Abstract
Biodegradable polymer double-wall microspheres (DWMS) are promising vehicles for macromolecular therapeutics such as proteins and peptides. Using precision particle fabrication (PPF) technology, uniform DWMS with outer diameter ~55 μm were fabricated comprising poly(lactide-co-glycolide) cores encapsulating bovine serum albumin (BSA) and ~10 μm thick, drug-free, poly(lactic acid) shells of varying PLA molecular weight. Also, monolithic single-wall microspheres (SWMS) were fabricated to mimic the BSA-loaded core. The use of relatively fast extracting ethyl acetate and slowly extracting dichloromethane as shell- and core-phase solvents, respectively, was found to produce DWMS with well-defined core-shell structure, high BSA encapsulation efficiency, and the desired localization of protein in the particle core. Initial protein distribution, particle erosion, and in vitro protein release from DWMS and SWMS were examined. The presence of a BSA-free shell in DWMS decreased the protein release rate and extended the duration of release from ~50 days to 70-80 days, demonstrating the capacity of such DWMS to provide enhanced control of protein delivery rates.
Keywords: monodisperse double-wall microspheres, poly(lactide-co-glycolide), poly(lactic acid), bovine serum albumin, controlled release
INTRODUCTION
Biodegradable polymer devices such as microspheres, rods and disks have been utilized as a means to deliver drugs in a controlled, predictable and minimally invasive manner 1-3. In particular, spherical microparticles with sizes ranging from a few to several hundred microns have been shown to provide controlled release of small molecule drugs as well as macromolecules 4-7, and several products have been commercialized. Biodegradable polymer particles offer several advantages such as minimally invasive administration, potential for high localized drug concentrations near the site of administration, relatively simple fabrication, and protection of fragile therapeutics. A limitation of these systems, however, is the difficulty of controlling drug release rates and, in particular, obtaining constant rate of release for prolonged times. Several approaches have been employed, with mixed success, to provide flexibility and control of drug release rates including (i) choice of polymer chemistry 8, (ii) conjugating drugs to the polymer 9, (iii) varying physical characteristics of particles 6,10-13, (iv) controlling particle size and size distribution 14-22, and (v) modifying particle structure 23-27.
Double-wall microspheres (DWMS), comprising a drug-loaded polymer core surrounded by a drug-free shell of the same or a different polymer, are promising devices for controlled release applications. DWMS are often fabricated by variations of the conventional emulsion/solvent extraction method, and formation of the core-shell structure is driven by phase separation of the two polymer components 28,29. Such methods typically produce DWMS with a broad distribution of size and shell thickness, and are limited to immiscible core and shell polymers at their thermodynamically stable configurations 30,31.
Using the precision particle fabrication technique (PPF) 32-36, however, we produced monodisperse DWMS with poly(lactide-co-glycolide) (PLG) cores and poly(lactic acid) (PLA) shells of uniform thickness. The model protein bovine serum albumin (BSA) was encapsulated in the PLG core phase, and the PLA shell did not contain drug. Also BSA-loaded PLG monolithic single-wall microspheres (SWMS) were produced to mimic the PLG core in DWMS. We hypothesized that the drug-free PLA layer of DWMS would provide better protein encapsulation efficiency as well as postpone protein release compared to SWMS.
In this study, we examined the influence of polymer solvents and molecular weights on the DWMS formation process, protein encapsulation and in vitro protein release rates. In addition, investigation of DWMS morphology, intraparticle protein distribution, and particle erosion provides insight into the mechanism of BSA release.
MATERIALS AND METHODS
Materials
Poly(lactide-co-glycolide) (PLG, Mw 4.2 kDa; lactide: glycolide 50:50), and poly(lactic acid) (PLA, Mw 43 kDa, 106 kDa and 192 kDa) were purchased from LACTEL Absorbable Polymers. Chromatography grade ethyl acetate (EtAc) and dichloromethane (DCM) were obtained from Sigma-Aldrich. Bovine serum albumin (BSA, Mw 66,700 Da) and dimethyl sulfoxide (DMSO) were purchased from Fisher Scientific. Fluorescent dye 5-(and-6)-carboxytetramethylrhodamine succinimidyl ester (TAMRA) was obtained from Molecular Probes. Poly(vinyl alcohol) (PVA, Mw 25,000 Da, 88% hydrolyzed) was purchased from Polysciences. Tween 80 was purchased from Acros Organics.
Double-wall and Single-wall Microsphere Fabrication
BSA (100 mg/mL in deionized water) was emulsified with PLG in DCM or EtAc (10% w/v) at a volumetric ratio of 1:10 aqueous: organic by sonication (CE Converter 102 C, Branson) at 60% amplitude for 1 min to form the core phase. The shell phase was 3% w/v PLA dissolved in DCM or EtAc. PVA water solution (0.5% w/v) was used as non-solvent carrier stream.
