invariant natural killer T; MDA, malondialdehyde; TNF, tumour necrosis factor.
We propose that the fundamental abnormality in AMD is a dysfunction of RPE cells; this can result from multiple types of ‘insult’ but the final common pathway is damage to an important metabolically active cell type that maintains homeostasis in the neuroretina. The importance of individual insults, especially in individual patients, in mediating RPE injury is unclear. Many of these insults, which can be both local and systemic, arise as a result of inappropriate immune activation, such as dysregulated complement activation, production of inflammasome activators and autoantibodies that are specific for retinal antigens.
Under normal conditions, the RPE layer continuously maintains immune suppression through both tight junction-mediated barrier integrity and anti-inflammatory cytokine production. Retinal microglia provide additional immune surveillance by clearing cell debris. Many different hazards can create initial foci of tissue damage and, importantly, also render the retina unable to removing dying, dead or stressed retinal cells. This concept is similar to that of other progressive age-related diseases (BOX 4). The inability to sufficiently cope with increased tissue damage leads to drusen accumulation, and the drusen function as concentrated immunostimulatory punctae. As drusen accumulate, the development of AMD depends on the type of immune response. In the neovascular form of AMD, breakdown of the blood–retinal barrier provides circulating immune cells with unusual access to a highly immunogenic environment, which results in macrophage recruitment and activation. When these macrophages encounter an avascular, metabolically demanding tissue, they initiate a VEGFA-dependent neovascular response. The complex mixture of pro-angiogenic cytokines that they produce results in a leaky, abnormal vascular network that is associated with fluid leakage and, if left untreated, in the eventual development of fibrosis108. In the case of geographic atrophy, circulating immune cells are denied access to the degenerating retina. Rather than a proliferative vascular response, the inability to repair the increasing tissue damage leaves large, confluent areas of degenerated RPE and photoreceptors. The degenerating area expands as diseased RPE cells damage neighbouring cells by releasing toxic inflammatory stimulators and cytokines.
Box 4 | Age-dependent degenerative diseases.
Most tissues require methods to clear dead cells and intracellular and extracellular waste. Necrosis and apoptosis are two examples of these methods, which can be triggered by ‘wear and tear’ (so-called normal ageing phenomena), ischaemia–reperfusion injury, trauma, foreign agents (microorganisms) and toxins. The complement system is one of the first (if not the first) responder to such events and is activated by necrosis and apoptosis150. It is involved in degenerative arthritis151 and atherosclerosis152,153, including complement activation by lipid particles, and there is also a role for the complement pathway in the development of abdominal aortic aneurysm. In this regard, stressed and damaged retinal pigmented epithelium (RPE) could be seen as analogous to cartilage loss predisposing an individual to osteoarthritis151. Relative to atherosclerosis, drusen accumulation could be viewed as comparable to extracellular lipid deposits154. Indeed, a recent collaborative genome-wide association study identified age-related macular degeneration (AMD) risk variants in loci that are also involved in atherosclerosis and lipid metabolism155. Although the nature of the debris, the generation of the inflammatory response and the clearance mechanisms are likely to have unique features in specialized tissues, the innate immune response and the degradative and clearance processes will have overlapping features92. Thus, the four great diseases of ageing — Alzheimer’s disease, atherosclerosis, AMD and degenerative arthritis — can all be viewed in this perspective: they feature a generally unknown age-dependent degenerative and destructive process, as well as an accumulation of self debris: amyloid (in Alzheimer’s disease), lipids (in atherosclerosis), drusen (in AMD) or glycosaminoglycans (in degenerative arthritis). The debris is not adequately eliminated and the dying cells (neurons, RPE cells or chondrocytes) are not easily replaceable. The innate immune response to this damage is insufficient. Initially, the innate immune response is presumably beneficial and promotes longevity, but at later stages in the disease process it is probably detrimental.
In this model, AMD arises from both immune hypoactivity in the case of inadequate tissue repair and immune hyperactivity in the case of complement-mediated tissue damage. One emerging theme from such a model is that pan-immunosuppression could potentially reduce late-stage AMD; however, by preventing endogenous immune-mediated tissue repair processes, new foci of dysfunction and disease might be created in the remaining functional retina. Another prediction from this model is that the most effective immunotherapies must be highly target- and context-specific to inhibit specific pathogenic factors but to only minimally affect tissue repair mechanisms. As the search for new and improved AMD therapeutics continues, we must not turn a blind eye to the Janus-faced nature of ocular immunity.
Immune-based therapy for AMD
Any discussion about the future potential for AMD therapeutics must be placed in the context of the current standard of care. The management of neovascular AMD has undergone a transformation over the past 10 years as a result of the astonishing success of VEGFA-targeted therapies (<10% of patients treated with VEGF-targeted agents exhibit considerable vision loss14). The ‘off-label’ use of bevacizumab (Avastin; Genentech/Roche) — a humanized monoclonal antibody targeting VEGFA — which improves or maintains vision in the majority of patients and is available in doses that cost in the order of tens of dollars18,109, has somewhat diminished future prospects for the development and commercialization of new drugs to treat neovascular AMD. Nonetheless, in cases in which VEGFA-based treatment is insufficient or ineffective, or in the case of geographic atrophy, combination therapies or immune-based therapies that are directed towards alternative targets are being explored.
