Abstract
In this issue, Oda et al. (2014. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201312014) use mutant analysis, protein tagging, and cryoelectron tomography to determine the detailed location of components in flagellar radial spokes—a complex of proteins that connect the peripheral microtubule doublets to the central pair. Remarkably, this approach revealed an interaction between radial spokes and the central pair based on geometry rather than a specific signaling mechanism, highlighting the importance of studying a system in three dimensions.
Eukaryotic flagella and motile cilia are protrusive organelles that enable cellular motility or generate flow of extracellular fluid. This organelle, composed of more than 600 proteins (Pazour et al., 2005), retains functions such as bending and switching of waveforms by calcium ions even after isolated from the cell body, indicating that flagella and cilia can function as a closed system. Understanding this highly compartmentalized system is inherently interesting because of the striking organization and structure of this organelle and also because of its medical relevance; defects in this organelle are responsible for a group of diseases termed ciliopathies (Fliegauf et al., 2007). In this issue, Oda et al. present a 3D arrangement of a subset of flagellar/ciliary proteins and provide insight into mechanical signaling in this organelle.
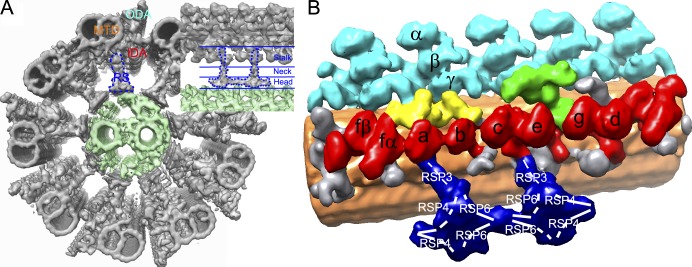
Most flagella and motile cilia share a common 9 + 2 axoneme structure (Fig. 1 A) with nine microtubule doublets surrounded by two singlet microtubules (called the central pair apparatus; Fig. 1 A, light green). Adjacent doublets are connected by isoforms of the microtubule-based motor protein dynein. Peripheral microtubule doublets and the central pair are linked by complexes of 23 proteins (Yang et al., 2006) called radial spokes. These structures form a beautifully ordered array along the microtubule doublets with 96-nm periodicity. Each radial spoke has a T-shaped structure with pseudo twofold symmetry (Pigino et al., 2011; Barber et al., 2012). The radial spokes and central pair are essential for the waveform behavior of cilia/flagella by regulating dynein force generation.
Figure 1.
3D structure of the axoneme and radial spokes. (A) Axoneme from C. reinhardtii flagella seen from the proximal end (the minus end of the microtubule). RS, radial spoke (in a blue dotted line); ODA, outer dynein arm; IDA, inner dynein arm; MTD, microtubule doublet. The central pair apparatus is shown in light green. The inset highlights one microtubule doublet, radial spoke, and central pair apparatus seen from the side (left, the proximal end). The head, neck, and stalk subdomains of the radial spoke are indicated. Modified from Bui and Ishikawa (2013). (B) 3D structure of the 96-nm periodic unit from C. reinhardtii flagella (EMDataBank accession no. EMD-2117). Proximal end is left. The color code is based on previous studies (Heuser et al., 2009; Pigino et al., 2011; Bui et al., 2012). Dark blue, radial spoke; light blue, outer dynein arm; red, inner dynein arm; green, dynein regulatory complex; yellow, intermediate and light chains of dynein f; orange, microtubule; gray, unidentified. Radial spoke proteins located in Oda et al. (2014) and dynein species (α, β, and γ are part of the outer dynein arm, and a–f form the inner dynein arm) based on Bui et al. (2012) are indicated.
Undoubtedly, the bona fide study of individual proteins is essential for the understanding of the flagellar/ciliary bending mechanism. Indeed, high resolution structural biology of dynein (Burgess et al., 2003; Kon et al., 2012; Roberts et al., 2012; Schmidt et al., 2012) has provided insight into the mechanism of force generation inside flagella/cilia. However, we also need to study how individual parts are integrated into the entire system. We must clarify how the individual components interact with each other to organize the highly orchestrated bending motion. This systems biology requires three-dimensional mapping of proteins in vivo.
