Abstract
Out-of-hospital cardiac arrest (OHCA) has attracted increasing attention over the past years because outcomes have improved impressively lately. The changes for neurological intact outcomes has been poor but several areas have achieved improving survival rates after adjusting their cardiac arrest care. The pre-hospital management is certainly key and decides whether a cardiac arrest patient can be brought back into a spontaneous circulation. However, the whole chain of resuscitation including the in-hospital care have improved also. This review describes aetiologies of OHCA, risk and potential protective factors and recent advances in the pre-hospital and in-hospital management of these patients.
Keywords: Out-of-hospital cardiac arrest, resuscitation, sudden cardiac arrest, ventricular fibrillation.
INTRODUCTION
Sudden cardiac arrest (SCA) and sudden cardiac death (SCD) refer to the sudden collapse of cardiac activity with hemodynamic compromise, either due to sustained ventricular tachycardia (VT)/ventricular fibrillation (VF), asystole or pulseless electric activity (PEA). There are also non-cardiac etiologies for sudden circulatory collapse (Table 1) [1]. The most common cause of cardiac arrest is acute or chronic coronary artery disease but various other cardiac and non-cardiac related diseases are also well recognised, as initially disputed (Table 1). Sudden out of hospital cardiac arrest accounts for around 0.5 - 1 deaths per 1000 population every year [2]. In North America alone, more than 300.000 deaths per year are attributed to SCA [3]. The survival rate has remained very low for several decades, with only 8-10 % of patients surviving to hospital discharge and in many rural areas survival is dramatically less [4]. Survival and long-term functional outcome are closely related to the underlying cause such as initial rhythm.
RISK FACTORS FOR SUDDEN CARDIAC DEATH
The high mortality associated with SCA emphasises the need for early identification of patients at risk. However, very little is known about risk factors. Since coronary artery disease is the most important cause of SCA, cardiovascular risk factors also increase the risk of OHCA and this is especially the case for diabetes and smoking but less so for obesity [8, 9].
However, our understanding of the relationship between acute myocardial ischemia and its most fatal immediateconsequence, cardiac arrhythmia, remains very limited. We know that ischemia alters repolarisation and it prolongs the QT interval [10]. The extent of QT prolongation during an acute coronary artery occlusion depends on the degree of collateralisation [10, 11]. Intriguingly, there is increasing evidence that the collateral circulation has a protective role during early ischemia. A study in 170 patients with acute anterior infarction showed lower incidence for malignant arrhythmias (defined as VF, VT or high degree atrioventricular (AV) block and lower mortality in patients with angiographically well-developed collaterals [12]. Furthermore, a well-developed collateral circulation has been associated with reduced risk for cardiac and all-cause mortality in patients with stable coronary artery disease in general [13].
ELECTROPHYSIOLOGICAL RISK ASSESSMENT
Testing of the electrical vulnerability to ventricular arrhythmia remains elusive. This is primarily due to the dynamic nature of the electrophysiological behavior of the myocardium. Although invasive electrophysiological testing to evaluate the inducibility of ventricular arrhythmia has been advocated, the validity of this approach is uncertain. Non-invasive investigations have been developed to estimate the susceptibility to arrhythmias [14]. These tests examine different aspects of myocardial electrophysiology as reflected on the surface electrocardiogram (ECG), namely change of autonomic function due to increased circulating catecholamines, delay in myocardial conduction, prolongation of repolarization and stretch-induced after-depolarisations.
The clinical usefulness of these tests is controversial. The highest diagnostic yield may be a combination of tests given the limited predictive value of each individual investigation [15].
THE RISK OF SCD IN ATHLETES
Overall, the risk of SCD is estimated to be between 1:50.000 and 1:300.000 in athletes over a 10-20 years period [16-18].
Due to their increased physical activity, athletes, and especially those involved in competitions, are at particular risk of SCA in the presence of conditions such as hypertrophic cardiomyopathy, abnormal origin of the coronary arteries, myocarditis, arrhythmic right ventricular cardiomyopathy (ARVC), mitral valve prolapse, aortic stenosis, coronary arteriosclerosis [19]. The European Society of Cardiology (ESC) has published guidelines for the pre-participation screening of young competitive athletes in 2005 [20]:
Complete personal and family history and physical examination
12 lead ECG
Any abnormal findings warrant further examination (e.g. echocardiography or cardiac magnetic resonance imaging (CMR)
Reevaluation after two years
Similarly, a family screening of patients who experienced unexplained OHCA has to be considered. An ECG and stress-testing, echocardiography or magnetic resonance imaging (MRI) but also genetic testing may be useful in this setting.