As our understanding of the role of immunity in AMD has advanced, we have gained an appreciation of the nuanced and conflicting roles of the immune system as a protector, an instigator and a driver of retinal degeneration. The complex cell type-, pathological context-, temporal- and pathway-specific aspects of immune regulation of retinal health demand targeted medicine. It is perhaps a lack of understanding or appreciation of these facts that has led to the numerous failures of immune-based therapies; for example, steroidal anti-inflammatory drugs have only achieved very modest success as an adjunct to VEGFA-targeted treatment110,111.
Complement-based diagnostics and therapeutics
Despite the knowledge that complement activation is a genetic risk factor for AMD development, so far the clinical predictive or therapeutic value of these findings has not been realized. Several clinical trials using complement inhibition-based treatment of AMD have produced disappointing primary outcome data that show minimal improvement in visual acuity or reduction in disease progression (TABLE 1). It is also worth noting that, despite millions of dollars having been spent and nearly a decade of intense research, the predictive value of genetic screening for AMD is limited by the simple reality that the relatively inexpensive procedure of taking a patient’s history and a routine eye examination provide more relevant information with respect to current and potential future clinical AMD development. Data about whether polymorphisms in complement genes and other AMD risk factor genes can be used to predict the response to VEGFA-targeted therapies are conflicting112,113; however, given the lack of alternative treatments that are available, these findings are unlikely to change the management of neovascular AMD. Similarly, genetic models fail to predict progression to geographic atrophy114,115 but without an approved therapy for geographic atrophy, once again, there is no immediate clinical use for such genetic information.
Table 1.
Current immune-based clinical trials for AMD
| Compound | Target | Structure | Indications | Trial status | Trial number |
|---|---|---|---|---|---|
| POT-4 (Alcon) | C3 | Cyclic peptide inhibitor | Neovascular AMD | Phase I has been completed | NCT00473928 |
| 93% of patients had no improvement in visual acuity (S. Bakri, personal communication) | |||||
| Eculizumab (Alexion) | C5 | Monoclonal antibody | Dry AMD (presence of drusen) and geographic atrophy | Phase II is ongoing | NCT00935883 |
| There was no improvement in mean visual acuity or area of geographic atrophy in the first 6 months of the trial (P. Rosenfeld, personal communication) | |||||
| LFG316 (Novartis) | C5 | Monoclonal antibody | Geographic atrophy Advanced neovascular AMD |
Phase II is ongoing (Phase I data are unpublished) |
NCT00709527 NCT00950638 |
| ARC1905 (Ophthotech) | C5 | Aptamer | Neovascular AMD Geographic atrophy |
Phase I has been completed (Phase I data are unpublished) Phase I is ongoing |
NCT00709527 NCT00950638 |
| FCFD4514S (Genentech) | Factor D | Monoclonal antibody (Fab fragment) | Geographic atrophy | Phase II is ongoing (Phase I data are unpublished) | NCT01602120 |
| Sonepcizumab (Lpath) | Sphingosine-1-phosphate | Monoclonal antibody | Neovascular AMD, ‘subresponders’ to VEGFA-targeted therapy | Phase II is ongoing (Phase I data are unpublished) | NCT01414153 |
| Glatiramer acetate (Teva) | Unknown anti-inflammatory target | Small peptide | Early and intermediate dry AMD | Phase II and Phase III are inactive | NCT00466076 |
| A small cohort (N = 8) showed reduction in drusen size156 | |||||
| RN6G (Pfizer) | Amyloid-β | Monoclonal antibody | Geographic atrophy | Phase II is ongoing (Phase I data are unpublished) | NCT01577381 |
| Daclizumab (Hoffman-La Roche) | IL-2 receptor subunit-α | Monoclonal antibody | Neovascular AMD, co-administered with VEGFA-targeted therapy | Phase II has been completed | NCT00304954 |
| It reduced the frequency of VEGFA-specific injections in four patients157 | |||||
| Bromfenac (ISTA) | Cyclooxygenase | NSAID | Neovascular AMD, co-administered with VEGFA-targeted therapy | Phase II has been completed | NCT00805233 |
| It reduced the number of VEGFA-specific injections in 16 patients but had no benefit for visual acuity158 | |||||
| Infliximab (Janssen) | TNF | Monoclonal antibody | Neovascular AMD, co-administered with VEGFA-targeted therapy | Phase II has been completed | NCT00304954 |
| It reduced the number of VEGFA-specific injections in three patients157 | |||||
| Adalimumab (Abbott) | TNF | Monoclonal antibody | Neovascular AMD, co-administered with VEGFA-targeted therapy | Phase II has been completed (data are unpublished) | NCT01136252 |
| Triamcinolone acetonide (Bristol-Myers Squibb) | Broad anti-inflammatory target | Corticosteroid | Neovascular AMD, co-administered with VEGFA-targeted therapy | Phase III has been completed | NCT00370370 |
| It reduced the number of VEGFA-specific injections but had no benefit for visual acuity159 | |||||
| Fluocinolone acetonide (Alimera Sciences) | Broad anti-inflammatory target | Corticosteroid | Geographic atrophy | Phase II (Phase I data are unpublished) | NCT00695318 |
AMD, age-related macular degeneration; C, complement component; IL-2, interleukin-2; NSAID, non-steroidal anti-inflammatory drug; TNF, tumour necrosis factor; VEGFA, vascular endothelial growth factor A.
