Abstract
Stroke is one of the main causes of death and disability in the world. Cardioembolic etiology accounts for approximately one fifth of all ischemic strokes whereas 25-30% remains undetermined even after an advanced diagnostic workup. Despite there is not any biomarker currently approved to distinguish cardioembolic stroke among other etiologies in clinical practice the use of biomarkers represents a promising valuable complement to determine stroke etiology reducing the number of cryptogenic strokes and aiding in the prescription of the most appropriated primary and secondary treatments in order to minimize therapeutic risks and to avoid recurrences. In this review we present an update about specific cardioembolic stroke-related biomarkers at a protein, transcriptomic and genetic level. Finally, we also focused on reported biomarkers associated with atrial fibrillation (a cardiac illness strongly related with cardioembolic stroke subtype) thus with a potential to become biomarkers to detect cardioembolic stroke in the future.
Keywords: Atrial, biomarker, cardioembolic, classification, etiology, fibrillation, stroke, miRNA.
INTRODUCTION
Stroke remains one of the most important neurological affection representing the second leading cause of preventable death and being one of the major causes of productivity impairment. In the US, on average, every 40 seconds, someone has a stroke and annually, 5.5 million people die worldwide, with 44 million disability-adjusted life-years lost [1, 2]. Furthermore, the prevalence of stroke is expected to become significantly larger as the world population older than 65 is increasing by 9 million people per year [3].
There exist two principle acute stroke subtypes. The main one, representing over 80-85% of all strokes, is ischemic stroke caused by brain arterial occlusion. On the other hand, 15-20% are due to bleeding into the brain as a consequence of an arterial rupture [1]. At present, stroke diagnosis is mainly based on clinical criteria supplemented by imaging data. In most countries, once diagnosed patients with acute ischemic stroke are treated with intravenous recombinant tissue plasminogen activator (r-tPA), a serine protease, as primary therapy. Unfortunately, with a narrow effective therapeutic time window of only 4.5 h, an early diagnostic becomes essential. Moreover, after overcoming diagnosis and hyperacute treatment, an accurate etiological classification is critical not only during the acute phase and its primary therapy, but also and mainly to select a suitable secondary treatment.
MAJOR STROKE ETIOLOGY CLASSIFICATION SYSTEMS
Since the Harvard Stroke Regisitry development in 1978 [4], several classifications of stroke subtypes have been developed, being the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification system the most widely used nowadays for stroke subtyping. The TOAST classification system determines five major subtypes of ischemic stroke: cardioembolic stroke (CE), large artery atherotrombotic stroke (LAA), small vessel disease (SVD), stroke of other determined cause (SOC) and stroke of undetermined cause [5]. As well as TOAST, all the other classification systems group patients into different main categories being, in some cases, further sub-classified in other subtypes. Using some of the currently available stroke classification systems there is an evident oversizing and a great heterogeneity of the undetermined category, especially in the eldest ones. This might probably be a direct consequence of the unavailability of modern diagnostic tools by the time that these classification systems were developed, hindering a complete evaluation of the patient [4-6].
In contrast, new classifications such as SSS-TOAST, its automated version CCS (Causative Classification of Stroke System) [7, 8], the Korean TOAST [9] and ASCO (Atherosclerosis, Small-vessel disease, Cardiac source, Other cause) [10, 11], have been developed taking into account underlying diseases associated with stroke and the existence of different phenotypes. As a consequence, the rate of strokes with an undetermined cause has been clearly reduced with the newest stroke classification systems when compared with the eldest ones.
The latest classification system, CISS (Chinese Ischemic Stroke Subtype Subclassification), is a two step system which was conceived in 2011. CISS first step aims at the etiology, considering five TOAST-based categories but including more accurate subgroups, whereas the second step classifies stroke patients by the mechanism that underlies the ischemic event [12]. Even though considering both etiological and pathophysiological causes of stroke, one of the most important limiting conditions of the CISS classification system is its dependence on modern imaging technology availability.
STROKE MANAGEMENT REGARDING ETIOLOGICAL SUBTYPES
Depending on the causes of the artery occlusion patients might be differently managed in the stroke unit. If the thrombus has a cardiac or atherosclerotic origin, those affected individuals who fulfil the inclusion criteria receive r-tPA as a primary treatment. On the contrary there exists controversy about thrombolysis on patients with lacunar strokes. Some authors are against the administration of intravenous r-tPA in patients without demonstrated artery occlusion [13], whereas others propose thrombolysis even on the presence of an undetectable fibrin clot in the arteriole [14, 15]. In addition, as hemorrhagic transformation risk is related to the volume of the infarcted tissue [16], that risk might be reduced in lacunar strokes. Nevertheless the degree to which thrombolytic primary therapy may improve outcome in these patients is still uncertain [17].
Regarding secondary prevention therapy, patients will receive different treatment depending on etiology: cardioembolic strokes are usually treated with anticoagulant drugs whereas atherosclerotic strokes follow an antiplatelet therapy or even surgery (e.g. carotid endarterectomy). Similarly antiplatelet agents are the treatment of choice for lacunar strokes, together with antihypertensive drugs.
In spite of the importance of the accurate stroke subtype classification, the etiology of approximately 30% of all stroke cases remains unknown even after a precise and advanced diagnostic workup has been conducted [18]. The use of biomarkers would ease the lowering of the rate of cryptogenic strokes and also could contribute to speed up the diagnostic process prescribing the most appropriated primary and secondary treatments in order to minimize therapeutic risks and to avoid recurrences.
BIOMARKERS OF CARDIOEMBOLIC STROKE
Stroke caused by cardiac embolism accounts for approximately one fifth of ischemic strokes each year and 6-12% patients experience recurrences within 2 weeks after the first embolism [19]. Thrombus formation in the cardiac chambers is mainly caused by blood stasis, leading to a fibrin rich clot which may be then ejected towards the arterial circulation. Cerebral ischemia appears when the blood flow through a cerebral artery turns impaired by this thrombus.
One of the purposes of the stroke etiological classification systems is to classify patients for the therapeutic decision-making but, in spite of their usefulness, these classification systems still have important limitations (i.e. high rates of undetermined etiology, neuroimaging dependence). Biomarkers of stroke etiology might have a great importance in the development of more precise and reliable classification schemes which may serve as a valuable research and diagnostic tool [20].
This review presents an update of the research done in stroke biomarkers focusing on the possibility of identifying stroke of cardioembolic etiology since a more aggressive management will reduce dramatically stroke recurrence rates. For that we performed a search in Medline database introducing the terms cardioembolic stroke AND biomarker and selected data on different available biomarkers that may be measured in blood, such as proteins and circulating microRNAs, or analyzed at genetic level as gene expression profile or polymorphisms.
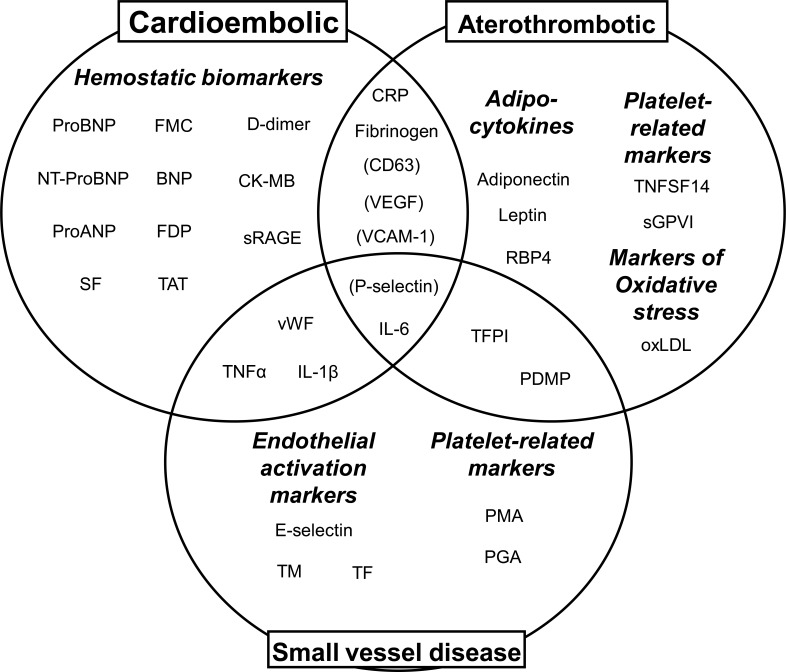
The most promising candidates to become biological markers for each stroke etiology are supposed to play a role in the pathophysiology of each stroke subtype (i. e. hemostasis, inflammation, immune system activation, endothelial damage or oxidative stress) [21]. Unfortunately several of these processes underlie in more than one stroke etiology, highlighting the complexity of the cerebrovascular disease. What is more, biomarkers might not even be specific to stroke since other clinical conditions, such as myocardial infarction, have common underlying pathophysiological mechanisms. Thus non-specific biomarkers should be employed with caution and this may hamper the application of these biomarkers in daily clinical practice. The most relevant studied biomarkers and their association with stroke etiologies are summarized in (Fig. 1).