Until now, location of component proteins in flagella/cilia has been mainly determined by comparing the structure of various deletion mutants of green algae Chlamydomonas reinhardtii using techniques of cryoelectron tomography and subtomogram averaging. With this approach, the location of all of the major dynein species was determined (Bui et al., 2008, 2012). The molecular arrangement of the radial spoke is less defined. Five proteins out of 23 are localized at the top part (called the head subdomain; Fig. 1 A, inset) of this T-shaped complex, close to the central pair apparatus. The rest of the proteins belong to the neck and stalk subdomains. Genetic depletion of one radial spoke head protein (RSP4) causes loss of the entire head, probably because it is essential for the assembly of the radial spoke head. Although a previous study using mutants (Pigino et al., 2011) identified the radial spoke proteins as a group, the exact position of the individual proteins has not been clarified. Oda et al. (2014) enabled a more detailed assignment by generating mutant strains. When RSP4 is expressed in the C. reinhardtii pf1 strain, which lacks the RSP4 gene and therefore the entire radial spoke head, the entire head domain is recovered. The authors expressed RSP4 tagged with biotin carboxyl carrier protein (BCCP). The density of the BCCP tag appears in cryoelectron tomography, indicating the position of RSP4 (Fig. 1 B). This approach enabled not only the location of the proteins to be elucidated but also provided information about protein orientations, as far as the resolution allows. The authors succeeded in identifying the location of the N and C termini of RSP4. They also applied this technology to additional radial spoke proteins and revealed how two copies of this complex of proteins are arranged in one head domain, which might support a model of preassembly proposed by Diener et al. (2011).
The authors went far beyond simple structural analysis. They tagged, instead of with BCCP, with proteins of various sizes. When relatively small peptides (BSA or His tag) were used as a tag, flagellar motility appeared to be relatively normal. However, attaching a large protein (streptavidin) to the C termini of RSPs 3, 4, and 6—all of which are exposed to the interface of the central pair apparatus according to their structural analysis—caused paralysis. In contrast, expression of a large protein tag coupled to a radial spoke protein rescued the motility of paralyzed mutants that lack proteins projecting from the central pair. This remarkable finding suggests an interaction between the central pair apparatus and the radial spoke that depends on geometry, which probably modulates mechanical interaction by collision, rather than inducing a particular signal transduction mechanism. Mechanical interaction has been proposed previously as a hypothesis, based on the geometrical relationship between the radial spoke and the central pair (Warner and Satir, 1974; Lindemann, 2003), the absence of signal transduction motifs in the radial spoke head domain (Pigino and Ishikawa, 2012), and the fact that the central pair twists in the axoneme (Mitchell and Nakatsugawa, 2004), which indicates that the interface with the radial spokes is not regular along the central pair apparatus. Oda et al. (2014) provide the first experimental proof of this model. This signaling mechanism and their methodology could be applicable to other scenarios in the cell.
This challenge of in vivo protein mapping of flagella/cilia will need further development and probably another breakthrough. Besides dynein and the radial spoke, only limited numbers of component proteins have been located and/or functionally analyzed even partially—dynein regulatory complex (Heuser et al., 2009), intermediate and light chains of inner arm dynein f (Heuser et al., 2012; Yamamoto et al., 2013), and an intermediate chain of the outer arm dynein (Oda and Kikkawa, 2013). More than 500 proteins of the 600 listed as flagella/cilia components are still unallocated, whereas many features in the tomogram are left unidentified. Systematic approaches to tag or label proteins in this organelle await development. Unfortunately, the design of mutants using homologous recombination in C. reinhardtii has not yet been reported. Knockdown by RNAi might be a promising approach to locate target proteins and analyze their function (Dymek et al., 2011; Rompolas et al., 2013). If knockdown and expression of mutant proteins are combined in the future, it will enable systematic mutant generation. Flagella/cilia research, which takes advantage of periodicity and symmetry/pseudosymmetry of the axoneme, will lead to in vivo structural and functional analysis of other cellular organelles, which will pave the way to three-dimensional systems biology.
Acknowledgments
Our work mentioned in this paper was funded by a grant from the Swiss National Science Foundation (NF31003A-125131/1).
The author declares no competing financial interests.