MANAGEMENT OF SCA
The European Resuscitation Council (ERC) updated their guidelines on the treatment of SCA in 2010 [21]. The main changes compared to the 2005 guidelines in Basic Life Support (BLS) were the introduction of a compression/ventilation ratio of 30:2 as compared to 15:2 to optimize maintenance of circulation and reduce the “hands-off” periods. There is increasing evidence that continuous chest compressions (“hands-only cardio- pulmonary resuscitation (CPR) without ventilation, might not entail adverse consequences on neurologic outcome, at least within the first few minutes after SCA [22].
However, the debate on the ratio of chest compressions and ventilations during CPR and the continuous adaption of guidelines may have confused lay people and prevented them from performing CPR. Nose-to-mouth or mouth-to-mouth ventilation recommended during CPR may further deter people fearing infection. Moreover, the use of automated external defibrillators (AED) is clearly recommended.
It is a matter of an ongoing debate, whether CPR should be applied immediately or whether early defibrillation should be preferred in patients after SCA. While current guidelines advocate immediate defibrillation, both approaches seem to have comparable results and in patients with a cardiac arrest >5 minutes, chest compressions before defibrillation may be superior [23].
Importantly, patient transfer with ongoing CPR results in reduced quality of chest compressions. However, with the new mechanical chest compression devices (e.g., LUCAS (Jolife, Lund, Sweden) and AutoPulse (Zoll Circulation, Chelmsford, Massachusetts, USA) adequate chest compression quality can be maintained during transport [24]. However, there is currently insufficient evidence for a clear advantage over manual chest compressions with regard to clinical outcomes when using the LUCAS device [25]. Similarly, the Circulation Improving Resuscitation Care (CIRC) trial assessing the effect of the AutoPulse automatic chest compression device in >4000 OHCA patients failed to show a survival benefit. However, the data of this trial are not published yet.
The primary aim of post cardiac arrest return of spontaenous circulation (post-ROSC) care is to optimize cardio-cerebral recovery. In-hospital aims include maintaining cardiac output and cerebral perfusion, optimising systemic haemodynamics and minimising ischaemia-reperfusion injury [26]. The importance of optimal post-resuscitation care is highlighted in the recent 2010 International Liaison Committee on Resuscitation (ILCOR) Consensus on CPR Science with Treatment Recommendations (CoSTR) and ERC Guidelines [27, 28]. Table 2 gives an overview of a recommended diagnostic work-up of patients who survived SCA.
MANAGEMENT OF OHCA
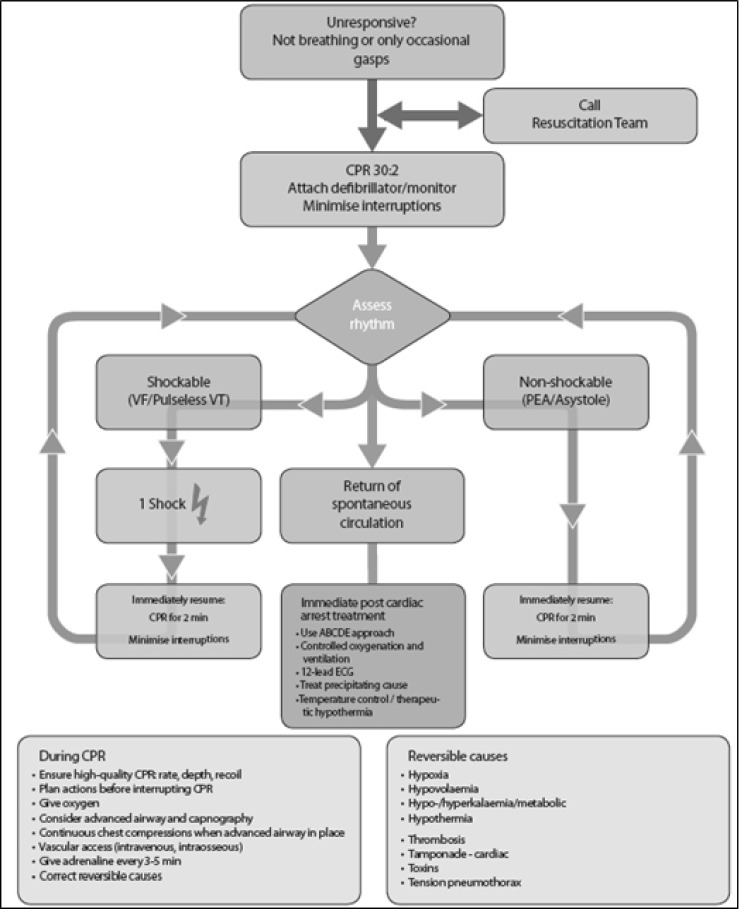
An updated Advanced Life Support (ALS) algorithm for medical professionals is shown in Fig. (1).
Fig. (1).
Algorithm for Advanced Life Support (ALS) [21].