For DWMS fabrication, a triple concentric nozzle system was used. The core phase PLG/BSA emulsion passed through an inner metal nozzle, and the shell phase PLA solution passed through a concentric glass nozzle. An outermost glass nozzle was for PVA non-solvent carrier stream, which allowed production of particles smaller than the nozzle opening 17,18. For SWMS, a double nozzle system was employed, in which the core phase PLG/BSA emulsion passed through an inner metal nozzle, and a concentric glass nozzle was used for the PVA carrier stream. A frequency generator (Agilent 33220A) and piezoelectric transducer (CV33, Sonic & Materials Inc.) generated an acoustic wave on the nozzle system to break the exiting polymer streams into uniform droplets (Supplemental Information Fig. S1). Nascent DWMS and SWMS were collected in a 500 mL beaker containing 200-500 mL of 0.5% w/v PVA solution and were stirred for 3 h for organic solvent extraction and evaporation. The particles were filtered (Filter Paper #4, Whatman), washed three times by deionized water, and lyophilized for 48 h. Samples were stored until use in a −20 °C freezer with desiccant.
Particle Size Distribution
The size distributions of nascent particles (wet particles before lyophilizing) were determined using a Coulter Multisizer III (Beckman Coulter Inc.) with a 200 μm aperture in Isoton II. More than 10,000 particles were measured for each sample.
Protein Loading
Samples of approximately 5 mg were dissolved in 100 μL DMSO. The solution was pipetted into 1 mL of phosphate-buffered saline (PBS, pH 7.4±0.05) then incubated for 1 h at 37 °C with shaking at 240 rpm. The mixture was centrifuged for 10 minutes at 10,000 rpm, and BSA concentration in the supernatant was determined using BCA assay (Pierce). All absorbance measurements were taken on a SpectraMax 340 PC reader equipped with SoFTMax Pro software. The loading equaled the mass of BSA per mass of particles. The encapsulation efficiency equaled the actual BSA loading divided by theoretical BSA loading multiplied by 100.
Scanning Electron Microscopy (SEM)
DWMS and SWMS were prepared for imaging by placing a droplet of an aqueous particle suspension on a silicon stub. The samples were dried overnight and sputter coated with gold and platinum prior to imaging. In order to image the cross-sections, microspheres were frozen in liquid nitrogen and fractured using a blade on a glass slide. The JEOL 6060 LV scanning electron microscope was used at an acceleration voltage of 5-20 kV.
Confocal Fluorescence Microscopy
Twenty milligrams of BSA were dissolved in 2 mL of sodium bicarbonate (Fisher) at pH 8.3±0.05. A solution of 1 mg TAMRA in 100 μL DMSO (Fisher) was then pipetted into a foil-wrapped vial containing the BSA solution. The solution was stirred for 60 minutes at room temperature and then separated using PD-10 desalting column (GE Healthcare). The labeled protein was collected from the column, frozen, and lyophilized. The degree of labeling (DoL, the number of TAMRA molecules attached to each protein molecule) as determined from the relative absorbances of TAMRA and BSA was 3.40. Particles were loaded with 5% of TAMRA-labeled BSA and 95% unlabeled BSA.
Fluorescence and transmitted light images of the protein-loaded DWMS and SWMS were obtained with a Leica SP2 laser confocal microscope with a 63x oil-immersion lens. Fluorescence was excited using a HeNe laser (543 nm) and emission collected with a 575-640 nm band-pass filter.
In Vitro BSA Release
For each batch of DWMS or SWMS, a sample of approximately 30 mg was suspended in 1.25 mL release buffer consisting of 0.05% (v/v) Tween 80 (to prevent particle agglomeration) and PBS, pH 7.4. These samples were incubated at 37 °C with shaking (240 rpm). At various time points, 1.0 mL supernatant was removed and replaced with fresh media in order to maintain constant pH sink condition. Blank DMWS or SWMS (same fabrication parameters, except no protein was added) were treated the same way and the supernatants at various time points were collected as controls. The release study was performed in triplicate, and BSA concentrations in the collected supernatants were measured using BCA assay (Pierce) with absorbance corrected by absorbance of blank microspheres.
Particle Degradation/Erosion Study
For each batch of DWMS or SWMS, a sample of approximately 5 mg was suspended in 1.25 mL release buffer consisting of 0.05% (v/v) Tween 80 and PBS. These samples were incubated at 37 °C with shaking (240 rpm). At various time points, all supernatant was removed and the samples were frozen and lyophilized for at least 48 h. The samples were prepared for SEM as described above.