Despite considerable interest, no complement-based therapeutic has yet progressed to a Phase III clinical trial. The early results from complement-based strategies have dampened enthusiasm for using these as targets and have highlighted our lack of basic understanding about the mechanisms by which complement factors influence AMD development. Indeed, one mechanism that is apparently involved in complement-mediated AMD development is the upregulation of VEGFA48,116 — this only reminds us that VEGFA-based therapies already do an excellent job in the majority of patients.
Other immune-based clinical trials
So far, immune-based therapies have exclusively evaluated anti-inflammatory agents. The successes of these therapies have been measured in terms of a reduction in the number of VEGFA-targeted injections that are needed for neovascular AMD treatment; it is possible that a positive outcome results from a better response to trauma from repeated intraocular injections rather than prevention of a fundamental disease process. Although reducing the number of injections is an important goal, no trial has reported improvements in visual acuity as a result of anti-inflammatory monotherapy or adjunctive therapy.
Ocular immunity is involved both in maintaining visual homeostasis and in driving AMD pathogenesis, so we support the development of new targeted therapies as the most promising approach to treat this disease; however, this awaits a more thorough investigation of the molecular mediators of AMD. Recent studies have identified new potential targets (such as IL-1β, IL-18 and inflammasome components) that merit further evaluation for potential intervention. The search for new targets will also benefit from a re-evaluation of current animal models of advanced AMD. Laser photocoagulation- induced neovascularization effectively models the VEGFA-dependent angiogenic component but probably does not mimic the inflammatory component of human neovascular AMD and has a timescale that differs by several orders of magnitude. Similarly, no animal model has yet been developed that mimics all of the changes that are associated with advanced geographic atrophy.
We postulate that the future of AMD-based therapies lies in pathomechanism-driven discovery and caution against demanding that putative targets be validated in GWASs. VEGFA is a prime example of a validated target for AMD that arose via mechanistic biology and which would not withstand the scrutiny of statistical genetics. Immunology-based insights are likely to have an important role in the future of clinical AMD management once a more thorough understanding of ‘friend’ and ‘foe’ in the context of AMD pathogenesis is realized.
Acknowledgements
J.A. is supported by US National Institutes of Health (NIH) grants (R01EY018836, R01EY020672 and R01EY022238), the Doris Duke Charitable Foundation, USA, the Ellison Medical Foundation, USA, the Burroughs Wellcome Fund, USA, the Reeves Foundation, USA, a Dr. E. Vernon Smith and Eloise C. Smith Endowment and a Research to Prevent Blindness Unrestricted Grant, USA. J.P.A. is supported by NIH grants (AI041592, AR007279, AR0483335, GM099111 and HL112303), the Edward N. and Della L. Thome Memorial Foundation, USA, and Alexion Pharmaceuticals, USA. B.D.G. is suported by the National Center for Advancing Translational Sciences, USA, grants UL1TR000117 and UL1TR000117. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- Retina
A highly organized and specialized neural network where light is converted into electrical impulses. Diseases of the retina such as age-related macular degeneration are leading causes of blindness in the developed world.
- Macula
A specialized region of the retina densely populated with cone photoreceptors, which is responsible for fine visual acuity. Degeneration of the macular photoreceptors following either atrophy of the retinal pigmented epithelium (geographic atrophy) or fluid leakage from choroidal neovessels (neovascular age-related macular degeneration (AMD)) is the cause of vision loss in AMD.
- Complement factor H
(CFH). A negative regulator of alternative complement pathway activation. Single nucleotide polymorphisms in CFH that reduce its inhibitory potential are responsible for a substantial proportion of the genetic risk for the development of age-related macular degeneration.
- Neovascular AMD
(also known as exudative or ‘wet’ age-related macular degeneration). Characterized by degeneration of the macula following fluid leakage from choroidal neovessels that have invaded the retina. The use of vascular endothelial growth factor A-targeted therapies has revolutionized the management of this disease, which accounts for the majority of blindness that results from AMD.
- Photoreceptors
Specialized neurons that are responsible for the conversion of light into biochemical signals.
- Blood–retinal barrier
A tightly controlled transport barrier that is maintained by tight junctions in the retinal capillary endothelium (comprising the inner blood–retinal barrier), and by the Bruch membrane and the retinal pigmented epithelium monolayer (comprising the outer blood–retinal barrier). Integrity of the blood–retinal barrier is important for control of fluid leakage, solute transport and immune quiescence, all of which support the functional homeostasis of the retina.