Fig. (1).
Most relevant biomarkers grouped by specific pathophysiologies related to the three main stroke etiologies. Molecules in brackets have been identified in patients with AF, strongly related with cardioembolic stroke. BNP: b-type natriuretic peptide; CK-MB: creatine kinase isoform MB; CRP: C reactive protein; FDP: fibrin/fibrinogen degradation products; FMC: fibrin monomer complex; IL-1β: interleukin 1 beta; IL-6: interleukin 6; oxLDL: oxidized low denisity lipoprotein; PDMP: plateled derived microparticles; ProANP: pro-atrial natriuretic peptide; RBP4: retinol binding protein; SF: soluble fibrin; sGPVI: soluble glycoprotein VI; sRAGE: soluble advanced glycation end products receptor ; TAT: thrombin-antithrombin complex; TFPI: tissue factor pathway inhibitor; TM: thrombomodulin; TNFα: tumour necrosis factor alpha; TNFSF14: tumour necrosis factor family member 14; vWF: von Willebrand factor.
STROKE ETIOLOGY AND CIRCULATING PROTEINS
In the subsections below we will focus on protein biomarkers associated with cardioembolic stroke grouped by the underlying pathophysiological mechanism. The biomarkers discussed are represented in (Fig. 2) and classified by the pathophysiologic mechanism they are involved in.
Fig. (2).
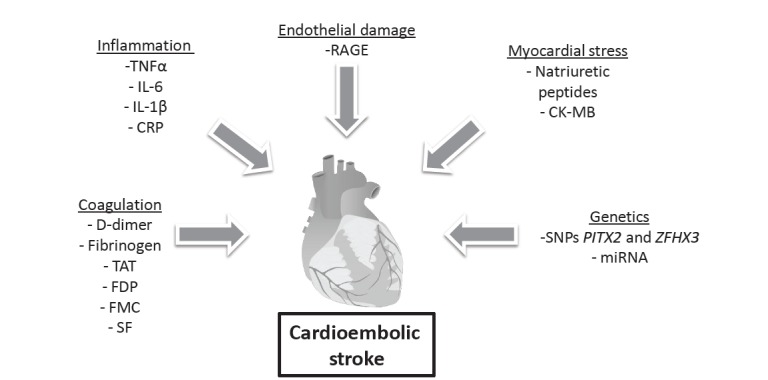
Illustration of the cardioembolic stroke pathophysiology and different areas represented by the biomarkers presented in the review.
Coagulation and Fibrinolytic Systems
Coagulation and fibrinolytic mechanisms are activated as a physiologic response after ischemia. D-dimer, a breakdown product of fibrin, is one of the basic markers of fibrinolytic system activity. It is not only elevated in plasma taken from patients with atrial fibrillation (AF) -most common cardiac abnormality leading to stroke- but also has been found raised in CE stroke patients compared with other etiologies [22-24]. A recent study, conducted by Alvarez-Perez and collaborators with 200 stroke patients and 50 controls, shows a thrombogenic profile in patients with CE stroke characterized by higher D-dimer levels than LAA, SVD or undetermined etiologies (p<0.0001) [25].
Raised fibrinogen and D-dimer/fibrinogen ratio in patients with CE stroke (p<0.0001 vs controls and patients with LAA and SVD strokes; p=0.004 vs patients with undetermined stroke) were also found in this study. D-dimer together with other hemostatic biomarkers, such as thrombin-antithrombin III complex (TAT), fibrinogen, fibrin/fibrinogen degradation products (FDPs), fibrin monomer complex (FMC) and soluble fibrin (SF), have been assayed lately among different subtypes of stroke [26]. CE stroke patients showed higher levels of FMC, SF, D-dimer and FDP than non-CE patients (LAA, SVD and others, p<0.05) after one day of hospitalization while fibrinogen was increased in LAA strokes when compared to CE. FMC and SF levels (both markers indicative of hypercoagulable state) were still different (p<0.05) two days after stroke onset, but regarding FDP (indicative of hyperfibrinolytic state), these differences remained even 7 days after stroke. Thus, measurement of these three hemostatic biomarkers could aid in the differentiation of CE and non-CE strokes in both acute and subacute phases.
Other markers for CE stroke have been further investigated. B-type natriuretic peptide (BNP) has an antifibrotic role in the heart and acts as a cardiac hormone. BNP is the main product of the cleavage of propeptide (proBNP), which also equimolarly release N-terminal peptide (NT-proBNP). All three peptides are considered to have a similar potential to discern CE stroke subtype among other etiologies [27]. Montaner and colleagues showed an association between high levels of BNP and D-dimer with CE stroke [24]. Plasma concentrations of both proteins were determined in a cohort of 707 patients. BNP higher than 76 pg/ml (OR: 2.3; 95% CI: 1.4-3.7; p=0.001) together with D-dimer higher than 0.96 μg/ml (OR: 2.2; 95% CI: 2.4-18.9; p=0.001) were independent predictors of CE stroke in a logistic regression model including NIHSS at baseline. This is important since CE strokes present larger infarcts and more pronounced deficits than other etiologies. Thus logistic-regression models should be adjusted by variables which reflect this involvement such as NIHSS, infarct size or Oxford Community Stroke Project classification (OCSP). More recently published studies report similar relation between BNP and cardioembolism and further confirm previous findings [24, 28-30]. Interestingly, BNP has also been found to be associated with AF (OR: 2.0; 95% CI; 1.6-2.5) in a cohort of 569 patients including all stroke subtypes. In the same study BNP was related with CE stroke subtype (p<0.001) and resulted an independent predictor of functional outcome ((OR, 0.5; 95% CI, 0.3-0.9) and mortality (OR, 3.05; 95% CI, 1.1-8.2) exclusively in patients with CE [31].
NT-proBNP has also been proposed for discerning between CE and other stroke etiologies in 92 patients with AF [27]. As authors discuss, NT-proBNP serum levels above 912 pg/mL (sensitivity 55.5%, specificity 97.9%, positive predictive value (PPV) 90.9 %, negative predictive value (NPV) 83.9%) can be used to identify those patients more prone to have AF in undetermined strokes and consequently it may aid to select subjects for prolonged cardiac rhythm monitoring in order to confirm paroxysmal AF. These patients who would probably suffer a secondary stroke with a cardiac origin may benefit from anticoagulant preventive treatment.
Regarding proBNP, Rodríguez-Yáñez and colleagues published a study including 262 patients from all TOAST etiologies and showed that plasma levels over 360 pg/mL of this peptide were independently associated with CE stroke (OR: 28.51; 95% CI: 5.90-136.75; p<0.0001) in a model that included basal NIHSS [32].
Von Willebrand Factor (vWF), a well studied hemostatic marker, has been found to be related with CE stroke. Raised levels of vWF were determined in CE patients by Licata and collaborators but only showed a trend (10 (5-12) ng/ml; p=0.0053) compared to other stroke subtypes [33]. Other studies showed that high levels of vWF are associated with AF and can predict cardiovascular events [34-36]. More recently, Hanson and collaborators examined vWF levels in patients with CE, LAA SVD or UND stroke [37]. Subject with CE and LAA stroke displayed higher vWF levels than SVD group (253 IU/dl [95% CI 230-279] and 263 IU/dl [95% CI 242-286], vs. 213 UI/dl [95% CI 197-229], respectively) during the acute phase, whereas at three months after the cerebrovascular event, only patients who suffered CE stroke showed higher vWF than those with SVD (240 IU/dl [95% CI 224-256] vs. 201 IU/dl [95% CI 189-215]). As authors discussed, these results agree with those from Ohira and colleagues, in which CE stroke patients also showed increased levels of vWF than patients with SVD (p<0.05) [38].