RESULTS
Production of Monodisperse BSA-loaded DWMS
To prepare DWMS, the PLG core and PLA shell materials were dissolved in either EtAc or DCM 37-39. Using DCM as both core and shell solvent (denoted as DCM(DCM), Fig. 1A), the particle size distribution was 54.8±1.4 μm. Using EtAc as shell solvent and using DCM as core solvent (EtAc(DCM), Fig. 1B), the particle size distribution was 55.1±2.0 μm. In both cases some particles smaller than the desired diameter were formed, but the volume percent of the main peaks were ~70%. However, when EtAc was used as core solvent with either DCM or EtAc as the shell solvent (DCM(EtAc), EtAc(EtAc)), the particle uniformity was poor (Supplemental Information Fig. S2).
Figure 1.
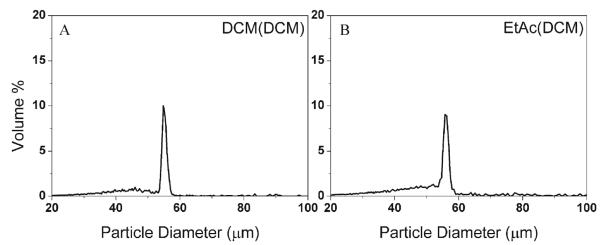
Size distributions of PLA(PLG) DWMS formed with different solvent configurations: (A) DCM(DCM); (B) EtAc(DCM).
We also investigated the effects of PLA molecular weight on particle fabrication and BSA encapsulation. Two sets of particles were produced: (i) EtAc(DCM) solvent configuration, increasing PLA shell molecular weight (43 kDa, 106 kDa); (ii) DCM(DCM) solvent configuration, increasing PLA shell molecular weight (43 kDa, 106 kDa, 192 kDa). PLG SWMS were also fabricated to mimic the PLG core in the DWMS.
Despite changing solvents and polymer molecular weights, the diameters of uniform DWMS were ~55 μm, and all samples were within 2 μm of each other (Table 1). Based on the measured outer diameter of DWMS, the core diameter as well as the shell thickness was calculated for DWMS (Table 1 and Supplemental Information). In all cases, the shell thickness was ~10 μm.
Table 1.
Dimensions of DWMS/SWMS
| Sample | Solvent Shell(Core) |
PLA Shell Mw (kDa) |
PLG core Mw (kDa) |
Outer Dia. Measured (μm) |
Core Dia. Calc. (μm) |
Shell Thickness Calc. (μm) |
|---|---|---|---|---|---|---|
| A1 | EtAc(DCM) | 43 | 4.2 | 55.1±2.0 | 35.8 | 9.7 |
| A2 | EtAc(DCM) | 106 | 4.2 | 56.8±2.8 | 36.9 | 10.0 |
| N/Aa | EtAc(DCM) | 192 | 4.2 | N/A | N/A | N/A |
| B1 | DCM(DCM) | 43 | 4.2 | 54.8±1.4 | 35.6 | 9.6 |
| B2 | DCM(DCM) | 106 | 4.2 | 55.4±1.7 | 36.0 | 9.7 |
| B3 | DCM(DCM) | 192 | 4.2 | 56.6±2.1 | 36.8 | 9.9 |
| O | (DCM) | N/A | 4.2 | 35.2 ±1.0 | 35.2 | N/A |
PLA Mw=192 kDa’s chirality changed from poly(D, L-lactide) to poly(L-lactide) and cannot be dissolved in EtAc
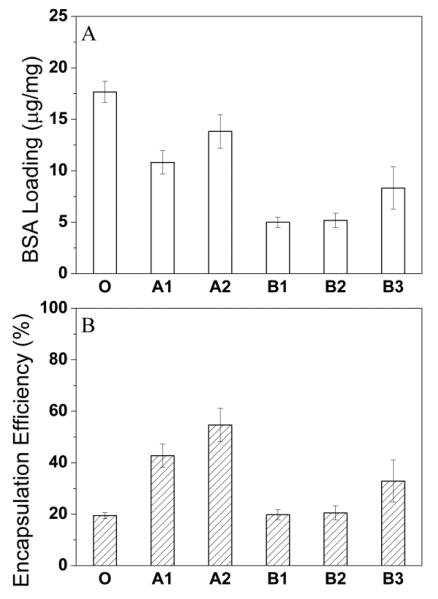
BSA loading and encapsulation efficiency of EtAc(DCM) DWMS were in general higher than DCM(DCM) DWMS (Fig. 2). This is likely due to faster extraction of the shell solvent, EtAc 40, which results in rapid formation of a polymer-rich shell preventing loss of BSA from the particle core. When DCM was used as shell solvent, the slower removal of DCM from both shell and core may allow BSA transport toward the particle surface. In general, BSA encapsulation efficiency increased with the shell polymer molecular weight, most likely due to increased PLA hydrophobicity and/or increased solution viscosity, which could better confine the BSA/water phase of the emulsion in the PLG core region 41. The encapsulation efficiency of single-wall microspheres (sample O) was lower than all DWMS. The lack of a shell layer may lead to easier transport and escape of BSA out of the microspheres.