- Retinal pigmented epithelium
(RPE). A monolayer of epithelial cells that has multiple essential roles in visual function, including recycling components of the visual cycle, secreting trophic factors and maintaining the outer blood–retinal barrier. The RPE is widely considered to be the focal point of age-related macular degeneration pathogenesis, in which breakdown of the RPE leads to secondary photoreceptor degeneration.
- Drusen
Discrete extracellular deposits that commonly precede the development of age-related macular degeneration, and that are comprised of numerous cellular and inflammatory factors.
- Geographic atrophy
(also known as end-stage ‘dry’ age-related macular degeneration (AMD) or atrophic AMD). A disease affecting the macula in which the retinal pigmented epithelium can no longer support photoreceptor function owing to spontaneous degeneration of large confluent regions. Although geographic atrophy occurs less frequently than neovascular AMD (approximately 50% as common), there are no currently approved therapies.
- Immune privilege
The property of a tissue being tolerant to antigen. Retinal immune privilege is maintained by blood–retinal barrier integrity and the absence of functional lymphatic circulation.
- Fundus photography
A common method for visualizing the retinal pigmented epithelium, retina and retinal circulation photographically. Funduscopy is used by ophthalmologists to diagnose retinal disorders such as age-related macular degeneration.
- Para-inflammation
A state in which tissue homeostasis is maintained by low-grade inflammatory-based clearance of noxious stimuli. In the retina, para-inflammation may persist and ultimately contribute to age-related macular degeneration through increased immune cell infiltration and activation at sites of tissue damage.
- Genome-wide association studies
(GWASs). The process by which genetic variations and disease phenotypes are statistically correlated. Linkage studies carried out with markers located across the entire genome that were traditionally carried out with approximately 300 markers of simple sequence-length repeats but that have been more recently carried out with approximately 1–5 million single nucleotide polymorphisms.
- Alternative pathway of complement activation
An evolutionarily ancient innate immune process by which microorganisms are destroyed through opsonization and activation of the membrane attack complex. According to the ‘complement hypothesis’, misactivation of, and/or the inability to appropriately inhibit, the alternative pathway results in retinal tissue damage and drives age-related macular degeneration pathology.
- Carboxyethylpyrrole-adducted proteins
Proteins that are modified through the oxidation of the fatty acid docosahexaenoic acid. These adducts are abundant in the retina. They can have direct pro-inflammatory effects through pattern recognition, and autoantibodies that recognize them are abundant in the circulation of patients with age-related macular degeneration.
- M1 macrophages
A pro-inflammatory or ‘classically activated’ subset of macrophages, which are characterized by phagocytic activity and expression of particular inflammatory cytokines (such as tumour necrosis factor) and inflammatory mediators (such as inducible nitric oxide synthase).
- M2 macrophages
A pro-angiogenic or ‘alternatively activated’ subset of macrophages, which are characterized by the expression of particular angiogenic cytokines (such as vascular endothelial growth factor A) and anti-inflammatory mediators (such as arginase).
Footnotes
Competing interests statement
The authors declare competing financial interests. See Web version for details.
FURTHER INFORMATION
Jayakrishna Ambati’s homepage: http://www.mc.uky.edu/angiogenesis/
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.The Global Economic Cost of Visual Impairment. AMD Alliance International. 2010 [online], http://www.icoph.org/resources/146/The-Global-Economic-Cost-of-Visual-Impairment.html. [Google Scholar]
- 2.Haines JL, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AO, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 4. Klein RJ, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. References 2–4 are landmark studies showing increased statistical risk of AMD in individuals who have a single CFH polymorphism these studies were the first of their kind for a complex human disease.
- 5.Hageman GS, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl Acad. Sci. USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuo J, Grob S, Zhang K, Chan CC. Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul. Immunol. Inflamm. 2012;20:27–36. doi: 10.3109/09273948.2011.628432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory statesthe examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv. Immunol. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- 8.Khandhadia S, Cipriani V, Yates JR, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2012;217:127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 9. Anderson DH, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. References 6–9 provide a comprehensive review and analysis of the role of the complement system in AMD.
- 10.Saint-Geniez M, et al. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS ONE. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bill A, Sperber G, Ujiie K. Physiology of the choroidal vascular bed. Int. Ophthalmol. 1983;6:101–107. doi: 10.1007/BF00127638. [DOI] [PubMed] [Google Scholar]
- 12.Hageman GS, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE — Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 15. Brown DM, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. References 14 and 15 report Phase III clinical trials showing the efficacy of VEGFA-targeted antibody therapy for neovascular AMD, which is the standard of care for this disease.