Other biological markers of cardiac damage such as proatrial natriuretic peptide (pro-ANP) together with creatine kinase MB (CK-MB) were analyzed and reported to be higher in patients with CE infarcts. Authors suggested a cutoff point of 2.6 ng/mL (sensitivity 62%, specificity 80%, p<0.0001) and 2266.6 fmol/mL (sensitivity 62%, specificity 70%, p<0.0001), for each marker respectively [32].
Very recently Santamarina and collaborators determined plasma concentrations of some of these previously studied biomarkers (BNP, D-dimer, CK-MB, troponin and myoglobin) in a cohort of 89 selected patients with undetermined stroke. After cardiological work-up, 49 of these patients with cryptogenic stroke were diagnosed as embolic. The most common findings were AF, severe aortic atheromatosis, patent foramen ovale, aneurysm of atrial septum and dilated cardiomyopathy. Cardioembolic stroke patients had higher plasma concentrations of BNP (121 [24-260.5] vs 26.8 [12.2-70.4], p=0.003), myoglobin (109 [66.9-177.7] vs 85.4 [61.4-125], p=0.028) and CK-MB (1.45 [0.5-2.27] vs 0.5 [0.5-1.4], p=0.004) than the ones that remained as cryptogenic. Finally, they constructed a predictive model adjusted by age, gender, risk factors, heart disease and stroke severity where BNP and CK-MB together with suffering of a previous stroke were independently associated to embolic abnormalities (OR 8.06, CI 95% 2.34-27.72; OR 6.52, CI 95% 1.44-29.5; OR 5.34, CI 95% 1.14-29.97; respectively). These results evidence the fact that some biomarkers contain the potential to improve the diagnosis of a stroke from an embolic source optimizing the performance of early cardiac explorations in those patients with undetermined stroke [39].
Systemic Inflammation and Endothelial Activation
High serum levels of immuno-inflammatory mediators such as TNFα, IL-6, IL-1β, selectins and adhesion molecules have been associated with ischemic stroke suggesting that both molecular and cellular inflammation mediates the mechanisms of cerebral injury and repair. Several studies have tried to clarify the role played by inflammation in the pathogenesis of different stroke etiologies. Licata and collaborators evaluated the levels of different cytokines, selectins and adhesion molecules among ischemic stroke subtypes [33]. In this study, patients classified as CE showed higher concentration of TNF-α (38.5 (22.2-46) pg/ml; p<0.0001), IL-6 (11 (5.5-19) pg/ml; p=0.0029) and IL-1β (11.5 (8-13) pg/ml; p<0.0001) in plasma compared to other TOAST categories. Although systemic inflammatory state have been further related with atherosclerosis, these results suggest that inflammation may also play a role in the pathological processes of cerebral cardioembolism [40]. Moreover, higher plasma levels of IL-18 [41], E-selectin [42], soluble vascular cell adhesion molecule-1 (sVCAM-I) [43], platelet factor-4 (PF-4) [44], sP-selectin [45], Platelet Microparticles (PMP) [46], asymmetric and symmetric dymethylarginine (ADMA, SDMA) [47, 48] found in different cohorts of patients with AF indicates that inflammation, platelet activation and oxidative stress are conditions not only related with LAA but might be also related with CE stroke.
C reactive protein (CRP) is a well-known acute phase reactant protein whose concentration rises in response to inflammation or infection. In spite of being one of the most studied inflammatory markers in stroke, several controversial results have been reported regarding its use as etiologic biomarker. Terruzzi and collaborators showed higher plasma levels of CRP in CE stroke patients when compared with other TOAST subtypes within the first 6 hours after symptoms onset [49]. However, as CRP has been suggested to promote platelet activation and foam cells generation through macrophages differentiation, it has been associated with large artery atherotrombotic events [50, 51]. Álvarez-Perez and colleagues showed a higher CRP in LAA when compared with SVD (p=0.010) and undetermined (p=0.003) strokes, but not with CE ones [52]. Adding more controversy to the role of CRP as etiologic biomarker, some authors detected no differences in CRP serum levels between etiology groups [53, 54]. Alternative approaches, such as meta-analysis or studies including greater cohorts of patients, are necessary to clarify the relation between CRP levels and different stroke etiologies.
Endothelial Damage
Micro and macroangiopathy affect endothelial walls thickness and reduce blood flow through small and large vessels. These processes are strongly related with hypertension and diabetes.
Reduced plasma levels of soluble advanced glycation end products receptor (sRAGE), a cell surface molecule member of the immunoglobulin superfamily, are considered to be related with the development of microangiopathy. Plasma levels of this protein were compared between stroke etiologies in a total of 482 enrolled patients by Yokota and collaborators. Distribution of sRAGE were significantly different among etiologies, being CE plasma concentrations the highest (1280 (271-4720) pg/mL, p=0.001) [55]. In this line of evidence, Montaner also showed significantly higher sRAGE levels in the group of patients with CE stroke (1.1 (0.7-1.9) ng/ml) etiologic [24].
Biomarkers which have been assayed in ischemic stroke patients and related with CE subtype are summarized in Table 1. Table 2 shows the CE stroke-related biomarkers with a highest sensitivity and specificity found in the literature.
Table 1.
Candidate makers of cardioembolic stroke.
| Name | Type of Biomarker | Protein Accession # | Sample size | Protein Levels Regarding Etiology | Main Function | Ref |
|---|---|---|---|---|---|---|
| Albumin | Protein | P02768 | 200 | CE < other etiologies | Regulates colloidal osmotic pressure of blood. | [52] |
| ANP | Protein | P01160 | 262 | CE > other etiologies | Cardiovascular homeostatic hormone. | [32] |
| BNP | Protein fragment | - | 1300 | CE > other etiologies | ProBNP cleavage product. Cardiac hormone that regulates cardiovascular homeostasis. | [24, 29, 31, 39] |
| CK-MB | Protein | P12277-P06732** | 262 | CE > other etiologies | Mediates energy transduction in tissues. Increased in heart damage diseases. | [32, 39] |
| CRP* | Protein | P02741 | 985 | CE > other etiologies; LAA > other etiologies; no difference among subtypes | Displays several functions associated with host defense. Risk marker of AF. | [49-54, 59] |
| D-dimer | Protein fragment | - | 1430 | CE > other etiologies | Fibrin degradation product. | [22-26, 52, 59, 60] |
| FDP | Group of proteins | - | 69 | CE > non-CE | Involved in coagulation/fibrinolysis processes. | [26] |
| Fibrinogen* | Protein | P02671 | 269 | CE < LAA; no difference among subtypes | Yields monomers that polymerize into fibrin and is a cofactor in platelet aggregation. | [25, 26] |
| FMC | Protein aggregate | - | 69 | CE > non-CE | Involved in coagulation/fibrinolysis processes. | [26] |
| IL-1β | Protein | P01584 | 227 | CE > other etiologies | Pro-inflammatory cytokine. | [33, 40] |
| IL-6 | Protein | P05231 | 227 | CE > other etiologies | Pro-inflammatory cytokine. | [33, 40] |
| NT-proBNP | Protein fragment | - | 92 | CE > non-CE | Pro-BNP cleavage product. High levels may help to select stroke patients with AF. | [27] |
| Pro-BNP | Protein | P16860 | 262 | CE > other etiologies | BNP precursor. ProBNP might be useful to reclassify undetermined stroke as of CE origin. | [32] |
| S100B | Protein | P04271 | 33 | CE > other etiologies | Involved in metal-ion binding and in the regulation of protein phosphorylation in brain tissue. | [61] |
| sCD40L* | Protein | P29965 | 132 | No difference among etiologies | Inflammatory marker. Elevated in AF patients. | [62, 63] |
| SF | Protein complex | - | 69 | CE > other etiologies | Involved in coagulation/fibrinolysis processes. | [26] |
| sRAGE | Protein | Q9UQ07 | 1189 | CE > other etiologies | Growth factor for several cell types. | [24,55] |
| TNF-α | Protein | P01375 | 227 | CE > other etiologies | Pro-inflammatory cytokine. | [33,40] |
| vWF | Protein | P04275 | 1551 | CE and LAA > other etiologies | Promotes platelet adhesion to the sub-endothelial matrix. Higher in patients with AF. | [33-38] |
ANP: atrial natriuretic peptide; AF: atrial fibrillation; BNP: b-type natriuretic peptide; CE: cardioembolic; CK-MB: creatine kinase MB; CRP: C-reactive protein; FDP: fibrin/ fibrinogen degradation products, FMC: fibrin monomer complex; IL-1β: interleukin 1-beta; IL-6: interleukin 6; LAA: large artery atherosclerosis; ; NT-proBNP: N-terminal part of pro-BNP; Pro-BNP: proform of BNP; SF: soluble fibrin; sRAGE: soluble receptor for advanced glycation end products; SVD: small vessel disease; TNF-α: tumour necrosis factor alpha;
Candidate marker with controversial results among studies.