Figure 2.
BSA loading (A) and encapsulation efficiency (B) of DWMS/SWMS: (O) (DCM), PLG Mw 4.2 Da; (A1) EtAc(DCM), PLG Mw 4.2 kDa and PLA Mw 43 kDa; (A2) EtAc(DCM), PLG Mw 4.2 kDa and PLA Mw 106 kDa; (B1) DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 43 kDa; (B2) DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 106 kDa; (B3) DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 192 kDa.
BSA Distribution in DWMS and SWMS
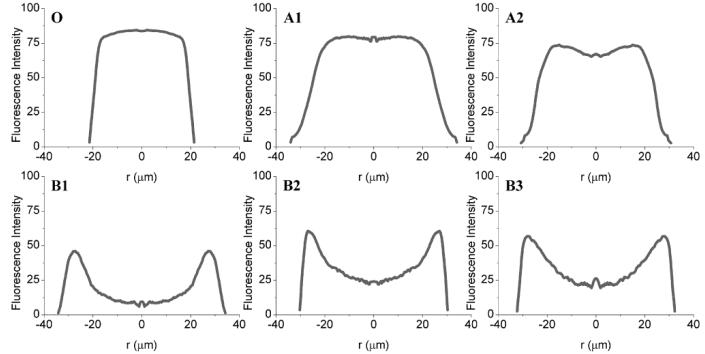
Confocal fluorescence microscopy allowed visualization of the spatial distribution of TAMRA-labeled BSA within SWMS and DWMS (Supplemental Information Fig. S3), and image analysis of the micrographs provided average radial fluorescence intensities of the particles (Fig. 3). TAMRA-BSA distribution within SWMS (Sample O) was relatively uniform across the particles, as expected. For EtAc(DCM) DWMS A1 and A2, the TAMRA-BSA fluorescence was concentrated in the core area and drug-free regions near the particle surface were observed. In DCM(DCM) DWMS, samples B1-B3, TAMRA-BSA was not confined in the core, but appeared to spread throughout the particles and tended to be concentrated near the surface.
Figure 3.
Average radial fluorescence intensity of TAMRA-BSA within DWMS/SWMS: (O) (DCM), PLG Mw 4.2 Da; (A1) EtAc(DCM), PLG Mw 4.2 kDa and PLA Mw 43 kDa; (A2) EtAc(DCM), PLG Mw 4.2 kDa and PLA Mw 106 kDa; (B1) DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 43 kDa; (B2) DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 106 kDa; (B3) DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 192 kDa.
BSA In Vitro Release
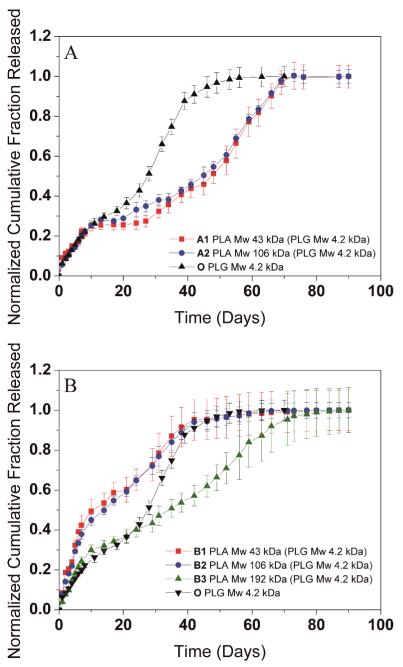
The release of protein from polymer microparticles is controlled by a combination of particle size, initial protein distribution, polymer degradation rates and other factors such as architecture of the microparticles and pore formation during degradation. We investigated the release of BSA from DWMS formed with EtAc(DCM) and DCM(DCM) solvent configurations and of varying PLA shell molecular weights (Fig. 4). In general, all samples exhibited a tri-phasic release: an initial rapid release, a lag phase exhibiting slow release, and a final steady release. Release from samples A1 and A2, prepared with EtAc(DCM) solvent configuration and differing PLA shell molecular weights, were almost identical (Fig. 4A). For SWMS of the same size as the PLG core (Sample O), complete release occurred at around 55 days, while for A1 and A2, complete release was delayed to 70 days. Also, the BSA release rates from particles A1 and A2 in the steady release phase were slower than that of O. These release profiles showed that the presence of the drug-free PLA shell postponed the BSA release rate from the PLG core, but the molecular weight of the PLA shell had no influence.