- 16.Bird AC. Therapeutic targets in age-related macular disease. J. Clin. Invest. 2010;120:3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunness JS, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–1779. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- 18.Martin DF, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marneros AG, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am. J. Pathol. 2005;167:1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sepp T, et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest. Ophthalmol. Visual Sci. 2006;47:536–540. doi: 10.1167/iovs.05-1143. [DOI] [PubMed] [Google Scholar]
- 21.Sofat R, et al. Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. Int. J. Epidemiol. 2012;41:250–262. doi: 10.1093/ije/dyr204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Droz I, et al. Genotype-phenotype correlation of age-related macular degeneration: influence of complement factor H polymorphism. Br. J. Ophthalmol. 2008;92:513–517. doi: 10.1136/bjo.2007.127811. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson KP, et al. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med. 2006;3:e5. doi: 10.1371/journal.pmed.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron DJ, et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle. 2007;6:1122–1125. doi: 10.4161/cc.6.9.4157. [DOI] [PubMed] [Google Scholar]
- 25.Sobrin L, et al. Heritability and genome-wide association study to assess genetic differences between advanced age-related macular degeneration subtypes. Ophthalmology. 2012;119:1874–1875. doi: 10.1016/j.ophtha.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young RW. Pathophysiology of age-related macular degeneration. Surv. Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 27.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 28.Sugita S. Role of ocular pigment epithelial cells in immune privilege. Arch. Immunol. Ther. Exp. (Warsz.) 2009;57:263–268. doi: 10.1007/s00005-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 29.Streilein JW. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilege in the eye. Eye (Lond.) 1995;9:236–240. doi: 10.1038/eye.1995.46. [DOI] [PubMed] [Google Scholar]
- 30.Morohoshi K, Goodwin AM, Ohbayashi M, Ono SJ. Autoimmunity in retinal degeneration: autoimmune retinopathy and age-related macular degeneration. J. Autoimmun. 2009;33:247–254. doi: 10.1016/j.jaut.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 32.Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS ONE. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Forrester JV, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS ONE. 2011;6:e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuo J, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 37.Espinosa-Heidmann DG, et al. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 38.Shen WY, Yu MJ, Barry CJ, Constable IJ, Rakoczy PE. Expression of cell adhesion molecules and vascular endothelial growth factor in experimental choroidal neovascularisation in the rat. Br. J. Ophthalmol. 1998;82:1063–1071. doi: 10.1136/bjo.82.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ambati J, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nature Med. 2003;9:1390–1397. doi: 10.1038/nm950. CCL2- and CCR2-deficient mice were the first animal model shown to reproduce numerous features of early, intermediate and neovascular AMD. This study also directly implicates macrophage migration in retinal degeneration.
- 40. Combadiere C, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. This study advanced the epidemiological finding that the Thr280Met CX3CR1 polymorphism confers AMD risk by identifying dysfunctional microglial migration in patients carrying the 280Met mutation.
- 41.Ross RJ, et al. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp. Res. 2008;86:675–683. doi: 10.1016/j.exer.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luhmann UF, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest. Ophthalmol. Vis. Sci. 2009;50:5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruban J, et al. CCR2/CCL2-mediated inflammation protects photoreceptor cells from amyloid-β-induced apoptosis. Neurobiol. Dis. 2011;42:55–72. doi: 10.1016/j.nbd.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Raoul W, et al. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res. 2008;40:115–119. doi: 10.1159/000119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuo J, et al. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS ONE. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, et al. Relationship between complement membrane attack complex, chemokine (C-C motif) ligand 2 (CCL2) and vascular endothelial growth factor in mouse model of laser-induced choroidal neovascularization. J. Biol. Chem. 2011;286:20991–21001. doi: 10.1074/jbc.M111.226266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie P, et al. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS ONE. 2011;6:e28933. doi: 10.1371/journal.pone.0028933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raychaudhuri S, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nature Genet. 2011;43:1232–1236. doi: 10.1038/ng.976. The initial clear-cut description of a rare variant in CFH with high penetrance, in which the functional studies demonstrate a defect as a result of this variant. Presumably, the first of many rare variants in regulators and activators of the alternative complement pathway that predispose to AMD.
- 51.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 52. Baudouin C, et al. Immunohistological study of subretinal membranes in age-related macular degeneration. Jpn. J. Ophthalmol. 1992;36:443–451. The histological examination in this study was the first to implicate complement disturbances in AMD, preceding GWASs by 13 years.
- 53.Clark SJ, et al. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. J. Biol. Chem. 2010;285:30192–30202. doi: 10.1074/jbc.M110.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sjöberg AP, et al. The factor H variant associated with age-related macular degeneration (His-384) and the non-disease-associated form bind differentially to C-reactive protein, fibromodulin, DNA, necrotic cells. J. Biol. Chem. 2007;282:10894–10900. doi: 10.1074/jbc.M610256200. [DOI] [PubMed] [Google Scholar]
- 55.Laine M, et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J. Immunol. 2007;178:3831–3836. doi: 10.4049/jimmunol.178.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson PT, et al. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc. Natl Acad. Sci. USA. 2006;103:17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollyfield JG, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doyle SL, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nature Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tarallo V, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. The first study to show that inflammasome activation in RPE is a crucial driver of geographic atrophy.