UniProt codes from CK-MM and CK-BB.
Table 2.
Markers with highest sensitivity / specificity for the identification of CE stroke.
| Marker | Sample Size | Cutoff Point | Sensitivity | Specificity | PPV | NPV | Ref |
|---|---|---|---|---|---|---|---|
| BNP | 707 | >76 pg/mL | 68% | 72% | 55% | 82% | [24] |
| D-dimer | 707 | >300 ug/L | 100% | 52% | 46% | 73% | [24] |
| BNP combined with D-dimer | 707 | - | 66.5% | 91.3% | - | - | [24] |
| Pro-BNP | 262 | >360 pg/mL | 87% | 83% | - | - | [32] |
| ANP | 262 | >2266.6 fmol/mL | 62% | 70% | - | - | [32] |
| NT-proBNP | 92 | >265 pg/mL | 71.4% | 73.7% | 77.8% | 66.6% | [27] |
| CK-MB | 89 | >1.5 ng/mL | 47.9% | 85% | 79.3% | 79.3% | [39] |
| BNP combined with CK-MB | 89 | - | 31.2% | 95% | 88.2% | 53.5% | [39] |
ANP: atrial natriuretic peptide; BNP: b-type natriuretic peptide; CK-MB: creatine kinase MB; NT-proBNP: N-terminal part of pro-BNP; NPV: Negative predictive value; PPV: Positive predictive value
OTHER CANDIDATES
Apart from stroke biomarkers strongly related with CE etiology, some other molecules are associated with high risk cardioembolic conditions and therefore remain as candidates to become markers for CE stroke. The most common of these cardiac conditions which may lead to a cardioembolic event are: atrial fibrillation, recent myocardial infarction, dilated myocardiopathy, and mitral rheumatic stenosis. Other major sources of cardioembolism are ineffective endocarditis, marantic endocarditis and atrial myxoma; and patent foramen ovale, atrial septal aneurysm, atrial or ventricular septal defects, calcific aortic stenosis, and mitral annular calcification as minor sources [56, 57]. These potential biomarkers of underlying pathological risks may provide ample basis for future studies and could become valuable indicators of neurovascular events allowing physicians to carry out a better etiological classification.
Table 3 shows those candidates which are associated to AF, the most common CE stroke related cardiac affection.
Table 3.
Candidate markers related with atrial fibrillation.
| Name | Protein Accession # | Sample Size | Biomarker Levels in AF | Main Function | Ref |
|---|---|---|---|---|---|
| Adiponectin | Q15848 | 384 | Persistent AF > paroxysmal AF and control subjects | Adipokine involved in the control of fat metabolism and insulin sensitivity. | [64, 65] |
| ADMA | - | 42 | Acute AF > chronic AF and controls | Endogen inhibitor of NOS. ADMA contributes to thromboembolism in AF. | [47] |
| Ang-2 | Q15123 | 59 | AF > control subjects | Angiogenic factor. | [66] |
| Apelin | Q9ULZ1 | 166 | AF < sinus rhythm or control subjects | May have a role in the control of body fluid homeostasis. | [67,68] |
| CD63* | P08962 | 121 | AF > control subjects | Platelet activation marker. | [69] |
| E-selectin* | P16581 | 145 | AF > control subjects | Marker of endothelial activation. | [42] |
| Fibrinopeptide A | P02671 | 83 | AF ↑ | Released as part of blood clotting process. It reflects thrombin activity. | [59] |
| IL-18 | Q14116 | 56 | Persistent AF > paroxysmal AF and sinus rhythm | Pro-inflammatory cytokine. | [41] |
| MMP-1 | P03956 | 48 | AF < control subjects | Cleaves collagen types I, II and III. | [70] |
| MMP-2 | P08253 | 364 | AF > sinus rhythm | Degrades extracellular matrix in remodeling of vasculature, angiogenesis and tissue repair. | [43,71] |
| MMP-3 | P08254 | 86 | AF > sinus rhythm | Degrades fibronectin, laminin, gelatins and collagens. | [71] |
| MMP-9 | P14780 | 364 | AF > control and sinus rhythm subjects | Proteolyses extracellular matrix. | [41,71,72] |
| NPY | P01303 | 222 | AF > control subjects | Implicated in the control of feeding and in secretion of gonadotrophin-releasing hormone. | [72] |
| Osteoprotegerin | O00300 | 2863 | Associated with AF | Neutralizes osteoclastogenesis. | [73] |
| PF-4 | P02776 | 26 | AF > control subjects | Marker of platelet activation in AF patients. | [44] |
| PMP | - | 20 | AF > control subjects | PMPs play a role in hemostatic response to vascular injury. | [46] |
| Prothrombin fragment 1.2 (F1+2) | - | 48 | AF > control subjects | Marker of thrombogenesis | [59] |
| p-selectin (CD62P) | P16109 | 121 | AF > control subjects | Mediates the interaction of activated endothelial cells or platelets with leukocytes | [69] |
| SDMA | - | 394 | AF > non-AF | It influences NO formation via inhibition of L-arginine uptake. | [48] |
| sVCAM-1 | P19320 | 278 | Associated with AF | Important in cell-cell mediation. | [43] |
| sP-selectin | P16109 | 90 | AF > control subjects | Mediates interaction of activated endothelial cells or platelets with leukocytes. | [45] |
| TGF-β | P01137 | 107 | AF < non-AF | Controls proliferation, differentiation and other functions in many cell types. | [74] |
| TIMP-1 | P01033 | 134 | AF > sinus rhythm patients and control subjects | Irreversibly inactivates metalloproteinases. | [70, 71] |
| Troponin I | P48788 | 6189 | AF ↑ | Inhibitory subunit of troponin. Increased troponin I associated with an increase in the risk of stroke or systemic embolism and vascular events. | [75] |
| VEGF | P15692 | 72 | Paroxysmal and persistent AF > control subjects | Pro-angiogenic factor. | [76] |
Ang: Angiopoietin; ADMA: Asymmetric dimethylarginine; SDMA: Symmetric dimethylarginine: CITP: Carboxy-terminal telopeptide of collagen type I; MMP: Matrix metalloproteinase; TIMP-I: Tissue inhibitor of metalloproteinases; IL: Interleukin; NPY: Neuropeptide Y; PF-4: Platelet factor 4; PMP: Platelet microparticle; TF: Transferrin; TGF-β: Transforming growth factor beta; VEGF: Vascular endothelial growth factor; sVCAM-1: soluble vascular cell adhesion molecule 1; sCD40L: soluble CD40 ligand; NOS: Nitric oxide synthase.
Candidate marker with controversial results among studies.
GENE EXPRESSION BIOMARKERS OF STROKE ETIOLOGY
Gene expression profile can also be used to discern between different stroke subtypes. In 2010, Jickling GC and colleagues reported the results of the complete CLEAR trial. The expression profile from a total number of 23 CE and 10 LAA patients were compared and, as a result, 40 genes whose expression was significantly different among both etiologies were identified [58]. 11 of these 40 genes are involved in cellular movement, cell-to-cell signalling and interaction, and tissue development.