ROLE OF HEART CATHETERIZATION AND PERCUTANEOUS INTERVENTION AFTER SCA
Importantly, current guidelines recommend immediate referral of patients after OHCA to a cardiac centre with onsite cardiac catheterisation facilities in patients after SCA [21]. The decision by the pre-hospital emergency medical service (EMS) provider where to admit the patient after an OHCA is crucial. Several non-randomised observational studies have demonstrated survival benefit from early angiography post-OHCA compared to no coronary angiography or percutaneous coronary intervention (PCI) [29, 30]. However, other studies have also highlighted an increased complication risk if early angiography is performed in these patients [31]. In our view, although the role of immediate coronary angiography is controversial, substantial information on the coronary circulation can guide in-hospital management beyond coronary intervention. For example, in patients with cardiogenic shock, an intra-aortic balloon pump (IABP) or other support devices (e.g. Impella 2.5) can be inserted at this occasion to augment cardiac output [32].
Moreover, approximately 80% of OHCA presenting with VF or VT are cardiac in origin and these patients may benefit from an early PCI [33]. Studies are currently under way to determine whether patients who fail to achieve return of spontaneous circulation (ROSC) at the scene and who are suspected to have obstructive CAD may benefit from PCI whilst receiving continuous CPR [34]. Importantly, PCI plays a major role in the improved survival rates [35, 36]. In a study of 714 OHCA patients referred to a tertiary centre in Paris, 435 (61%) had no obvious extracardiac cause. This subgroup underwent early coronary angiography and 70% of those had at least one significant coronary lesion [30].
To facilitate decision making, an ECG should be recorded as soon as possible after ROSC to assess for ST-elevation or (new) LBBB [37]. However, the ECG has a limited accuracy in the setting of SCA. The absence of ST-segment elevation does not exclude the presence of critical coronary stenoses. In approximately 50% of OHCA survivors despite the absence of ST-segment elevation in the post-arrest ECG, a significant coronary artery stenosis can be found; [29, 30] However, even though these coronary artery stenoses were regarded “significant”, it remains unclear whether these stenoses are actually the cause for the cardiac arrest and whether revascularising these lesions can improve the clinical outcome. Unfortunately, the evidence in this area is very scant, cardiac arrest patients have been excluded from most acute myocardial infarction trials, which has created a gap of evidence for these patients. While non-cardiac arrest patients with ST elevation infarctions clearly benefit from immediate angiography/ PCI, we lack data for patients after a cardiac arrest.
HYPOTHERMIA
Based on experience with avalanche victims who had good neurological outcome despite very prolonged circulatory arrest under the circumstance of hypothermia, the concept of therapeutic hypothermia has been proposed in patients with OHCA. As to date, it is the only post-ROSC intervention shown to improve survival from OHCA [38]. Two major randomised clinical trials (the Hypothermia After Cardiac Arrest trial from Europe [39] and a smaller trial from Australia [40]) have demonstrated the efficacy of this intervention. The exact mechanism of the protective effects of mild therapeutic hypothermia (MTH) remains to be determined but probably includes a reduction of ischaemia-reperfusion injury and a reduction in oxidative stress. During cardiac arrest, brain tissue becomes ischaemic. Following ROSC, rapid re-oxygenation leads to oxygen free radical production, which can lead to secondary cell death. MTH has pleiotropic neuroprotective effects. MTH slows down cellular metabolism, altering biochemical and signaling pathways and reduces oxygen demand. Comatose (i.e., lack of meaningful response to verbal commands) adult patients with ROSC after out-of-hospital VF cardiac arrest should be cooled to 32°C to 34°C for at least 12 to 24 hours. MTH can also be considered following OHCA with non-shockable initial rhythms such as PEA or asystole but the benefit in these patients is less clear [28, 41].
Many questions surrounding MTH remain unanswered. The timing of initiation and the optimal duration of cooling are unclear. Should MTH be initiated pre-hospital or is it sufficient to start this therapy in hospital? Some experimental studies suggest that early cooling improves outcome; but a randomised clinical trial failed to show superiority for pre-hospital cooling versus in-hospital initiated cooling [42]. The time frame during which MTH can be initiated is unknown. The length of time MTH should be maintained is also uncertain. In the Australian trial patients were cooled for 12 hours [40], while in the Hypothermia After Cardiac Arrest (HACA) trial they were cooled for 24 hours [39]. Longer duration may be superior. [43]
The optimal cooling method is not yet clear [38]. External cooling methods include cold pads and cooling caps, whilst invasive cooling methods include cold intra-venous saline or intra-vascular cooling catheters. Recently, intra-nasal evaporative cooling has been shown to produce effective pre-hospital cooling [44]. However, 30 mL/kg of intravenous 4°C saline or Hartmann’s solution is the simplest and most cost effective method for pre-hospital setting. The target core temperature is 33 ± 1°C. Intravascular devices have been shown to be effective in inducing and maintaining this target temperature; however, there can be delays in inserting the intravascular catheter and this is more invasive than other techniques. Surface cooling, using cooling blankets and ice packs to the axilla, groin and neck, has also been advocated. These devices are simple, require less operator experience, and are inexpensive, but initial cooling may be slower [45]. One study has shown that both intravascular and surface cooling are equivalent in their effectiveness to reach and maintain core temperatures [46], although another showed better temperature control with an intravascular device [47]. Whichever method is used, a feedback loop is advocated to ensure target temperature compliance and to prevent overcooling. Devices with feedback control also enable better control of rewarming (usually at 0.25oC/h).