- 60.Kaneko H, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng WA, et al. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kauppinen A, et al. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells-Implications for age-related macular degeneration (AMD) Immunol. Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Liu RT, et al. Inflammatory mediators induced by amyloid-β in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2013;54:2225–2237. doi: 10.1167/iovs.12-10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleinman ME, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinman ME, et al. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol. Ther. 2012;20:101–108. doi: 10.1038/mt.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavalette S, et al. Interleukin-1β inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am. J. Pathol. 2011;178:2416–2423. doi: 10.1016/j.ajpath.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N. Engl. J. Med. 2008;359:1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zareparsi S, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum. Mol. Genet. 2005;14:1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- 70.Cho Y, et al. Toll-like receptor polymorphisms and age-related macular degeneration: replication in three case-control samples. Invest. Ophthalmol. Vis. Sci. 2009;50:5614–5618. doi: 10.1167/iovs.09-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiose S, et al. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J. Biol. Chem. 2011;286:15543–15555. doi: 10.1074/jbc.M111.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.West XZ, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujimoto T, et al. Choroidal neovascularization enhanced by Chlamydia pneumoniae via Toll-like receptor 2 in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2010;51:4694–4702. doi: 10.1167/iovs.09-4464. [DOI] [PubMed] [Google Scholar]
- 74.Patel N, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115:422–430. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 1990;228:270–274. doi: 10.1007/BF00920033. References 57 and 75 provided the first evidence, in mice and humans, that autoantibodies are involved in AMD.
- 76.Cherepanoff S, Mitchell P, Wang JJ, Gillies MC. Retinal autoantibody profile in early age-related macular degeneration: preliminary findings from the Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2006;34:590–595. doi: 10.1111/j.1442-9071.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl Acad. Sci. USA. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandes AF, et al. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J. Biol. Chem. 2008;283:20745–20753. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bian Q, et al. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012;53:1298–1307. doi: 10.1016/j.freeradbiomed.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sparrow JR, Zhou J, Cai B. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue-light illumination. Invest. Ophthalmol. Vis. Sci. 2003;44:2245–2251. doi: 10.1167/iovs.02-0746. [DOI] [PubMed] [Google Scholar]
- 81.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue lightinduced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- 82.Radu RA, et al. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J. Biol. Chem. 2011;286:18593–18601. doi: 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iriyama A, et al. A2E, a component of lipofuscin, is pro-angiogenic in vivo. J. Cell. Physiol. 2009;220:469–475. doi: 10.1002/jcp.21792. [DOI] [PubMed] [Google Scholar]
- 84.Catala A. Lipid peroxidation of membrane phospholipids in the vertebrate retina. Front. Biosci. (Schol Ed) 2011;3:52–60. doi: 10.2741/s131. [DOI] [PubMed] [Google Scholar]
- 85.Hoppe G, O’Neil J, Hoff HF, Sears J. Products of lipid peroxidation induce missorting of the principal lysosomal protease in retinal pigment epithelium. Biochim. Biophys. Acta. 2004;1689:33–41. doi: 10.1016/j.bbadis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp. Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 87.Weismann D, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma A, et al. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch. Biochem. Biophys. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-β is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vision. 2003;9:184–190. [PubMed] [Google Scholar]
- 90.Ding JD, et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl Acad. Sci. USA. 2011;108:e279–e287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu XC, Liu XF, Jian CX, Li CJ, He SZ. IL-33 is induced by amyloid-β stimulation and regulates inflammatory cytokine production in retinal pigment epithelium cells. Inflammation. 2012;35:776–784. doi: 10.1007/s10753-011-9379-4. [DOI] [PubMed] [Google Scholar]
- 92.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond.) 1990;4:613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 94.Lopez PF, et al. Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am. J. Ophthalmol. 1991;112:647–656. doi: 10.1016/s0002-9394(14)77270-8. [DOI] [PubMed] [Google Scholar]
- 95.Oh H, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest. Ophthalmol. Vis. Sci. 1999;40:1891–1898. [PubMed] [Google Scholar]
- 96.Seregard S, Algvere PV, Berglin L. Immunohistochemical characterization of surgically removed subfoveal fibrovascular membranes. Graefes Arch. Clin. Exp. Ophthalmol. 1994;232:325–329. doi: 10.1007/BF00175983. [DOI] [PubMed] [Google Scholar]
- 97.Tsutsumi-Miyahara C, et al. The relative contributions of each subset of ocular infiltrated cells in experimental choroidal neovascularisation. Br. J. Ophthalmol. 2004;88:1217–1222. doi: 10.1136/bjo.2003.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br. J. Ophthalmol. 2010;94:918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 99.Grossniklaus HE, et al. Correlation of histologic 2-dimensional reconstruction and confocal scanning laser microscopic imaging of choroidal neovascularization in eyes with age-related maculopathy. Arch. Ophthalmol. 2000;118:625–629. doi: 10.1001/archopht.118.5.625. [DOI] [PubMed] [Google Scholar]
- 100. Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. References:36, 37, 99 and 100 show the diverging roles of macrophages in neovascular AMD.