Especially interesting genes are ENPP2 (Ectonucleotide pyrophoshpatase/phosphodiesterase family member 2) and GRM5 (Glutamate receptor metabotropic 5). ENPP2 protein is involved in angiogenesis and neurite growth, whereas GRM5 encodes the glutamate receptor metabotropic 5, which is involved in normal brain function. Other genes such as LHFP, TMEM19 and EBF1 are involved in cardiac proliferation, cardiovascular development and haematological system function [77]. The proposed 40 genes expression profile predicted correctly 9 out of 10 patients known to be CE and when the same gene profile was used in 36 patients with an undetermined etiology, 58% of them were reclassified as CE or LAA with a probability greater than 90%. These results strongly suggest that gene expression profile could be used to complement diagnostic tests to determine the etiology in cryptogenic patients.
On the other hand, in the same study a different 37 genes expression profile was found to distinguish patients with CE stroke due to AF and non-AF causes with a probability higher than 90%. This 37 genes list may provide physicians an additional tool for the identification of patients more prone to suffer AF, as its detection strongly depends on cardiac monitoring, not always available for all patients. Moreover, paroxysmal AF detection requires a more prolonged follow-up period making more difficult the diagnosis of AF, thus this 37 gen expression profile analysis at baseline could be very helpful to identify these individuals.
In a later study, the same authors followed a similar strategy in a cohort of patients with lacunar and non-lacunar stroke. The study reported 41 genes whose expression profile allowed to distinguish both groups and predict etiology in small deep infarcts of undetermined cause [78].
Finally, these two gene profiles, the 40-genes list that distinguished CE and LAA strokes and the 41-genes list that distinguished lacunar and non-lacunar strokes, were integrated and combined with infarct location assessed by neuroimaging to predict the most probable cause of stroke in a cohort of 131 patients with cryptogenic strokes. Of these 131 undetermined strokes, 76 (58%) were predicted to be CE, 24 (18,3%) were LAA, 15 (11,5%) were lacunar and 16 (12,2%) remained unknown [77].
As authors discussed, these studies have some limitations being the small population size the most important. However, they evidenced that gene expression profile could allow us to distinguish between different causes of stroke. With the development of point-of-care devices, based on fast analysis, cryptogenic stroke clarification and preventive stroke treatments could be further improved.
STROKE ETIOLOGY AND CIRCULATING microRNA
microRNAs (miRNAs) are small (19-25 nt length) non-coding RNAs involved in the post-transcriptional regulation of genes by inhibition or degradation of mRNA. miRNAs are single stranded molecules of RNA whose high stability allows its detection in serum or plasma. Moreover, their expression patterns may reflect underlying pathophysiological processes. These important features make miRNAs very attractive biomarkers for human diseases [79].
Unfortunately, just a few studies have attempted to find differential miRNA levels between patients and controls. The study conducted by Gan and colleagues is an example. They measured blood levels of miRNA-145 (a modulator of vascular smooth muscle cells phenotype) and showed an up-regulation in stroke [80]. On the other hand, Zeng analyzed blood miRNA-210 and found it decreased in patients with ischemic stroke when compared to healthy controls (p=0.001) even after 14 days from stroke onset [81].
Among etiologies, until now only one study has focused on finding differences on miRNAs expression [82]. This study, conducted by Tan and collaborators, assessed differential miRNA profile and reported a list of 132 miRNAs with altered expression depending on stroke etiology. Some of them were up-regulated with a fold change >2 in only one subtype. This is the case of 7 miRNAs (miR-130b, -29b, -301a, -339-5p, -532-5p, - 634 and 886-5p) more expressed in SVD. Moreover, cluster analysis on different miRNA profiles among etiologies showed that undetermined stroke profiles were similar to SVD, suggesting that studied patients with undetermined etiology have resulted from SVD. Hence it might be assumed that miRNA profiling could be used to differentiate CE, LAA or SVD strokes from each other and to resolve undetermined strokes.
In contrast to the small number of published studies of miRNA focused on stroke etiologies, numerous studies have documented a relation between some specific miRNA and AF, strongly related with CE stroke [83]. Very recently, Liu Z and collaborators examined miRNA expression profiles in 3 groups: 5 patients with paroxysmal AF, 5 with persistent AF and 5 healthy controls. They found substantially lower levels of miRNA-150 in both groups of AF patients when compared with controls (p<0.001) and was identified as a predictor of AF (OR 1.96, 95% CI 1.5 to 3.57, p<0.001) [84]. Other authors have evidenced the involvement of miRNAs in basic mechanisms of AF. Adam and colleagues found an increased expression on miRNA-21 and a decreased expression of protein Sprouty1 (downstream target of miRNA-21) in left atria of patients with AF [85]. They also showed a reduction of AF by miRNA-21 inhibition, evidencing its important role in atrial fibrosis regulation.
The results of these studies suggest, on one hand, the use of miRNA as plasma biomarkers for AF detection and consequently the identification of an embolic source in undetermined strokes; on the other hand, they open the door to potential therapeutic targets by direct miRNA silencing.
STROKE ETIOLOGY AND GENE POLYMORPHISMS
To date several studies have been performed to further understand the genetic basis of ischemic stroke. The most promising results have been obtained from projects that performed Genome Wide Analysis (GWA) approaches. The International Stroke Genetics Consortium (ISGC) and the Wellcome Trust Case Control Consortium 2 (WTCCC2) conducted a GWA study on three stroke subtypes: LAA, SVD and CE stroke [86]. This was a large study with a discovery association phase conducted in 3548 affected individuals and 5972 controls, and a second replication phase performed in 5859 cases and 6281 controls. Some previously reported associations were replicated in this analysis such as the single nucleotide polymorphisms (SNPs) rs1906599 and rs12932445, located in the PITX2 (Paired-like homeodomain 2) and ZFHX3 (Zinc finger homeobox 3) genes respectively, and both related with CE etiology; or the SNP rs2383207 located in the CDKN2A/CDKN2B (Cyclin-dependent kinase inhibitor 2) gene, related with LAA stroke. Interestingly, a previously unknown association between LAA stroke and the SNP rs11984041 (located within the final intron of the HDAC9 (Histone deacetylase 9) gene) was firstly identified in this study. Finally, a third additional verification step was performed in 735 LAA cases and 28583 controls which further confirmed previous data. Taken together, the results from the discovery and two-steps verification phases provide strong evidence for association between the new SNP rs11984041 and LAA stroke (p=1.87x10-11) and further confirmed the association between CE stroke etiology and previously identified polymorphism in genes PITX2 and ZHF3.
Other studies have been carried out with approaches different from GWA, identifying other SNPs related to stroke etiology subtypes, e.g. rs2020918 related with SVD [87, 88], or rs315934, rs1180243, rs2071373 associated with a decrease in LAA stroke [89]. In spite of being size-limited and needing replication in further steps, these studies provide helpful information as a basis for future GWA of polymorphisms related to stroke etiologies.
Other polymorphisms different from single nucleotide ones have been analyzed and observed to be specifically associated with a stroke subtype. For instance, a 32 base-pair deletion in CCR5 (Chemokine C-C motif receptor 5) gene (Δ32 polymorphism) is considered to exert a protective effect against CE stroke as it has lower frequency in this subtype (OR, 0.4; 95% CI, 0.24-0.79; p=0.008) than in LAA, SVD or cryptogenic strokes [90].
Although blood proteins, nucleic acids and even gene expression profiles may aid in the diagnosis and decision-making during acute and subacute phases of stroke, genetic biomarkers specific for each etiology provide valuable evidence before stroke occurrence allowing physicians to prescribe the most suitable preventive treatment. Furthermore, the identification of genetic signatures of each etiology may be of main interest in guiding undetermined stroke diagnosis, especially during subacute phase when it becomes primordial an optimal secondary prevention to minimize recurrences. Table 4 shows a compilation of CE stroke and AF related genes, miRNA and polymorphisms.
Table 4.