Despite strong evidence and clear guideline recommendations, MTH is still under-used in some regions of the world. In a recent trial of an impedance threshold device for OHCA in the United States and Canada, just 48% of the 2289 enrolled patients admitted hospital were treated with therapeutic hypothermia [48]. On the other hand, in the UK 86% of intensive care units had implemented hypothermia by 2009 [49]. Lack of resources and cost of MTH are commonly cited as barriers to implementation, despite relatively cheap options being available.
However, in patients with MTH several side effects may occur. MTH may affect the coagulation cascade and platelet function, eventually leading to an increased bleeding risk [50, 51]. In case of bleeding, the patient should be rewarmed >35°C body temperature. Moreover, leucocyte function may be decreased resulting in an increased risk for infections [39]. On the ECG, bradycardia and a prolongation of the QT interval may be detected [52]. In rare cases, severe arrhythmias are provoked by MTH. Further, due to renal side effects, the electrolyte balance may be altered and careful monitoring is mandatory [53, 54]. Additionally, the metabolisation of several drugs may be altered by MTH [54].
SECONDARY PREVENTION OF SCA
In patients who survived SCA, strategies are needed to prevent future, potentially fatal events. The mechanisms of death were evaluated in several studies. The main cause of death in these trials was low cardiac output due to progressive heart failure (45-50%), severe arrhythmia (20-35%) and non-cardiac related death (e.g. renal disease, ca. 20-30%) [55, 56]. Table 3. refers to the European Society of Cardiology (ESC) guidelines on decision making on implantable cardioverter defibrillator (ICD) implantation for secondary prevention of SCA due to severe arrhythmia [57, 58]. Additional therapies including VT ablation or permanent antiarrhythmic therapy may be warranted in selected cases. However, as outlined previously severe arrhythmias are closely related to an impaired left ventricular function, and an optimal heart failure management will be mandatory in this patient population [59].
PROGNOSIS AFTER SCA
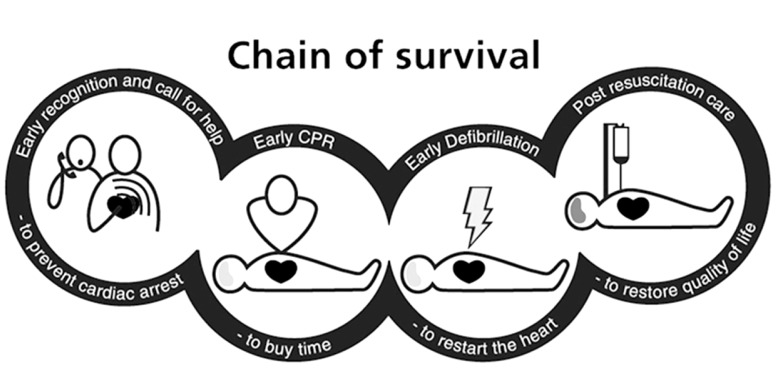
The Time to defibrillation and other factors such as bystander CPR, has not improved over precise time [60]. The chain of survival (Fig. 2) is changing over time and it is likely that it will soon include pre-hospital induction of hypothermia and early coronary intervention. Overall, such changes have increased the survival rate. In Sweden for instance, the survival rate after OHCA has doubled over the last years [61].
The prognosis after cardiac arrest is determined by several factors. In case of asystole or PEA as the initial rhythm, a prolonged cardiac arrest must be assumed and therefore only 10% of patients will survive until hospital admission [62, 63]. In contrast, outcome is much better in patients with ventricular arrhythmias, especially in those with a witnessed cardiac arrest [64]. In the vast majority of patients, ventricular fibrillation will not terminate spontaneously and the probability to survive will decline by 10% per minute of ongoing VF [65]. Ideally, a regular pulse is restored within 10 minutes of CPR [66].