- 101.Cao X, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol. Int. 2011;61:528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasegawa E, et al. IL-27 inhibits pathophysiological intraocular neovascularization due to laser burn. J. Leukoc. Biol. 2012;91:267–273. doi: 10.1189/jlb.1110603. [DOI] [PubMed] [Google Scholar]
- 103.Matsumura N, et al. Low-dose lipopolysaccharide pretreatment suppresses choroidal neovascularization via IL-10 induction. PLoS ONE. 2012;7:e39890. doi: 10.1371/journal.pone.0039890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J. Clin. Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou J, et al. Neutrophils promote experimental choroidal neovascularization. Mol. Vision. 2005;11:414–424. [PubMed] [Google Scholar]
- 106.Zhou J, et al. Neutrophils compromise retinal pigment epithelial barrier integrity. J. Biomed. Biotechnol. 2010;2010:289360. doi: 10.1155/2010/289360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Penfold PL, Provis JM, Billson FA. Age-related macular degeneration: ultrastructural studies of the relationship of leucocytes to angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 1987;225:70–76. doi: 10.1007/BF02155808. [DOI] [PubMed] [Google Scholar]
- 108.Luo L, et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano. 2013;7:3264–3275. doi: 10.1021/nn305958y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin DF, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jonas JB, Degenring RF, Kreissig I, Friedemann T, Akkoyun I. Exudative age-related macular degeneration treated by intravitreal triamcinolone acetonide. A prospective comparative nonrandomized study. Eye (Lond.) 2005;19:163–170. doi: 10.1038/sj.eye.6701438. [DOI] [PubMed] [Google Scholar]
- 111.Gillies MC, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: oneyear results. Arch. Ophthalmol. 2003;121:667–673. doi: 10.1001/archopht.121.5.667. [DOI] [PubMed] [Google Scholar]
- 112.Smailhodzic D, et al. Cumulative effect of risk alleles in CFH, ARMS2, and VEGFA on the response to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2012;119:2304–2311. doi: 10.1016/j.ophtha.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 113.Hagstrom SA, et al. Pharmacogenetics for genes associated with age-related macular degeneration (AMD) in the comparison of AMD treatments trials (CATT) Ophthalmology. 2013;120:593–599. doi: 10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scholl HP, et al. CFH, C3 and ARMS2 are significant risk loci for susceptibility but not for disease progression of geographic atrophy due to AMD. PLoS ONE. 2009;4:e7418. doi: 10.1371/journal.pone.0007418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klein ML, et al. Progression of geographic atrophy and genotype in age-related macular degeneration. Ophthalmology. 2010;117:1554–1559. doi: 10.1016/j.ophtha.2009.12.012. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nozaki M, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl Acad. Sci. USA. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Curcio CA, et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, yopography, and biogenesis model. Retina. 2013;33:265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson LV, et al. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc. Natl Acad. Sci. USA. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang AL, et al. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS ONE. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Munch IC, et al. Small, hard macular drusen and peripheral drusen: associations with AMD genotypes in the Inter99 Eye Study. Invest. Ophthalmol. Vis. Sci. 2010;51:2317–2321. doi: 10.1167/iovs.09-4482. [DOI] [PubMed] [Google Scholar]
- 121.Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest. Ophthalmol. Vis. Sci. 2009;50:586–591. doi: 10.1167/iovs.08-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol. Vis. 1999;5:28. [PubMed] [Google Scholar]
- 123.Crabb JW, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl Acad. Sci. USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hollyfield JG, Salomon RG, Crabb JW. Proteomic approaches to understanding age-related macular degeneration. Adv. Exp. Med. Biol. 2003;533:83–89. doi: 10.1007/978-1-4615-0067-4_11. [DOI] [PubMed] [Google Scholar]
- 125.Johnson LV, et al. The Alzheimer’s Aβ -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and agerelated macular degeneration. Proc. Natl Acad. Sci. USA. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klein ML, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1026–1031. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 127.Friese MA, et al. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol. Immunol. 1999;36:809–818. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 128.Spencer KL, et al. Deletion of CFHR3 and CFHR1 genes in age-related macular degeneration. Hum. Mol. Genet. 2008;17:971–977. doi: 10.1093/hmg/ddm369. [DOI] [PubMed] [Google Scholar]
- 129.Heurich M, et al. Common polymorphisms in C3, factor B, factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl Acad. Sci. USA. 2011;108:8761–8766. doi: 10.1073/pnas.1019338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takeda A, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang H, et al. Upregulation of CCR3 by age-related stresses promotes choroidal endothelial cell migration via VEGF-dependent and -independent signaling. Invest. Ophthalmol. Vis. Sci. 2011;52:8271–8277. doi: 10.1167/iovs.11-8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mo FM, Proia AD, Johnson WH, Cyr D, Lashkari K. Interferon γ-inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2010;51:4226–4236. doi: 10.1167/iovs.09-3910. [DOI] [PubMed] [Google Scholar]
