Geriatric depression has reported prevalence rates of 1.6 to 15% of the population1. New effective fast-acting treatment modalities in this population would be cost saving and alleviate suffering and disability for the patients and their families. The rapid onset of antidepressant effect produced by subanesthetic intravenous (IV) ketamine provides such a promise. Single2, 3 and repeat 4-7 ketamine infusions have been shown to produce an antidepressant effect within hours with a sustained effect lasting days to weeks. But not all depressed patients who are administered ketamine have a response. Using a 50% reduction in baseline symptoms as the response criteria, response rates of 43-90% are reported within 40 minutes, 24 hours, and 72 hours post-infusion8. Although a large number of patients do respond, studies to accurately predict who responds are at an early stage. Here, we report a case series where IV ketamine infusions in geriatric patients with Treatment Resistant Depression (TRD) did not prove beneficial.
Four patients (mean (± SD) age, 72.25 (± 5.38) years) admitted to an inpatient psychiatric unit due to a relapse in their Major Depressive Disorder (MDD), with histories of multiple failed medication trials and failed electroconvulsive therapy (ECT), were offered IV ketamine treatments, as described elsewhere9. An Institutional Review Board approved retrospective chart review was conducted for this case series. After the initial inpatient infusion demonstrated no physiological concerns, the infusions were continued on an outpatient basis. Prescribed medications were continued throughout the infusions.
Case Series
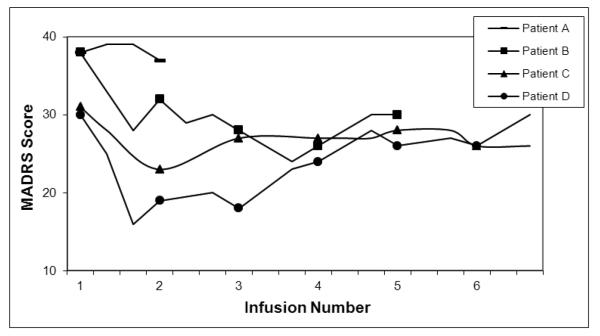
Patient A was diagnosed with MDD and Generalized Anxiety Disorder (GAD), as well as Parkinson’s-Plus. Since the onset of his depression in his early 70’s, he failed to respond to several pharmacological trials, cognitive-behavioral therapy, acupuncture, and a series of 15 ECT treatments. Previous neuropsychological testing indicated possible executive impairment and a magnetic resonance imaging (MRI) scan demonstrated some T2 white matter changes. Family history was significant for mood disorders. During the first infusion, he reported “going through a lot of worlds” and being cold. The patient reported no decrease in his depressive symptoms. On the second infusion, at a slightly reduced dose in an effort to avoid side effects, he reported feeling outside his body and some confusion. Again, he reported no response in his mood (Figure 1). The patient decided to terminate the treatments due to the adverse effects and the lack of benefit.
Figure 1.
Response to ketamine treatments over a series of infusions in four geriatric depressed patients. Each marker indicates a treatment. MADRS scores at or below 8 indicate remission.
Patient B was diagnosed with MDD and GAD. In her early 60’s, she experienced a poorly defined personality change, with subsequent onset of a severe depression one year later. After failing multiple medication trials, she initially responded to ECT, but subsequently failed despite a total of 55 treatments over three years. Previous neuropsychological testing indicated cognitive impairment consistent with severe depression. An MRI revealed mild diffuse atrophy and volume loss and a positron emission tomograph revealed mild diffuse hypometabolism. Family history was significant for mood disorders. The patient went through an acute series of five infusions with reported transient and tolerable symptoms of double vision, paresthesia, tinnitus, and a sensation of floating, but no improvement in her depressive symptoms (Figure 1).
Patient C was diagnosed with MDD and GAD. His depression began in his late 30’s with four distinct lifetime episodes, each lasting approximately one year. He had failed multiple medications and a series of 12 ECTs. No previous neuropsychological testing or neuroimaging had been performed. Family history was significant for mood disorders. The patient underwent six infusions and reported feeling fearful as well as experiencing paresthesia, and perceptual changes. He reported no improvement in his depressive symptoms (Figure 1).
Patient D was diagnosed with atypical MDD. His depression began in his early 50’s, with unsuccessful treatments including pharmacological trials, a series of 17 ECTs, and a vagal nerve stimulator. No previous neuropsychological testing or neuroimaging had been performed. Family history was significant for mood disorders. The patient went through a series of six infusions. He did report a decrease in his depressive symptoms after the first infusion, although not enough to meet the response criterion of 50% reduction. His depressive symptoms increased with subsequent infusions until he was back to his baseline level after infusion six (Figure 1). Somnolence was the only side effect.
Discussion
Recent research has focused on the glutamatergic system in MDD and the rapid antidepressant effect of medications targeting this system. Ketamine has been shown to be a rapid, safe, and efficacious alternative to more traditional forms of treatment2-7, 9 and has demonstrated similar effects when given repeatedly over an extended period of time4-7, 9. However, most, if not all, of the cases reported are in patients under the age of 65. Our cases describe four heterogeneous presentations of depression in patients over the age of 65 where ketamine treatments did not prove beneficial.