In a study including 200 patients presenting with ventricular fibrillation and successful early defibrillation, 72% of patients survived until hospital admission. However, only 40% of these patients were discharged with no or mild neurologic impairments [64]. Therefore, angiography is often delayed until the neurological status of the patient can be determined accurately. Blood markers may help to estimate the impact of hypoxic brain injury, such as the levels of the protein “neurone specific enolase”, “S-100” or “IL-8”, although predictive value and accuracy varies widely in different studies [67, 68]. More precise tools to predict outcomes would help tremendously for optimal resource allocation [69].
CARDIAC ARREST CENTRES
There is variation in outcome for OHCA patients depending on the hospital they are admitted to [70] and there is some evidence that mortality is lower among those admitted to intensive care units (ICUs) that treat a high volume of post-cardiac arrest patients [71]. A specialised multidisciplinary team approach to post-resuscitation care is essential. Post-resuscitation care is started by the EMS on scene and in some systems this may include pre-hospital induction of hypothermia. It is important to designate specific and clear roles to each team involved in the care and management of OHCA patients (emergency medicine, critical care, cardiology, neurology).
Trauma-centres have dramatically improved the outcomes of severely-injured patients. This model might be applicable to other medical conditions such as OHCA or stroke. Based on this premise, regionalised, coordinated resuscitation centres to care for post-OHCA patients has been proposed [72]. EMS providers should transport patients to those hospitals that are best suited for caring for OHCA victims - such hospitals would provide therapeutic hypothermia, 24/7 access to PCI facilities and availability of dedicated neurological investigations [72].
CONCLUSIONS
There have been multiple recent advances in the care of OHCA patients which may have a synergistic effect. The development of cardiac arrest centres, post-OHCA management protocols, further advances in therapeutic hypothermia and primary percountaneous intervention (PPCI) in post-OHCA are likely to further improve outcomes in the future. We are moving away from the perception that survival of OHCA victims is a fortunate rare event towards a renewed sense that OHCA is often a treatable event with an increasing chance for neurologically intact survival.
Fig. (2).
The chain of survival.
Table 1.
Causes of OHCA [5-7]
| Cardiac causes | Non-cardiac causes (≈5-12%) |
|---|---|
Ischemia (≈70%)
|
Electrolyte disorders
|
Structural heart disease
|
Neurological illnesses
|
Electric disorders
|
Drugs and drug interactions
|
Table 2.
Diagnostic Investigations for Patients After OHCA
History and physical examination
|
Laboratory evaluation
|
Electrocardiogram
|
Echocardiography
|
Coronary angiography
|
Cardiac magnetic resonance imaging
|
Table 3.
Secondary Prevention of SCA. Decision Making on ICD Implantation
| Reversible causes of OHCA ICD not primarily recommended |
Non-reversible causes of OHCA ICD recommended |
|---|---|
| Acute myocardial ischemia | LVEF ≤ 35%, not in context of acute ischaemia |
| Electrolyte disorders in the presence /or not of antiarrhythmic drug therapy proven to be the cause of SCA | LVEF > 35% Grey area Electrophysiology studies potentially helpful Individualised decision for ICD (hypertrophic cardiomyopathy, long QT, etc.) |
| Arrhythmia related to acquired long QT syndrome Patient must avoid exposure to drugs |
|
| Fulminant myocarditis | |
| WPW syndrome as a trigger for VF: ablation |
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ABBREVIATIONS
- ROSC
= Return of Spontaneous Circulation
- ALS
= Advanced Life Support
- ARVC
= Arrhythmic Right Ventricular Cardiomyopathy
- AED
= Automated External Defibrillators
- BLS
= Basic Life Support
- CPR
= Cardiopulmonary Resuscitation
- ECG
= Electrocardiogram
- EMS
= Emergency Medical Service
- ERC
= European Resuscitation Council
- ESC
= European Society of Cardiology
- ICD
= Implantable Cardioverter Defibrillator
- ICU
= Intensive Care Unit
- IABP
= Intra-Aortic Balloon Pump
- LBBB
= Left Bundle Branch Block
- MRI
= Magnetic Resonance Imaging
- MTH
= Mild Therapeutic Hypothermia
- OHCA
= Out-of-Hospital Cardiac Arrest
- PCI
= Percutaneous Coronary Intervention
- PPCI
= Primary Percutaneous Coronary Intervention
- PEA
= Pulseless Electric Activity
- SCA
= Sudden Cardiac Arrest
- SCD
= Sudden Cardiac Death
- VF
= Ventricular Fibrillation
- VT
= Ventricular Tachycardia
- WPW
= Wolf-Parkinson-White syndrome
REFERENCES