Figure 4.
In vitro release of BSA from DWMS/SWMS. (A) Sample A1, A2, O (B) Sample B1, B2, B3, O
DCM(DCM) particles exhibited different behavior (Fig. 4B). BSA release profiles from B1 and B2 were similar to each other despite the varying molecular weight of the PLA shell, and release was relatively fast compared to SWMS. For sample B3, however, the release rate was much slower, and near constant release was maintained from 10-75 days.
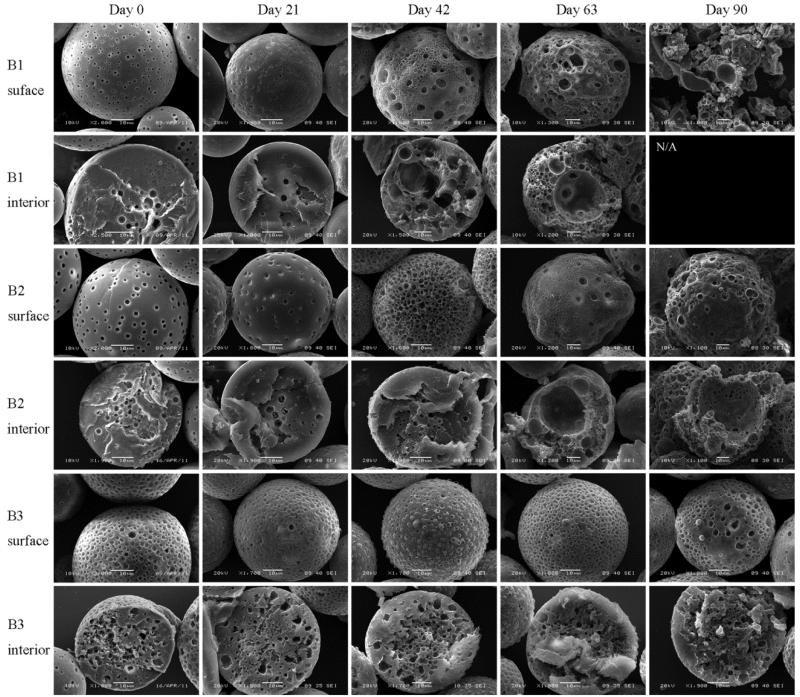
Particle Morphology During In Vitro DWMS Degradation/Erosion
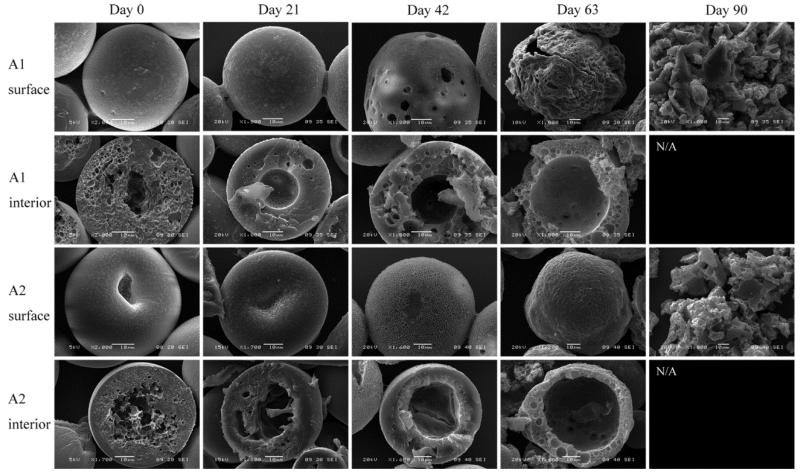
DWMS were incubated in PBS at 37 °C for a period of three months and samples were removed periodically for visualization by SEM. Initially, small pores were apparent on the surface of A1 and A2, which were probably caused by EtAc extraction. Large concave indentions were observed on the surfaces of A2, which might be caused by the dense PLA shell collapsing toward the porous PLG inner core during particle hardening. The cores of A1 and A2 were clearly porous, in accordance with confocal images showing BSA concentrated in the PLG core. The less porous shell areas appeared to be approximately 10 μm thick, as calculated (Table 1). A1 and A2 developed surface pores as degradation progressed. Both A1 and A2 also developed hollow cores by day 21. The hollow region grew larger and the shells became thinner through day 63. By day 90, the DWMS lost their particle morphology and appeared to break into pieces (Fig. 5). Overall, A2 appeared to erode slower than A1, likely due to the higher molecular weight of PLA (106 kDa compared to 43 kDa) in A2 shell phase.
Figure 5.