- 133.Sharma NK, et al. New biomarker for neovascular age-related macular degeneration: eotaxin-2. DNA Cell Biol. 2012;31:1618–1627. doi: 10.1089/dna.2012.1786. [DOI] [PubMed] [Google Scholar]
- 134.Mizutani T, Ashikari M, Tokoro M, Nozaki M, Ogura Y. Suppression of laser-induced choroidal neovascularization by a CCR3 antagonist. Invest. Ophthalmol. Vis. Sci. 2013;54:1564–1572. doi: 10.1167/iovs.11-9095. [DOI] [PubMed] [Google Scholar]
- 135.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch. Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 136.Dankbar B, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 137.Izumi-Nagai K, et al. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol. 2007;170:2149–2158. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leung KW, Barnstable CJ, Tombran-Tink J. Bacterial endotoxin activates retinal pigment epithelial cells and induces their degeneration through IL-6 and IL-8 autocrine signaling. Mol. Immunol. 2009;46:1374–1386. doi: 10.1016/j.molimm.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 139.Fujimura S, et al. Angiostatic effect of CXCR3 expressed on choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2012;53:1999–2006. doi: 10.1167/iovs.11-8232. [DOI] [PubMed] [Google Scholar]
- 140.Arakawa S, et al. Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population. Nature Genet. 2011;43:1001–1004. doi: 10.1038/ng.938. [DOI] [PubMed] [Google Scholar]
- 141.Nakata I, et al. Association of genetic variants on 8p21 and 4q12 with age-related macular degeneration in Asian populations. Invest. Ophthalmol. Vis. Sci. 2012;53:6576–6581. doi: 10.1167/iovs.12-10219. [DOI] [PubMed] [Google Scholar]
- 142.Lichtlen P, Lam TT, Nork TM, Streit T, Urech DM. Relative contribution of VEGF and TNF-α in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest. Ophthalmol. Vis. Sci. 2010;51:4738–4745. doi: 10.1167/iovs.09-4890. [DOI] [PubMed] [Google Scholar]
- 143.Olson JL, Courtney RJ, Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch. Ophthalmol. 2007;125:1221–1224. doi: 10.1001/archopht.125.9.1221. [DOI] [PubMed] [Google Scholar]
- 144.Shi X, et al. Inhibition of TNF-α reduces laser-induced choroidal neovascularization. Exp. Res. 2006;83:1325–1334. doi: 10.1016/j.exer.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 145.de Oliveira Dias JR, et al. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br. J. Ophthalmol. 2011;95:1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 146.Sengupta N, et al. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest. Ophthalmol. Vis. Sci. 2005;46:343–348. doi: 10.1167/iovs.04-0153. [DOI] [PubMed] [Google Scholar]
- 147.Lee E, Rewolinski D. Evaluation of CXCR4 inhibition in the prevention and intervention model of laser-induced choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2010;51:3666–3672. doi: 10.1167/iovs.09-3802. [DOI] [PubMed] [Google Scholar]
- 148.Guerin E, et al. SDF1-α is associated with VEGFR-2 in human choroidal neovascularisation. Microvasc. Res. 2008;75:302–307. doi: 10.1016/j.mvr.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 149.Bhutto IA, McLeod DS, Merges C, Hasegawa T, Lutty GA. Localisation of SDF-1 and its receptor CXCR4 in retina and choroid of aged human eyes and in eyes with age related macular degeneration. Br. J. Ophthalmol. 2006;90:906–910. doi: 10.1136/bjo.2006.090357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mevorach D. Clearance of dying cells and systemic lupus erythematosus: the role of C1q and the complement system. Apoptosis. 2010;15:1114–1123. doi: 10.1007/s10495-010-0530-8. [DOI] [PubMed] [Google Scholar]
- 151.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nature Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu G, et al. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ. Res. 2009;104:550–558. doi: 10.1161/CIRCRESAHA.108.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Szeplaki G, Varga L, Fust G, Prohaszka Z. Role of complement in the pathomechanism of atherosclerotic vascular diseases. Mol. Immunol. 2009;46:2784–2793. doi: 10.1016/j.molimm.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 154.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 155.Fritsche LG, et al. Seven new loci associated with age-related macular degeneration. Nature Genet. 2013;45:433–439. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Landa G, Butovsky O, Shoshani J, Schwartz M, Pollack A. Weekly vaccination with Copaxone (glatiramer acetate) as a potential therapy for dry age-related macular degeneration. Curr. Res. 2008;33:1011–1013. doi: 10.1080/02713680802484637. [DOI] [PubMed] [Google Scholar]
- 157.Nussenblatt RB, et al. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30:1579–1587. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomi F, Sawa M, Tsujikawa M, Nishida K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 2012;32:1804–1810. doi: 10.1097/IAE.0b013e31825be87f. [DOI] [PubMed] [Google Scholar]
- 159.Ahmadieh H, et al. Intravitreal bevacizumab versus combined intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration: six-month results of a randomized clinical trial. Retina. 2011;31:1819–1826. doi: 10.1097/IAE.0b013e31820d58f2. [DOI] [PubMed] [Google Scholar]
- 160.Lachmann PJ. The amplification loop of the complement pathways. Adv. Immunol. 2009;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]