- 1.Buxton AE, Calkins H, Callans DJ , et al. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation. 2006;114(23):2534–70. doi: 10.1161/CIRCULATIONAHA.106.180199. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM , et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichol G, Thomas E, Callaway CW , et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3(1):63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 5.Demirovic J, Myerburg RJ. Epidemiology of sudden coronary death: an overview. Prog Cardiovasc Dis. 1994;37(1):39–48. doi: 10.1016/s0033-0620(05)80050-7. [DOI] [PubMed] [Google Scholar]
- 6.Drory Y, Turetz Y, Hiss Y , et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol. 1991;68(13):1388–92. doi: 10.1016/0002-9149(91)90251-f. [DOI] [PubMed] [Google Scholar]
- 7.Green D, Roberts PR, New DI, Kalra PA. Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis. 2011;57(6):921–9. doi: 10.1053/j.ajkd.2011.02.376. [DOI] [PubMed] [Google Scholar]
- 8.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes glucose level and risk of sudden cardiac death. Eur Heart J. 2005;26(20):2142–7. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 9.Hallstrom AP, Cobb LA, Ray R. Smoking as a risk factor for recurrence of sudden cardiac arrest. N Engl J Med. 1986;314(5):271–5. doi: 10.1056/NEJM198601303140502. [DOI] [PubMed] [Google Scholar]
- 10.Meier P, Gloekler S, de Marchi SF, Zbinden R, Delacretaz E, Seiler C. An indicator of sudden cardiac death during brief coronary occlusion: electrocardiogram QT time and the role of collaterals. Eur Heart J. 2010;31(10):1197–204. doi: 10.1093/eurheartj/ehp576. [DOI] [PubMed] [Google Scholar]
- 11.Meier P, Seiler C. Sudden cardiac arrest during acute coronary occlusion -who is at risk?. Cardiology. 2010;117(2):124–7. doi: 10.1159/000320092. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Castellano N, Garcia EJ, Abeytua M , et al. Influence of collateral circulation on in-hospital death from anterior acute myocardial infarction. J AmColl Cardiol. 1998;31(3):512–8. doi: 10.1016/s0735-1097(97)00521-4. [DOI] [PubMed] [Google Scholar]
- 13.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a metaanalysis. Eur Heart J. 2012;33(5):614–21. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 14.Goldberger JJ, Cain ME, Hohnloser SH , et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118(14):1497–518. [PubMed] [Google Scholar]
- 15.Fishman GI, Chugh SS, Dimarco JP , et al. Sudden cardiac death prediction and prevention: report from a National Heart Lung and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122(22):2335–48. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical demographic and pathological profiles JAMA. 1996;276(3):199–204. [PubMed] [Google Scholar]
- 17.Maron BJ, Poliac LC, Roberts WO. Risk for sudden cardiac death associated with marathon running. J Am Coll Cardiol. 1996;28(2):428–431. doi: 10.1016/0735-1097(96)00137-4. [DOI] [PubMed] [Google Scholar]
- 18.Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in Air Force recruits. A 20-year review JAMA. 1986;256(19):2696–9. [PubMed] [Google Scholar]
- 19.Maron BJ, Thompson PD, Ackerman MJ , et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition Physical Activity and Metabolism endorsed by the American College of Cardiology. Foundation Circulation. 2007;115(12):1643–55. doi: 10.1161/CIRCULATIONAHA.107.181423. [DOI] [PubMed] [Google Scholar]
- 20.Corrado D, Pelliccia A, Bjørnstad HH , et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol.Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516–24. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- 21.Nolan JP, Soar J, Zideman DA , et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1.Executive summary. Resuscitation. 2010;81(10):1219–76. doi: 10.1016/j.resuscitation.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Seethala RR, Abella BS. To ventilate or not to ventilate during cardiopulmonary resuscitation: that is the question. Heart. 2010;96(8):577–8. doi: 10.1136/hrt.2009.183756. [DOI] [PubMed] [Google Scholar]
- 23.Meier P, Baker P, Jost D , et al. Chest compressions before defibrillation for out-of-hospital cardiac arrest: a meta-analysis of randomized controlled clinical trials. BMC Med. 2010;8:52. doi: 10.1186/1741-7015-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins GD, Brace S, Gates S. Mechanical chest-compression devices: current and future roles. Curr Opin Crit Care. 2010;16(3):203–10. doi: 10.1097/MCC.0b013e328339cf59. [DOI] [PubMed] [Google Scholar]
- 25.Gates S, Smith JL, Ong GJ, Brace SJ, Perkins GD. Effectiveness of the LUCAS device for mechanical chest compression after cardiac arrest: systematic review of experimental observational and animal studies. Heart. 2012;98(12):908–13. doi: 10.1136/heartjnl-2011-301571. [DOI] [PubMed] [Google Scholar]