SEM images of A1, A2 degradation/erosion study. Sample A1, EtAc(DCM), PLG Mw 4.2 kDa and PLA Mw 43 kDa; Sample A2, EtAc(DCM), PLG Mw 4.2 kDa and PLA Mw 106 kDa.
The surfaces of DCM(DCM) particles B1-B3 were initially much more porous than A1 and A2. No clearly defined core and shell regions could be identified. Erosion rates of B1, B2 and B3 decreased with increasing the PLA molecular weight. Sample B1 lost particulate morphology by day 90 while B2 and B3 appeared intact. For B1 and B2 hollow core regions appeared by day 63. For B3, due to the high PLA molecular weight, no hollow core areas were observed throughout three months (Fig. 6).
Figure 6.
SEM images of B1, B2 and B3 degradation study. Sample B1, DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 43 kDa; Sample B2, DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 106 kDa; Sample B3, DCM(DCM), PLG Mw 4.2 kDa and PLA Mw 192 kDa.
DISCUSSION
Core-shell DWMS provide a more complex delivery system compared to conventional monolithic microspheres providing more flexibility to achieve desired properties and release profiles. By using precision particle fabrication technique, we were able to produce uniform DWMS comprising PLA and PLG as the shell and core phases, respectively, with BSA loaded within the PLG core, overall diameters of ~55 μm, and 10-μm thick shells. Because the calculated core diameters of DWMS were ~35-37 μm, SWMS with a diameter of 35 μm were also produced. Thus, we can exclude the influence of differing particle sizes and structure on the release of BSA and can elucidate the impact of BSA initial distribution and polymer degradation/erosion on the final BSA release rate.
In the confocal micrographs, samples A1 and A2 exhibited high fluorescence intensity within 20 μm of the particle center, which gradually diminished closer to the surface (Fig. 3). In addition, SEM showed that the cores of these particles were highly porous, and the porosity was less near the particle surfaces (Fig. 5). These data suggest that the BSA was encapsulated primarily in the ~35 μm core with ~10 μm PLA shell containing much less protein. The presence of the PLA shell increased the BSA loading and encapsulation efficiency compared to SWMS (Fig. 2A and B), presumably by providing a barrier through which the BSA-containing dispersed phase of the emulsion must travel to reach the particle surface and allow BSA to escape. Most importantly, the presence of the drug-free PLA shell reduced the rate of BSA release and extended the duration of the release profile from ~55 to ~70 days (Fig. 4A). Surprisingly, however, release from samples A1 and A2 was essentially identical despite the difference in PLA molecular weight, most likely due to the deformed surfaces of A2.
For all three samples fabricated with both PLA and PLG in DCM, B1-B3, confocal fluorescence microscopy revealed that BSA was localized preferentially near the particle surface (Fig. 3). This is most likely due to the relatively slow removal of DCM from both the core and the shell, allowing time for the BSA/water droplets of the primary emulsion to coalesce and migrate toward the particle surface. The porous surfaces of these particles support this explanation (Fig. 6). As a result of the redistribution of BSA toward the surface, loading and encapsulation efficiency were lower for B1-B3 compared to A1 and A2. In addition, the BSA distribution appears to have resulted in faster release from B1 and B2, as might be expected. Release from sample B3, comprising a 192 kDa poly(L-lactide) shell, was significantly slower despite a similar localization of BSA near the particle surface. This slower release is most likely due to the very high molecular weight of the PLA (and perhaps exacerbated by the presumed (semi)-crystallinity of poly(L-lactide)42,43, though Tg was not determined here), both of which provide a dense, hydrophobic polymer matrix that degrades slowly (Fig. 6).
CONCLUSIONS
In this study, monodisperse double-wall microspheres as well as monolithic microspheres were fabricated using precision particle fabrication technology to investigate the potential of the core-shell morphology for controlling protein release rates. Production of the desired core-shell particles required appropriate choice of solvents. DCM(DCM) as well as EtAc(DCM) solvent configurations resulted in good DWMS uniformity. In addition, the solvent choice, together with the PLA molecular weight in the shell, influenced the rate of particle erosion, intraparticle protein distribution and in vitro protein release. Fabrication of DWMS with a faster-extracting solvent for the shell phase (EtAc(DCM)) was critical for production of DWMS with clear core-shell structures and encapsulation of protein primarily within the PLG core, leading to delayed protein release compared to SWMS. Due to the slower particle hardening process, protein redistributed to regions near the surface of DCM(DCM) DWMS, resulting in relatively fast protein release.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health Grant EB005181 and GM085222. Scanning electron micrographs were obtained in the Frederick Seitz Materials Research Laboratory Central Facilities, University of Illinois. Confocal microscopy measurements were obtained in the Beckman Institute Imaging Technology Group at University of Illinois.