Studies have shown that the N-methyl-D-aspartate (NMDA) receptors appear to be more affected than other glutamate receptors by the aging process, such that there is an overall decline in their number and functionality (although this is not uniform across all brain regions)10. Ketamine exerts its effects by blocking NMDA receptors and stimulating α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors11, 12. It is possible that those individuals with geriatric depression do not have sufficient NMDA receptors present for effective binding by ketamine, or that the NMDA receptors that are present have functionally changed.
Moreover, age-related sensitivity to anesthetic agents has been reported13, possibly due to age-related changes in NMDA receptors. Ketamine’s usage as an anesthetic is well-established14. Such age related sensitivity may result in a heightened response to ketamine and produce more intense side effects than described in younger patients. In our case series, patients were administered ketamine at 0.5 mg/kg of ideal body weight as described in the literature and it was evident that they experienced subjectively severe dissociative states. They reported being “in another world”, “floating”, and being “outside my body”. Patient A terminated the treatment and Patient C reported being frightened by his experience. Further investigation into this age-related anesthesia sensitivity may help to establish appropriate dosage guidelines for administration in geriatric patients with TRD, as well as reduce potential side effects.
It is known that late-life and late-onset depression differs from that of early-onset depression in many ways15, 16. The lack of well-designed clinical trials of novel treatments for late-life depression makes it difficult to define who would actually benefit from novel, fast-acting antidepressants like ketamine. The likelihood of co-morbid medical illness makes treatment for depression more complex and comorbid anxiety disorders in the elderly can increase treatment resistance17, further complicating the treatment of this group. It is possible that in two of our patients (A & B), their depression, and lack of response, was the result of a subcortical dementia or a fronto-temporal variant dementia, respectively. Revisiting diagnoses that may better account for the lack of treatment response to ketamine in geriatric TRD patients is necessary. Moreover, the aging process in general, as well as the age-related brain sensitivity to anesthetic drugs, makes it more difficult to determine the dose of ketamine that should be used. The patients described in this case series were refractory to multiple treatment modalities as are, we believe, all of the patients described in other ketamine reports2-7, 9. Given this current state of knowledge, it would be unwise at this time to extrapolate the results seen here to more typical or initial presentations of late-life depression. We would argue that more comprehensive studies of ketamine in older adults with TRD are needed to determine whether this treatment is worthwhile in this population. Our experience suggests that, if geriatric patients are being considered for ketamine therapy, they be given an initial dosage of ketamine that is less than 0.5 mg/kg of ideal body weight, with the hope of avoiding difficult side effects such as dissociation. Once psychological safety is established the dosage could be gradually increased to assess for potential benefit.
Footnotes
Conflicts of Interest and Source of Funding: Dr. Dale has received speaker’s fees from Astra Zeneca. All other authors declare that they have no conflicts of interest. There is no source of funding for this project.
References
- 1.Ciraulo DA, Evans JA, Qui WQ, et al. Antidepressant treatment of geriatric depression. In: Ciraulo DA, Shader RI, editors. Pharmacotherapy of Depression. 2nd Humana Press; New York: 2011. pp. 125–83. [Google Scholar]
- 2.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 3.Zarate CA, Singh JB, Carlson PH, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 4.Messer MM, Haller IV. Maintenance ketamine treatment produces long-term recovery from depression. Primary Psychiatry. 2010;17:48–50. [Google Scholar]
- 5.Messer MM, Haller IV, Larson P, et al. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- 6.aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry epub ahead of print. doi: 10.1016/j.biopsych.2012.06.022. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharm Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szymkowicz SM, Finnegan N, Dale RM. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J Affect Disorders. 2013;147:416–420. doi: 10.1016/j.jad.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during aging. Front Ag Neurosci. 2010;2:1–15. doi: 10.3389/fnagi.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeng S, Zarate CA, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson KR, Scanga C, Wagner AE, et al. Changes in anesthetic sensitivity and glutamate receptors in the aging canine brain. J Gerontol A Sci Med Sci. 2000;55:B448–454. doi: 10.1093/gerona/55.9.b448. [DOI] [PubMed] [Google Scholar]
- 14.Green SM, Li J. Ketamine in adults: What emergency physicians need to know about patient selection and emergence reactions. Acad Emerg Med. 2000;7:278–281. doi: 10.1111/j.1553-2712.2000.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 15.Sachs-Ericsson N, Corsentino E, Moxley J, et al. A longitudinal study of differences in late- and early-onset geriatric depression: Depressive symptoms and psychosocial, cognitive, and neurological functioning. Aging & Mental Health. 2013;17:1–11. doi: 10.1080/13607863.2012.717253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517–526. doi: 10.2147/ndt.s7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenze EJ. Comorbidity of depression and anxiety in the elderly. Curr Psychiatry Rep. 2003;5:62–67. doi: 10.1007/s11920-003-0011-7. [DOI] [PubMed] [Google Scholar]