- 26.Gaieski DF, Band RA, Abella BS , et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Nolan JP, Hazinski MF, Billi JE , et al. Part 1: Executive summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation. 2010;81(Suppl 1 ):e1–25. doi: 10.1016/j.resuscitation.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deakin CD, Nolan JP, Soar J , et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305–52. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Spaulding CM, Joly LM, Rosenberg A , et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336(23):1629–33. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 30.Dumas F, Cariou A, Manzo-Silberman S , et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3(3):200–7. doi: 10.1161/CIRCINTERVENTIONS.109.913665. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen N, Hovdenes J, Nilsson F , et al. Outcome. timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–34. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 32.Seyfarth M, Sibbing D, Bauer I , et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intraaortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–8. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 33.Jolly SS, Shenkman H, Brieger D , et al. Quantitative troponin and death. cardiogenic sock.cardiac arrest and new heart failure in patients with non-ST-segment elevation acute coronary syndromes (NSTE ACS)insights from the Global Registry of Acute Coronary Events. Heart. 2011;97(3): 197–202. doi: 10.1136/hrt.2010.195511. [DOI] [PubMed] [Google Scholar]
- 34.Wagner H, Terkelsen CJ, Friberg H , et al. Cardiac arrest in the catheterisation laboratory: a 5-year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation. 2010;81(4):383–7. doi: 10.1016/j.resuscitation.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Nolan JP, Lyon RM, Sasson C, Rossetti AO, Lansky AJ, Fox KA, Meier P. Advances in the hospital management of patients following an out of hospital cardiac arrest. Heart. 2012;98(16):1201–6. doi: 10.1136/heartjnl-2011-301293. [DOI] [PubMed] [Google Scholar]
- 36.Perkins GD, Brace SJ, Smythe M, Ong G, Gates S. Out-of-hospital cardiac arrest: recent advances in resuscitation and effects on outcome. Heart. 2011;98(7):529–35. doi: 10.1136/heartjnl-2011-300802. [DOI] [PubMed] [Google Scholar]
- 37.Arntz HR, Bossaert LL, Danchin N, Nikolaou NI. European Resuscitation Council Guidelines for Resuscitation 2010 Section 5.Initial management of acute coronary syndromes. Resuscitation. 2010;81(10):1353–63. doi: 10.1016/j.resuscitation.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Walters JH, Morley PT, Nolan JP. The role of hypothermia in postcardiac arrest patients with return of spontaneous circulation: A systematic review. Resuscitation. 2011;82(5):508–16. doi: 10.1016/j.resuscitation.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 39.The Hypothermia after Cardiac Arrest Study Group.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 40.Bernard SA, Gray TW, Buist MD , et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 41.Deakin CD, Morrison LJ, Morley PT , et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2010;81(Suppl 1 ):e93–e174. doi: 10.1016/j.resuscitation.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Bernard SA, Smith K, Cameron P , et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122(7):737–42. doi: 10.1161/CIRCULATIONAHA.109.906859. [DOI] [PubMed] [Google Scholar]
- 43.Nagao K, Kikushima K, Watanabe K , et al. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ J. 2010;74(1):77–85. doi: 10.1253/circj.cj-09-0502. [DOI] [PubMed] [Google Scholar]
- 44.Castren M, Nordberg P, Svensson L , et al. Intra-arrest transnasal evaporative cooling: a randomized. prehosp. tal.;multicenter study (PRINCE:Pre–ROSC IntraNasal Cooling Effectiveness). doi: 10.1161/CIRCULATIONAHA.109.931691. [DOI] [PubMed] [Google Scholar]
- 45.Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM. Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation. 2010;81(9):1117–22. doi: 10.1016/j.resuscitation.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Tomte O, Draegni T, Mangschau A, Jacobsen D, Auestad B, Sunde K. A comparison of intravascular and surface cooling techniques in comatose cardiac arrest survivors. Crit Care Med. 2011;39(3):443–9. doi: 10.1097/CCM.0b013e318206b80f. [DOI] [PubMed] [Google Scholar]
- 47.Hoedemaekers CW, Ezzahti M, erritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo-and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. 2007;11(4):R91. doi: 10.1186/cc6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aufderheide TP, Nichol G, Rea TD , et al. A trial of an impedance threshold device in out-ofhospital cardiac arrest. N Engl J Med. 2011;365(9):798–806. doi: 10.1056/NEJMoa1010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binks AC, Murphy RE, Prout RE , et al. Therapeutic hypothermia after cardiac arrest -implementation in UK intensive care units. Anaesthesia. 2010;65(3):260–5. doi: 10.1111/j.1365-2044.2009.06227.x. [DOI] [PubMed] [Google Scholar]