REFERENCES
- 1.Pillai O, Panchagnula R. Polymers in drug delivery. Current opinion in chemical biology. 2001;5(4):447–451. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 2.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388(6645):860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 3.Okada H, Toguchi H. Biodegradable microspheres in drug delivery. Critical reviews in therapeutic drug carrier systems. 1995;12(1):1–99. doi: 10.1615/critrevtherdrugcarriersyst.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- 4.Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. Journal of controlled release : official journal of the Controlled Release Society. 2003;90(3):261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 5.Gombotz WR, Pettit DK. Biodegradable Polymers for Protein and Peptide Drug Delivery. Bioconjugate Chem. 1995;6(4):332–351. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 7.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nature biotechnology. 2003;21(10):1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 8.Bezemer JM, Radersma R, Grijpma DW, Dijkstra PJ, van Blitterswijk CA, Feijen J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers: 2. Modulation of release rate. Journal of Controlled Release. 2000;67(2-3):249–260. doi: 10.1016/s0168-3659(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 9.Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Preparation and characterization of poly (D,L-lactide-co-glycolide) microspheres for controlled release of poly(L-lysine) complexed plasmid DNA. Pharm Res-Dord. 1999;16(4):509–513. doi: 10.1023/a:1018862827426. [DOI] [PubMed] [Google Scholar]
- 10.Amsden BG, Goosen MFA. An examination of factors affecting the size, distribution and release characteristics of polymer microbeads made using electrostatics. Journal of Controlled Release. 1997;43(2-3):183–196. [Google Scholar]
- 11.Dang W, Saltzman WM. Controlled release of macromolecules from a degradable polyanhydride matrix. Journal of biomaterials science Polymer edition. 1994;6(3):297–311. doi: 10.1163/156856294x00374. [DOI] [PubMed] [Google Scholar]
- 12.Leong KW, Brott BC, Langer R. Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. Journal of biomedical materials research. 1985;19(8):941–955. doi: 10.1002/jbm.820190806. [DOI] [PubMed] [Google Scholar]
- 13.Rafati H, Coombes AGA, Adler J, Holland J, Davis SS. Protein-loaded poly(dl-lactide-co-glycolide) microparticles for oral administration: formulation, structural and release characteristics. Journal of Controlled Release. 1997;43(1):89–102. [Google Scholar]
- 14.Sansdrap P, Moës AJ. Influence of manufacturing parameters on the size characteristics and the release profiles of nifedipine from poly(DL-lactide-co-glycolide) microspheres. Int J Pharmaceut. 1993;98(1–3):157–164. [Google Scholar]
- 15.Men Y, Thomasin C, Merkle HP, Gander B, Corradin G. A single administration of tetanus toxoid in biodegradable microspheres elicits T cell and antibody responses similar or superior to those obtained with aluminum hydroxide. Vaccine. 1995;13(7):683–689. doi: 10.1016/0264-410x(94)00046-p. [DOI] [PubMed] [Google Scholar]
- 16.Shiga K, Muramatsu N, Kondo T. Preparation of poly(D,L-lactide) and copoly(lactide-glycolide) microspheres of uniform size. J Pharm Pharmacol. 1996;48(9):891–895. doi: 10.1111/j.2042-7158.1996.tb05995.x. [DOI] [PubMed] [Google Scholar]
- 17.Berkland C. Chemical and Biomolecular Engineering. University of Illinois at Urbana-Champaign; Urbana-Champaign: 2003. Control of Micro- and Nano- Sphere Size Distributions: Implications in Drug Delivery. [Google Scholar]
- 18.Berkland C, Kim K, Pack DW. PLG microsphere size controls drug release rate through several competing factors. Pharm Res. 2003;20(7):1055–1062. doi: 10.1023/a:1024466407849. [DOI] [PubMed] [Google Scholar]
- 19.Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release. 2001;73(1):59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 20.Berkland C, King M, Cox A, Kim K, Pack DW. Precise control of PLG microsphere size provides enhanced control of drug release rate. J Control Release. 2002;82(1):137–147. doi: 10.1016/s0168-3659(02)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkland C, Kipper MJ, Narasimhan B, Kim KK, Pack DW. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J Control Release. 2004;94(1):129–141. doi: 10.1016/j.jconrel.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Berkland C, Pollauf E, Raman C, Silverman R, Kim K, Pack DW. Macromolecule release from monodisperse PLG microspheres: control of release rates and investigation of release mechanism. J Pharm Sci. 2007;96(5):1176–1191. doi: 10.1002/jps.20948. [DOI] [PubMed] [Google Scholar]