Genes, miRNA and polymorphisms candidates to become biomarkers of CE stroke:
| Genes associated with CE stroke | ||||
|---|---|---|---|---|
| Candidate Molecule | Entrez Gene | Sample Size | Reason for Being a Candidate | Ref |
| ENPP2 | 5168 | 33 | Regulates lysophosphatidic acid production. It has Angiogenic properties. | [77] |
| GRM5 | 2915 | 33 | Modulates normal brain function. | [77] |
| LHFP | 10186 | 33 | Involved in cardiac proliferation. | [77] |
| TMEM19 | 55256 | 33 | Cardiovascular development and haematological system function. | [77] |
| EBF1 | 13591 | 33 | Involved in haematological system development and function. | [77] |
| miRNA Associated with CE Stroke | ||||
| Candidate Molecule | Accession Number | Sample size | Reason for Being a Candidate | Ref |
| miRNA-145 | MI0000461 | 32 | Regulates the smooth muscle cells differentiation. Up-regulated in ischemic stroke patients when compared to controls. | [80] |
| miRNA-210 | MI0000286 | 112 | It is strongly linked to hypoxia pathways, and it is up-regulated in response to hypoxia-inducible factors. Decreased expression in ischemic stroke compared with control subjects. | [81] |
| miRNA associated with AF | ||||
| Candidate molecule | Accession number | Sample size | Reason for being a candidate | Ref |
| miRNA-150 | MI0000479 | 10 | Regulates hematopoiesis by modulating stem cell differentiation to megakaryocytes. Lower levels in AF patients than control subjects | [84] |
| miRNA-21 | MI0000077 | 10 | Regulates atrial fibrosis. Increased plasma levels than control subjects. | [85] |
| Polymorphisms Associated with CE stroke | ||||
| Candidate Gene | SNP | Sample Size | Reason for Being a Candidate | Ref |
| PITX2 | rs1906599 | 3548 | Controls cell proliferation in a tissue-specific manner and is involved in morphogenesis. SNP associated with CE stroke. | [86] |
| ZFHX3 | rs12932445 | 3548 | Transcriptional repressor. Regulator of myoblasts differentiation. SNP associated with CE stroke. | [86] |
| CCR5 | - | 478 | δ32 polymorphism (32 bp deletion) associated with lower risk of CE stroke than LAA, SVD and cryptogenic subtypes. | [90] |
ENPP2: Ectonucleotide pyrophoshpatase/phosphodiesterase family member 2; GRM5: Glutamate receptor metabotropic 5; LHFP: lipoma HMGIC fusion partner; TMEM19: transmembrane protein 19; EBF1: early B-cell factor 1; PITX2: Paired-like homeodomain 2; ZFHX3: Zinc finger homeobox 3; CCR5: Chemokine C-C motif receptor 5; SNP: single nucleotide polymorphism; CE: cardioembolic; LAA: large artery aterothrombotic; SVD: small vessel disease.
FUTURE PERSPECTIVES
During the last years, a great number of studies have been conducted aiming to find new biomarkers that could aid in the differentiation of ischemic stroke etiologies. Although some of these studies have shown markers with high sensitivity and specificity, in a large cohort of patients and proving to be independent predictors of ischemic stroke, there are still some hurdles to take over before implementing them to daily clinical routine. Table 5 shows the general strengths and shortcomings of the studies on stroke etiology biomarkers.
Table 5.
General pro’s and con’s of studies conducted aiming to find ischemic stroke etiology biomarkers.
| Strenghts | Shortcommings |
|---|---|
|
|
Recent advances achieved in proteomics in the last few years have brought new approaches for the identification of biomarkers. The use of quantitative proteomics has proven to be a promising strategy for identifying potential biomarkers for a number of diseases such as cancer, infectious diseases or autoimmunity. The stroke field can also take benefit from this fast development since recent studies have reported very promising results mainly directed at diagnosis. Dawson and collaborators explored the urinary proteome from ischemic stroke and transient ischemic attack patients versus controls. They developed two biomarker-based classifiers with identified proteins and validated them in independent blinded samples, showing a clear association of these biomarkers and stroke [91]. Dayon and colleagues also analyzed proteins from brain extracellular fluid and identified stroke-related proteins (i.e. glutathione S-transferase, or peroxiredoxin-I) that were further validated [92]. The same strategies could be followed on selected patients with clear etiologic profile to detect new candidate markers for each stroke subtype. Once replicated and validated, the combination of different biomarkers in a diagnostic test would provide the most valuable approach to accurately identify stroke etiology. However, neither biomarker nor panel of biomarkers has still showed enough sensitivity and specificity to improve clinical predictor models. New statistical tools, such as net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indexes, have been developed to evaluate the added value of a biomarker or combination of biomarkers to the clinical basis [93, 94]. If biomarkers demonstrated its role, these improved prediction models would help to reclassify patients according with the risk of suffering a cardiac embolism. Thus the rate of undetermined strokes might be reduced and a faster decision-making in pharmacological secondary prevention could be done.
At a genetic level, recent studies in transcriptomic and specially those performed through GWA approaches have provided new stroke and even etiology-related markers. These newly described genetic markers may not only aid in the diagnosis or patient etiologic classification, but also may serve as future therapeutic targets for each stroke etiologic subtype.
Despite having found many encouraging candidates by all these approaches their clinical application requires several careful validation steps. Multi-centric studies, with greater cohorts of patients, supported by multiple replications and performed with tools for massive molecular and genetic analysis will be the base to increase the output of potential biomarkers that may be implemented in daily clinical practice.
ACKNOWLEDGEMENTS
Neurovascular Research Laboratory takes part into the Spanish stroke research network INVICTUS (RD12/ 0014/0005) and the European Stroke Network (EUSTROKE 7FP Health F2-08-202213) and is partially funded by grants from the Fondo de Investigaciones Sanitarias (FIS 11/176) for stroke biomarkers studies. I.F-C is supported by Miguel Servet senior research contracts (CP12/03298), V.L. by a pre-doctoral fellowship from VHIR and T.G-B. by a pre-doctoral fellowship (FI09/00017) from the Carlos III Health Institute.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL , et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina EV, Tong X, George MG, Bansil P. Epidemiology and prevention of stroke: a worldwide perspective. Expert Rev Neurother. 2012;12(2):199–208. doi: 10.1586/ern.11.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 ):S85–90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Mohr JP, Caplan LR, Melski JW , et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurol-ogy. 1978;28(8):754–62. doi: 10.1212/wnl.28.8.754. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP Jr, Bendixen BH, Kappelle LJ , et al. Classification of subtype of acute ischemic stroke.Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Sacco RL, Ellenberg JH, Mohr JP , et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25(4):382–90. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 7.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58(5):688–97. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 8.Ay H, Benner T, Arsava EM , et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38(11):2979–84. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 9.Han SW, Kim SH, Lee JY , et al. A new subtype classification of ischemic stroke based on treatment and etiologic mechanism. Eur Neurol. 2007;57(2):96–102. doi: 10.1159/000098059. [DOI] [PubMed] [Google Scholar]
- 10.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis. 2009;27(5):493–501. doi: 10.1159/000210432. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27(5):502–8. doi: 10.1159/000210433. [DOI] [PubMed] [Google Scholar]