- 50.Michelson AD, MacGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71(5):633–40. [PubMed] [Google Scholar]
- 51.Reed RL, Bracey AW, Hudson JD, Miller TA, Fischer RP. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32(2):141–52. [PubMed] [Google Scholar]
- 52.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations side effects and cooling methods. Crit Care Med. 2009;37(3):1101–20. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 53.Polderman KH, Peerdeman SM, Girbes AR. Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. J Neurosurg. 2001;94(5):697–705. doi: 10.3171/jns.2001.94.5.0697. [DOI] [PubMed] [Google Scholar]
- 54.van den Broek MP, Groenendaal F, Egberts AC, Rademaker CM. Effects of hypothermia on pharmacokinetics and pharmacodynamics: a systematic review of preclinical and clinical studies. Clin Pharmacokinet. 2010;49(5):277–94. doi: 10.2165/11319360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Causes of death in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J Am Coll Cardiol. 1999;34(5):1552–9. doi: 10.1016/s0735-1097(99)00376-9. [DOI] [PubMed] [Google Scholar]
- 56.Grubman EM, Pavri BB, Shipman T, Britton N, Kocovic DZ. Cardiac death and stored electrograms in patients with third-generation implantable cardioverter-defibrillators. J Am Coll Cardiol. 1998;32(4):1056–62. doi: 10.1016/s0735-1097(98)00359-3. [DOI] [PubMed] [Google Scholar]
- 57.Zipes DP, Camm AJ, Borggrefe M , et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006;48(5):e247–346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Epstein AE, DiMarco JP, Ellenbogen KA , et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 59.Sheldon R, Connolly S, Krahn A, Roberts R, Gent M, Gardner M. Identification of patients most likely to benefit from implantable cardioverter-defibrillator therapy: the Canadian Implantable Defibrillator Study. Circulation. 2000;101(14):1660–4. doi: 10.1161/01.cir.101.14.1660. [DOI] [PubMed] [Google Scholar]
- 60.Holmgren C, Bergfeldt L, Edvardsson N , et al. Analysis of initial rhythm. witnessed status and delay totreatment among survivors of out-of-hospital cardiac arrest in Sweden. . Heart. 2010;96(22):1826–30. doi: 10.1136/hrt.2010.198325. [DOI] [PubMed] [Google Scholar]
- 61.Adielsson A, Hollenberg J, Karlsson T , et al. Increase in survival and bystander CPR in out-of-hospital shockable arrhythmia: bystander CPR and female gender are predictors of improved outcome.Experiences from Sweden in an 18-year perspective. Heart. 2011;97(17):1391–6. doi: 10.1136/hrt.2011.222711. [DOI] [PubMed] [Google Scholar]
- 62.Weaver WD, Cobb LA, Hallstrom AP, Fahrenbruch C, Copass MK, Ray R. Factors influencing survival after out-of-hospital cardiac arrest. J Am Coll Cardiol. 1986;7(4):752–7. doi: 10.1016/s0735-1097(86)80332-1. [DOI] [PubMed] [Google Scholar]
- 63.Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301–6. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 64.Bunch TJ, White RD, Gersh BJ , et al. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N Engl J Med. 2003;348(26):2626–33. doi: 10.1056/NEJMoa023053. [DOI] [PubMed] [Google Scholar]
- 65.Rea TD, Eisenberg MS, Becker LJ, Murray JA, Hearne T. Temporal trends in sudden cardiac arrest: a 25-year emergency medical services perspective. Circulation. 2003;107(22):2780–5. doi: 10.1161/01.CIR.0000070950.17208.2A. [DOI] [PubMed] [Google Scholar]
- 66.van Walraven C, Forster AJ, Parish DC , et al. Validation of a clinical decision aid to discontinue in-hospital cardiac arrest resuscitations. JAMA. 2001;285(12):1602–6. doi: 10.1001/jama.285.12.1602. [DOI] [PubMed] [Google Scholar]
- 67.Storm C, Nee J, Jörres A, Leithner C, Hasper D, Ploner CJ. Serial measurement of neuron specific enolase improves prognostication in cardiac arrest patients treated with hypothermia: a prospective study. Scand J Trauma Resusc Emerg Med. 2012;20:6. doi: 10.1186/1757-7241-20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE. S100.IL-8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation. 2007;75(2):219–28. doi: 10.1016/j.resuscitation.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Chamberlain D. Predictors of survival from out-of-hospital cardiac arrest. Heart. 2010;96(22):1785–6. doi: 10.1136/hrt.2010.207076. [DOI] [PubMed] [Google Scholar]
- 70.Stub D, Smith K, Bray JE, Bernard S, Duffy SJ, Kaye DM. Hospital characteristics are associated with patient outcomes following out-of-hospital cardiac arrest. Heart. 2011;97(18):1489–94. doi: 10.1136/hrt.2011.226431. [DOI] [PubMed] [Google Scholar]
- 71.Carr BG, Kahn JM, Merchant RM, Kramer AA, Neumar RW. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80(1):30–4. doi: 10.1016/j.resuscitation.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Nichol G, Aufderheide TP, Eigel B , et al. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation. 2010;121(5):709–29. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]