- 23.Berkland C, Pollauf E, Pack DW, Kim K. Uniform double-walled polymer microspheres of controllable shell thickness. J Control Release. 2004;96(1):101–111. doi: 10.1016/j.jconrel.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Berkland C, Cox A, Kim K, Pack DW. Three-month, zero-order piroxicam release from monodispersed double-walled microspheres of controlled shell thickness. J Biomed Mater Res A. 2004;70(4):576–584. doi: 10.1002/jbm.a.30114. [DOI] [PubMed] [Google Scholar]
- 25.Pekarek KJ, Jacob JS, Mathiowitz E. Double-walled polymer microspheres for controlled drug release. Nature. 1994;367(6460):258–260. doi: 10.1038/367258a0. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Wang J, Wang C-H. Double-walled microspheres for the sustained release of a highly water soluble drug: characterization and irradiation studies. Journal of Controlled Release. 2002;83(3):437–452. doi: 10.1016/s0168-3659(02)00235-3. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y-Y, Shi M, Goh S-H, Moochhala SM, Ng S, Heller J. POE/PLGA composite microspheres: formation and in vitro behavior of double walled microspheres. Journal of Controlled Release. 2003;88(2):201–213. doi: 10.1016/s0168-3659(02)00491-1. [DOI] [PubMed] [Google Scholar]
- 28.Pekarek KJ, Jacob JS, Mathiowitz E. One-step preparation of double-walled microspheres. Adv Mater. 1994;6(9):684–687. [Google Scholar]
- 29.Kokai LE, Tan H, Jhunjhunwala S, Little SR, Frank JW, Marra KG. Protein bioactivity and polymer orientation is affected by stabilizer incorporation for double-walled microspheres. Journal of Controlled Release. 2010;141(2):168–176. doi: 10.1016/j.jconrel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Torza S, Mason SG. Three-phase interactions in shear and electrical fields. Journal of Colloid and Interface Science. 1970;33(1):67–83. [Google Scholar]
- 31.Hobbs SY, Dekkers MEJ, Watkins VH. Effect of interfacial forces on polymer blend morphologies. Polymer. 1988;29(9):1598–1602. [Google Scholar]
- 32.Foster CA, Kim K, Turnbull RJ, Hendricks CD. Apparatus for producing uniform solid spheres of hydrogen. Rev Sci Instrum. 1977;48(6):625–631. [Google Scholar]
- 33.Kim NK, Kim K, Payne DA, Upadhye RS. Fabrication of hollow silica aerogel spheres by a droplet generation method and sol-gel processing. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 1989;7(3):1181–1184. [Google Scholar]
- 34.Kim K, Jang KY, Upadhye RS. Hollow Silica Spheres of Controlled Size and Porosity by Sol-Gel Processing. J Am Ceram Soc. 1991;74(8):1987–1992. [Google Scholar]
- 35.Gilliard RP, Kim K, Turnbull RJ. Spherical hydrogen pellet generator for magnetic confinement fusion research. Rev Sci Instrum. 1981;52(2):183–190. [Google Scholar]
- 36.Xu Q, Xia Y, Wang C-H, Pack DW. Monodisperse double-walled microspheres loaded with chitosan-p53 nanoparticles and doxorubicin for combined gene therapy and chemotherapy. Journal of Controlled Release. 2012;163(2):130–135. doi: 10.1016/j.jconrel.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langer R. New methods of drug delivery. Science. 1990;249(4976):1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 38.Bindschaedler C, Leong K, Mathiowitz E, Langer R. Polyanhydride microsphere formulation by solvent extraction. J Pharm Sci-Us. 1988;77(8):696–698. doi: 10.1002/jps.2600770811. [DOI] [PubMed] [Google Scholar]
- 39.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther. 2004;4(1):35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 40.Meng FT, Ma GH, Qiu W, Su ZG. W/O/W double emulsion technique using ethyl acetate as organic solvent: effects of its diffusion rate on the characteristics of microparticles. J Control Release. 2003;91(3):407–416. doi: 10.1016/s0168-3659(03)00273-6. [DOI] [PubMed] [Google Scholar]
- 41.Cam D, Hyon SH, Ikada Y. Degradation of high molecular weight poly(L-lactide) in alkaline medium. Biomaterials. 1995;16(11):833–843. doi: 10.1016/0142-9612(95)94144-a. [DOI] [PubMed] [Google Scholar]
- 42.Kim HK, Park TG. Comparative study on sustained release of human growth hormone from semi-crystalline poly(L-lactic acid) and amorphous poly(D,L-lactic-co-glycolic acid) microspheres: morphological effect on protein release. J Control Release. 2004;98(1):115–125. doi: 10.1016/j.jconrel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Perego G, Cella GD, Bastioli C. Effect of molecular weight and crystallinity on poly(lactic acid) mechanical properties. J Appl Polym Sci. 1996;59(1):37–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.