- 12.Gao S, Wang YJ, Xu AD, Li YS, Wang DZ. Chinese ischemic stroke subclassification. Front Neurol. 2011;2:6. doi: 10.3389/fneur.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Saver JL, Liebeskind DS , et al. Safety of intravenous fibrinolysis in imaging-confirmed single pene-trator artery infarcts. Stroke. 2010;41(11):2587–91. doi: 10.1161/STROKEAHA.110.586248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsia AW, Sachdev HS, Tomlinson J, Hamilton SA, Tong DC. Efficacy of IV tissue plasminogen activator in acute stroke: does stroke subtype really matter?. Neurology. 2003;61(1):71–5. doi: 10.1212/01.wnl.0000071228.56362.36. [DOI] [PubMed] [Google Scholar]
- 15.Mustanoja S, Meretoja A, Putaala J , et al. Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke. 2011;42(1):102–6. doi: 10.1161/STROKEAHA.110.597534. [DOI] [PubMed] [Google Scholar]
- 16.Singer OC, Kurre W, Humpich MC , et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40(8):2743–8. doi: 10.1161/STROKEAHA.109.550111. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL. Improving reperfusion therapy for acute ischaemic stroke. J Thromb Haemost. 2011;9(Suppl 1 ):333–43. doi: 10.1111/j.1538-7836.2011.04371.x. [DOI] [PubMed] [Google Scholar]
- 18.Wolf ME, Sauer T, Alonso A, Hennerici MG. Comparison of the new ASCO classification with the TOAST classification in a population with acute ischemic stroke. J Neurol. 2012;259(7):1284–9. doi: 10.1007/s00415-011-6325-1. [DOI] [PubMed] [Google Scholar]
- 19.Cardiogenic brain embolism.Cerebral Embolism Task Force. Arch Neurol. 1986;43(1):71–84. [PubMed] [Google Scholar]
- 20.García-Berrocoso T, Fernández-Cadenas I, Delgado P, Rosell A, Montane J. Blood biomarkers to identify ischemic stroke etiologies. Therapy. 2010;7(4):337–53. [Google Scholar]
- 21.García-Berrocoso T, Fernández-Cadenas I, Delgado P, Rosell A, Montaner J. Blood biomarkers in cardioembolic stroke. Curr Cardiol Rev. 2010;6(3):194–201. doi: 10.2174/157340310791658767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isenegger J, Meier N, Lämmle B , et al. D-dimers predict stroke subtype when assessed early. Cerebrovasc Dis. 2010;29(1):82–6. doi: 10.1159/000256652. [DOI] [PubMed] [Google Scholar]
- 23.Tombul T, Atbas C, Anlar O. Hemostatic markers and platelet aggregation factors as predictive markers for type of stroke and neurological disability following cerebral infarction. J Clin Neurosci. 2005;12(4):429–34. doi: 10.1016/j.jocn.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Montaner J, Perea-Gainza M, Delgado P , et al. Etiologic diagnosis of ischemic stroke subtypes with plasma bi-omarkers. Stroke. 2008;39(8):2280–7. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Usefulness of measurement of fibrinogen. D-dimer. D-dimer/fibrinogen ratio C reactive protein and erythrocyte sedimentation rate to assess the pathophysiology and mech-anism of ischaemic stroke. J Neurol Neurosurg Psychiatr. 2011;82(9):986–92. doi: 10.1136/jnnp.2010.230870. [DOI] [PubMed] [Google Scholar]
- 26.Hirano K, Takashima S, Dougu N , et al. Study of hemostatic biomarkers in acute ischemic stroke by clinical subtype. J Stroke Cerebrovasc Dis. 2012;21(5):404–10. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca AC, Matias JS, Pinho e Melo T, Falcão F, Canhão P, Ferro JM. N-terminal probrain natriuretic peptide as a biomarker of cardioembolic stroke. Int J Stroke. 2011;6(5):398–403. doi: 10.1111/j.1747-4949.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 28.Naya T, Yukiiri K, Hosomi N , et al. Brain natriuretic peptide as a surrogate marker for cardioembolic stroke with paroxysmal atrial fibrillation. Cerebrovasc Dis. 2008;26(4):434–40. doi: 10.1159/000155640. [DOI] [PubMed] [Google Scholar]
- 29.Shibazaki K, Kimura K, Iguchi Y, Okada Y, Inoue T. Plasma brain natriuretic peptide can be a biological marker to distinguish cardioembolic stroke from other stroke types in acute ischemic stroke. Intern Med. 2009;48(5):259–64. doi: 10.2169/internalmedicine.48.1475. [DOI] [PubMed] [Google Scholar]
- 30.Yukiiri K, Hosomi N, Naya T , et al. Plasma brain natriuretic peptide as a surrogate marker for cardioembolic stroke. BMC Neurol. 2008;8:45. doi: 10.1186/1471-2377-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rost NS, Biffi A, Cloonan L , et al. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43(2):441–5. doi: 10.1161/STROKEAHA.111.629212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Yáñez M, Sobrino T, Blanco M , et al. High serum levels of pro-brain natriuretic peptide (pro BNP) identify cardioembolic origin in undetermined stroke. Dis Markers. 2009;26(4):189–95. doi: 10.3233/DMA-2009-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Licata G, Tuttolomondo A, Di Raimondo D, Corrao S, Di Sciacca R, Pinto A. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost. 2009;101(5):929–37. [PubMed] [Google Scholar]
- 34.Freestone B, Gustafsson F, Chong AY , et al. Influence of atrial fibrillation on plasma von willebrand factor soluble E-selectin and N-terminal pro B-type natriuretic peptide levels in systolic heart failure. Chest. 2008;133(5):1203–8. doi: 10.1378/chest.07-2557. [DOI] [PubMed] [Google Scholar]
- 35.Folsom AR, Rosamond WD, Shahar E , et al. Prospective study of markers of hemostatic function with risk of ischemic stroke.The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100(7):736–42. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 36.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GDO, Fowkes FGR. Relative value of inflammatory. hemosttic.and rheological factors for incident myocardial infarction and stroke the Edinburgh Artery Study. . Circulation. 2007; 115(16):2119–27. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 37.Hanson E, Jood K, Karlsson S, Nilsson S, Blomstrand C, Jern C. Plasma levels of von Willebrand factor in the etiologic subtypes of ischemic stroke. J Thromb Haemost. 2011;9(2):275–81. doi: 10.1111/j.1538-7836.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 38.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37(10):2493–8. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 39.Santamarina E, Penalba A, García-Berrocoso T , et al. Biomarker level improves the diagnosis of embolic source in ischemic stroke of unknown origin. J Neurol. 2012;259(12):2538–45. doi: 10.1007/s00415-012-6532-4. [DOI] [PubMed] [Google Scholar]
- 40.Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Inflammation in ischemic stroke subtypes. Curr Pharm Des. 2012;18(28):4289–310. doi: 10.2174/138161212802481200. [DOI] [PubMed] [Google Scholar]
- 41.Luan Y, Guo Y, Li S , et al. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace. 2010;12(12):1713–8. doi: 10.1093/europace/euq321. [DOI] [PubMed] [Google Scholar]
- 42.Freestone B, Chong AY, Nuttall S, Blann AD, Lip GYH. Soluble E-selectin von Willebrand factor. soluble thrombomodlin.and total body nitrate/nitrite product as indices of endothelial damage/dysfunction in paroxysmal persistent and permanent atrial fibrillation. Chest . 2007; 132(4):1253–8. doi: 10.1378/chest.07-1185. [DOI] [PubMed] [Google Scholar]
- 43.Ehrlich JR, Kaluzny M, Baumann S, Lehmann R, Hohnloser SH. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011;100(11):1029–36. doi: 10.1007/s00392-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi K, Furui H, Taniguchi N, Sotobata I. Plasma beta-thromboglobulin and platelet factor 4 concentrations in patients with atrial fibrillation. Jpn Heart J. 1986;27(4):481–7. doi: 10.1536/ihj.27.481. [DOI] [PubMed] [Google Scholar]
- 45.Fu R, Wu S, Wu P, Qiu J. A study of blood soluble P-selectin. fibringen.and von Willebrand factor levels in idiopathic and lone atrial fibrillation. Europace. 2011;13(1):31–6. doi: 10.1093/europace/euq346. [DOI] [PubMed] [Google Scholar]
- 46.Azzam H, Zagloul M. Elevated platelet microparticle levels in valvular atrial fibrillation. Hematology. 2009;14(6):357–60. doi: 10.1179/102453309X12473408860460. [DOI] [PubMed] [Google Scholar]
- 47.Cengel A, Sahinarslan A, Biberoglu G , et al. Asymmetrical dimethylarginine level in atrial fibrillation. Acta Cardiol. 2008;63(1):33–7. doi: 10.2143/AC.63.1.2025329. [DOI] [PubMed] [Google Scholar]