References
- Barber C.F., Heuser T., Carbajal-González B.I., Botchkarev V.V., Jr, Nicastro D. 2012. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol. Biol. Cell. 23:111–120 10.1091/mbc.E11-08-0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui K.H., Ishikawa T. 2013. 3D structural analysis of flagella/cilia by cryo-electron tomography. Methods Enzymol. 524:305–323 10.1016/B978-0-12-397945-2.00017-2 [DOI] [PubMed] [Google Scholar]
- Bui K.H., Sakakibara H., Movassagh T., Oiwa K., Ishikawa T. 2008. Molecular architecture of inner dynein arms in situ in Chlamydomonas reinhardtii flagella. J. Cell Biol. 183:923–932 10.1083/jcb.200808050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui K.H., Yagi T., Yamamoto R., Kamiya R., Ishikawa T. 2012. Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme. J. Cell Biol. 198:913–925 10.1083/jcb.201201120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S.A., Walker M.L., Sakakibara H., Knight P.J., Oiwa K. 2003. Dynein structure and power stroke. Nature. 421:715–718 10.1038/nature01377 [DOI] [PubMed] [Google Scholar]
- Diener D.R., Yang P., Geimer S., Cole D.G., Sale W.S., Rosenbaum J.L. 2011. Sequential assembly of flagellar radial spokes. Cytoskeleton (Hoboken). 68:389–400 10.1002/cm.20520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek E.E., Heuser T., Nicastro D., Smith E.F. 2011. The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol. Biol. Cell. 22:2520–2531 10.1091/mbc.E11-03-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M., Benzing T., Omran H. 2007. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8:880–893 10.1038/nrm2278 [DOI] [PubMed] [Google Scholar]
- Heuser T., Raytchev M., Krell J., Porter M.E., Nicastro D. 2009. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 187:921–933 10.1083/jcb.200908067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser T., Barber C.F., Lin J., Krell J., Rebesco M., Porter M.E., Nicastro D. 2012. Cryoelectron tomography reveals doublet-specific structures and unique interactions in the I1 dynein. Proc. Natl. Acad. Sci. USA. 109:E2067–E2076 10.1073/pnas.1120690109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon T., Oyama T., Shimo-Kon R., Imamula K., Shima T., Sutoh K., Kurisu G. 2012. The 2.8 Å crystal structure of the dynein motor domain. Nature. 484:345–350 10.1038/nature10955 [DOI] [PubMed] [Google Scholar]
- Lindemann C.B. 2003. Structural-functional relationships of the dynein, spokes, and central-pair projections predicted from an analysis of the forces acting within a flagellum. Biophys. J. 84:4115–4126 10.1016/S0006-3495(03)75136-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.R., Nakatsugawa M. 2004. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J. Cell Biol. 166:709–715 10.1083/jcb.200406148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Kikkawa M. 2013. Novel structural labeling method using cryo-electron tomography and biotin-streptavidin system. J. Struct. Biol. 183:305–311 10.1016/j.jsb.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Oda T., Yanagisawa H., Yagi T., Kikkawa M. 2014. Mechanosignaling between central apparatus and radial spokes controls axonemal dynein activity. J. Cell Biol. 204:807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Agrin N., Leszyk J., Witman G.B. 2005. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170:103–113 10.1083/jcb.200504008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G., Ishikawa T. 2012. Axonemal radial spokes: 3D structure, function and assembly. BioArchitecture. 2:50–58 10.4161/bioa.20394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G., Bui K.H., Maheshwari A., Lupetti P., Diener D., Ishikawa T. 2011. Cryoelectron tomography of radial spokes in cilia and flagella. J. Cell Biol. 195:673–687 10.1083/jcb.201106125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.J., Malkova B., Walker M.L., Sakakibara H., Numata N., Kon T., Ohkura R., Edwards T.A., Knight P.J., Sutoh K., et al. 2012. ATP-driven remodeling of the linker domain in the dynein motor. Structure. 20:1670–1680 10.1016/j.str.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P., Azimzadeh J., Marshall W.F., King S.M. 2013. Analysis of ciliary assembly and function in planaria. Methods Enzymol. 525:245–264 10.1016/B978-0-12-397944-5.00012-2 [DOI] [PubMed] [Google Scholar]
- Schmidt H., Gleave E.S., Carter A.P. 2012. Insights into dynein motor domain function from a 3.3-Å crystal structure. Nat. Struct. Mol. Biol. 19:492–497: S1 10.1038/nsmb.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F.D., Satir P. 1974. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J. Cell Biol. 63:35–63 10.1083/jcb.63.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R., Song K., Yanagisawa H.-A., Fox L., Yagi T., Wirschell M., Hirono M., Kamiya R., Nicastro D., Sale W.S. 2013. The MIA complex is a conserved and novel dynein regulator essential for normal ciliary motility. J. Cell Biol. 201:263–278 10.1083/jcb.201211048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Diener D.R., Yang C., Kohno T., Pazour G.J., Dienes J.M., Agrin N.S., King S.M., Sale W.S., Kamiya R., et al. 2006. Radial spoke proteins of Chlamydomonas flagella. J. Cell Sci. 119:1165–1174 10.1242/jcs.02811 [DOI] [PMC free article] [PubMed] [Google Scholar]