Abstract
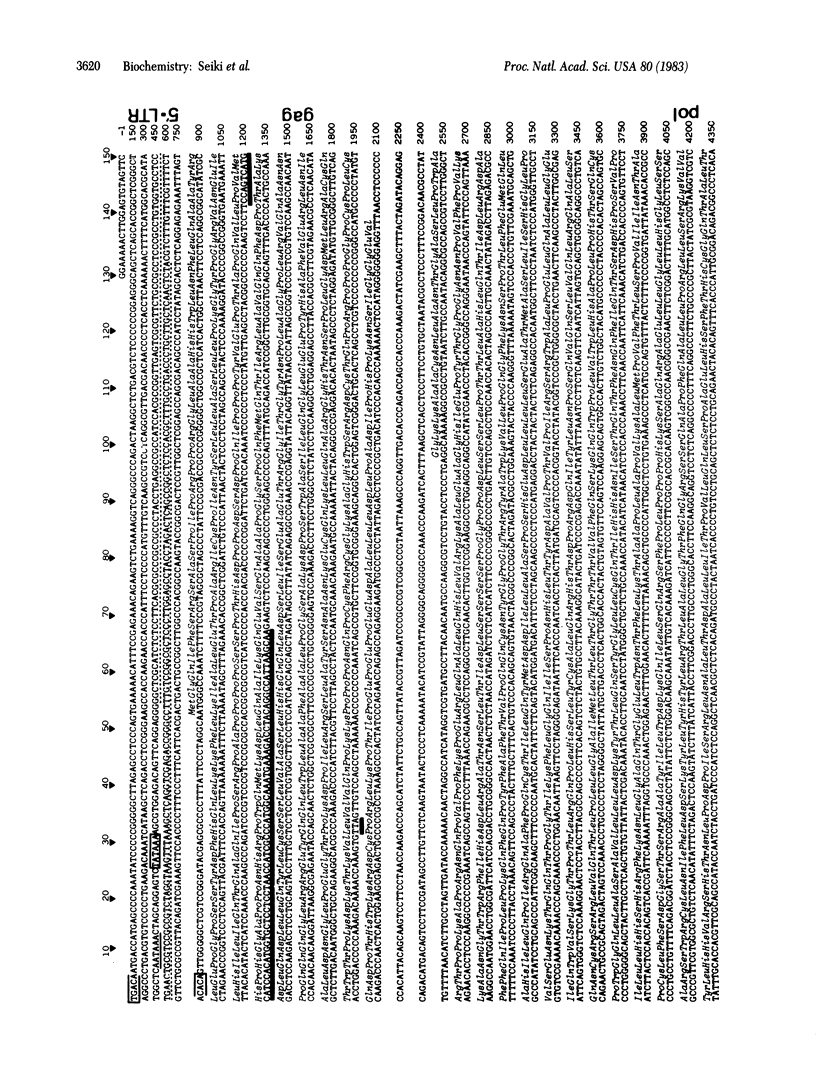
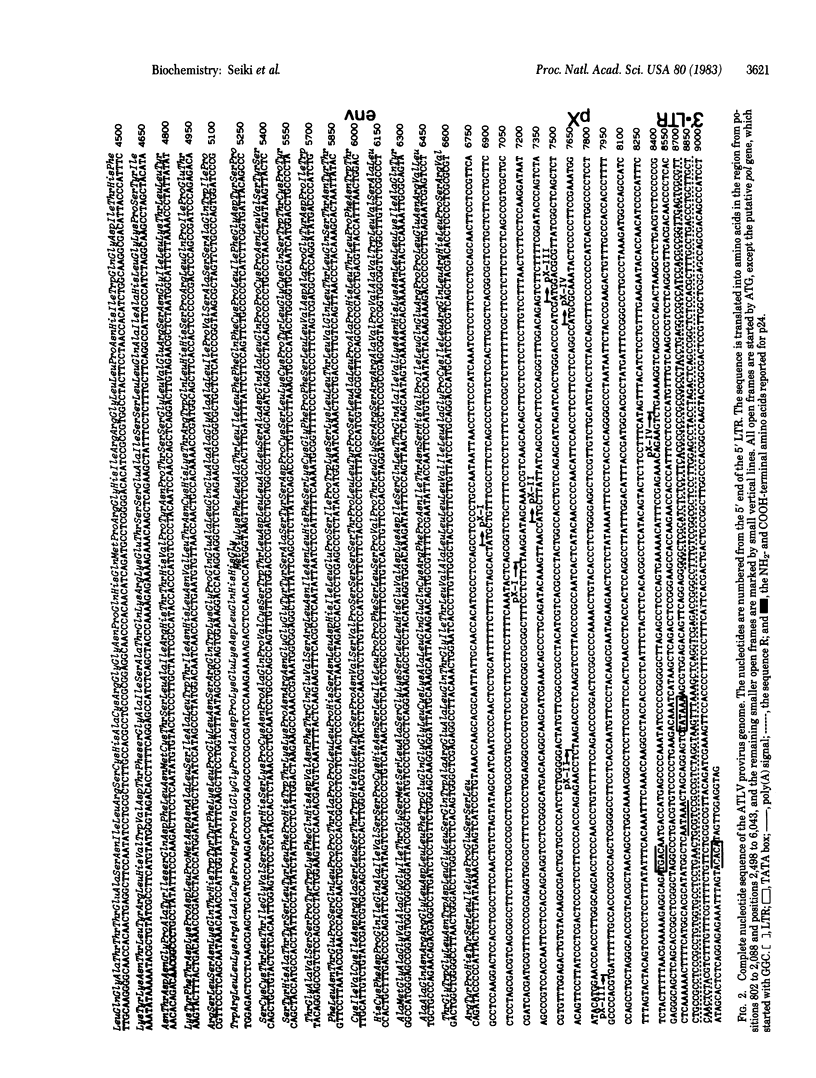
Human retrovirus adult T-cell leukemia virus (ATLV) has been shown to be closely associated with human adult T-cell leukemia (ATL) [Yoshida, M., Miyoshi, I. & Hinuma, Y. (1982) Proc. Natl. Acad. Sci. USA 79, 2031-2035]. The provirus of ATLV integrated in DNA of leukemia T cells from a patient with ATL was molecularly cloned and the complete nucleotide sequence of 9,032 bases of the proviral genome was determined. The provirus DNA contains two long terminal repeats (LTRs) consisting of 755 bases, one at each end, which are flanked by a 6-base direct repeat of the cellular DNA sequence. The nucleotides in the LTR could be arranged into a unique secondary structure, which could explain transcriptional termination within the 3' LTR but not in the 5' LTR. The nucleotide sequence of the provirus contains three large open reading frames, which are capable of coding for proteins of 48,000, 99,000, and 54,000 daltons. The three open frames are in this order from the 5' end of the viral genome and the predicted 48,000-dalton polypeptide is a precursor of gag proteins, because it has an identical amino acid sequence to that of the NH2 terminus of human T-cell leukemia virus (HTLV) p24. The open frames coding for 99,000- and 54,000-dalton polypeptides are thought to be the pol and env genes, respectively. On the 3' side of these three open frames, the ATLV sequence has four smaller open frames in various phases; these frames may code for 10,000-, 11,000-, 12,000-, and 27,000-dalton polypeptides. Although one or some of these open frames could be the transforming gene of this virus, in preliminary analysis, DNA of this region has no homology with the normal human genome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. W., Jr, Wydro R. M., Nadal-Ginard B., Dina D. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature. 1980 Dec 25;288(5792):665–669. doi: 10.1038/288665a0. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Chen R. Complete amino acid sequence and glycosylation sites of glycoprotein gp71A of Friend murine leukemia virus. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5788–5792. doi: 10.1073/pnas.79.19.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Nakao Y., Ito Y., Aoki T., Gallo R. C. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Sarngadharan M. G., Copeland T. D., Kalyanaraman V. S., Gilden R. V., Gallo R. C. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Reitz M. S., Jr, Sarngadharan M. G., Robert-Guroff M., Kalyanaraman V. S., Nakao Y., Miyoshi I., Minowada J., Yoshida M., Ito Y. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982 Nov 4;300(5887):63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Yoshida M. Human adult T-cell leukemia virus: molecular cloning of the provirus DNA and the unique terminal structure. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6899–6902. doi: 10.1073/pnas.79.22.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Hackett P. B., Oppermann H., Ullrich A., Levintow L., Bishop J. M. Cell-free translation of avian sarcoma virus RNA: suppression of the gag termination codon does not augment synthesis of the joint gag/pol product. Cell. 1978 Oct;15(2):607–614. doi: 10.1016/0092-8674(78)90029-6. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]