- 48.Schulze F, Carter AM, Schwedhelm E , et al. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis. 2010;208(2):518–23. doi: 10.1016/j.atherosclerosis.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Terruzzi A, Valente L, Mariani R, Moschini L, Camerlingo M. C-reactive protein and aetiological subtypes of cerebral infarction. Neurol Sci. 2008;29(4):245–9. doi: 10.1007/s10072-008-0975-5. [DOI] [PubMed] [Google Scholar]
- 50.Yeh ETH, Khan BV. The potential role of antiplatelet agents in modulating inflammatory markers in atherothrom-bosis. J Thromb Haemost. 2006;4(11):2308–16. doi: 10.1111/j.1538-7836.2006.02202.x. [DOI] [PubMed] [Google Scholar]
- 51.Mullenix PS, Andersen CA, Starnes BW. Atherosclerosis as inflammation. Ann Vasc Surg. 2005;19(1):130–8. doi: 10.1007/s10016-004-0153-z. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Albumin level and stroke.Potential association between lower albumin level and cardioembolic aetiology. Int J Neurosci. 2011;121(1):25–32. doi: 10.3109/00207454.2010.523134. [DOI] [PubMed] [Google Scholar]
- 53.Turgut B, Turgut N, Celik Y, Tekgündüz E, Pamuk GE, Demir M. Differences in platelet-leukocyte aggregates among subtypes of acute cerebral ischemia. J Neurol Sci. 2011;305(1-2):126–30. doi: 10.1016/j.jns.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 54.Cucchiara BL, Messe SR, Sansing L , et al. Lipoprotein-associated phospholipase A2 and C-reactive protein for risk-stratification of patients with TIA. Stroke. 2009;40(7):2332–6. doi: 10.1161/STROKEAHA.109.553545. [DOI] [PubMed] [Google Scholar]
- 55.Yokota C, Minematsu K, Tomii Y , et al. Low levels of plasma soluble receptor for advanced glycation end products are associated with severe leukoaraiosis in acute stroke patients. J Neurol Sci. 2009;287(1-2):41–4. doi: 10.1016/j.jns.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Arboix A, Alió J. Cardioembolic Stroke: Clinical Features. Specific Cardiac Disorders and Prognosis. Curr Cardiol Rev. 2010;6(3):150–61. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arboix A, Alió J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther. 2011;9(3):367–79. doi: 10.1586/erc.10.192. [DOI] [PubMed] [Google Scholar]
- 58.Jickling GC, Xu H, Stamova B , et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. 2010;68(5):681–92. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker RC. Biomarkers in atrial fibrillation: investigating biologic plausibility cause and effect. J Thromb Thrombolysis. 2005;19(1):71–5. doi: 10.1007/s11239-005-0943-3. [DOI] [PubMed] [Google Scholar]
- 60.Skoloudík D, Bar M, Sanák D , et al. D-dimers increase in acute ischemic stroke patients with the large artery occlusion but do not depend on the time of artery recanalization. J Thromb Thrombolysis. 2010;29(4):477–82. doi: 10.1007/s11239-009-0372-9. [DOI] [PubMed] [Google Scholar]
- 61.Petzold A, Michel P, Stock M, Schluep M. Glial and axonal body fluid biomarkers are related to infarct volume. seveity.and outcome. . J Stroke Cerebrovasc Dis. 2008; 17(4):196–203. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Tuttolomondo A, Di Raimondo D, Di Sciacca R , et al. Fetuin-A and CD40 L plasma levels in acute ischemic stroke: Differences in relation to TOAST subtype and correlation with clinical and laboratory variables. Atherosclerosis. 2010;208(1):290–6. doi: 10.1016/j.atherosclerosis.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 63.Osmancik P, Peroutka Z, Budera P, Herman D, Stros P, Straka Z. Changes in cytokine concentrations following successful ablation of atrial fibrillation. European Cytokine Network. 2010;21(4):278–84. doi: 10.1684/ecn.2010.0216. [DOI] [PubMed] [Google Scholar]
- 64.Shin SY, Yong HS, Lim HE , et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22(6):647–55. doi: 10.1111/j.1540-8167.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 65.Shimano M, Shibata R, Tsuji Y , et al. Circulating adiponectin levels in patients with atrial fibrillation. Circ J. 2008;72(7):1120–4. doi: 10.1253/circj.72.1120. [DOI] [PubMed] [Google Scholar]
- 66.Choudhury A, Freestone B, Patel J, Lip GYH. Relationship of soluble CD40 ligand to vascular endothelial growth factor angiopoietins and tissue factor in atrial fibrillation: a link among platelet activation angiogenesis and thrombosis?. Chest. 2007;132(6):1913–9. doi: 10.1378/chest.07-1565. [DOI] [PubMed] [Google Scholar]
- 67.Falcone C, Buzzi MP, D’Angelo A , et al. Apelin plasma levels predict arrhythmia recurrence in patients with persistent atrial fibrillation. Int J Immunopathol Pharmacol. 2010;23(3):917–25. doi: 10.1177/039463201002300328. [DOI] [PubMed] [Google Scholar]
- 68.Ellinor PT, Low AF, Macrae CA. Reduced apelin levels in lone atrial fibrillation. Eur Heart J. 2006;27(2):222–6. doi: 10.1093/eurheartj/ehi648. [DOI] [PubMed] [Google Scholar]
- 69.Choudhury A, Chung I, Blann AD, Lip GYH. Platelet surface CD62P and CD63. mean platelet volume and soluble/platelet P-selectin as indexes of platelet function in atrial fibrillation: a comparison of healthy control subjects and disease control subjects in sinus rhythm. J Am Coll Cardiol. 2007;49(19):1957–64. doi: 10.1016/j.jacc.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 70.Marín F, Roldán V, Climent V, Garcia A, Marco P, Lip GYH. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor.TIMP-1?. . Stroke. 2003;34(5):1181–6. doi: 10.1161/01.STR.0000065431.76788.D9. [DOI] [PubMed] [Google Scholar]
- 71.Kalogeropoulos AS, Tsiodras S, Rigopoulos AG , et al. Novel association patterns of cardiac remodeling markers in patients with essential hypertension and atrial fibrillation. BMC Cardiovasc Disord. 2011;11:77. doi: 10.1186/1471-2261-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gartner W, Zierhut B, Mineva I , et al. Brain natriuretic peptide correlates with the extent of atrial fibrillation-associated silent brain lesions. Clin Biochem. 2008;41(18):1434–9. doi: 10.1016/j.clinbiochem.2008.09.096. [DOI] [PubMed] [Google Scholar]
- 73.Schnabel RB, Larson MG, Yamamoto JF , et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104(1):92–6. doi: 10.1016/j.amjcard.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Behnes M, Hoffmann U, Lang S , et al. Transforming growth factor beta 1 (TGF-beta 1) in atrial fibrillation and acute congestive heart failure. Clin Res Cardiol. 2011;100(4):335–42. doi: 10.1007/s00392-010-0248-1. [DOI] [PubMed] [Google Scholar]
- 75.Hijazi Z, Oldgren J, Andersson U , et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012;125(13):1605–16. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- 76.Scridon A, Morel E, Nonin-Babary E, Girerd N, Fernandez C, Chevalier P. Increased intracardiac vascular endothelial growth factor levels in patients with paroxysmal. but not persistent atrial fibrillation. Europace. 2012;14(7):948–53. doi: 10.1093/europace/eur418. [DOI] [PubMed] [Google Scholar]
- 77.Jickling GC, Stamova B, Ander BP , et al. Prediction of cardioembolic arterial and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke. 2012;43(8):2036–41. doi: 10.1161/STROKEAHA.111.648725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jickling GC, Stamova B, Ander BP , et al. Profiles of lacunar and nonlacunar stroke. Ann Neurol. 2011;70(3):477–85. doi: 10.1002/ana.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reid G, Kirschner MB, Van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol Hematol. 2011;80(2):193–208. doi: 10.1016/j.critrevonc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet Mol Res. 2012;11(1):147–52. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- 81.Zeng L, Liu J, Wang Y , et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 2011;3:1265–72. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 82.Tan KS, Armugam A, Sepramaniam S , et al. Expression profile of MicroRNAs in young stroke patients. PLoS ONE. 2009;4(11):e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z, Lu Y, Yang B. MicroRNAs and atrial fibrillation: new fundamentals. Cardiovasc Res. 2011;89(4):710–21. doi: 10.1093/cvr/cvq350. [DOI] [PubMed] [Google Scholar]
- 84.Liu Z, Zhou C, Liu Y , et al. The expression levels of plasma micoRNAs in atrial fibrillation patients. PLoS ONE. 2012;7(9):e44906. doi: 10.1371/journal.pone.0044906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adam O, Löhfelm B, Thum T , et al. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107(5):278. doi: 10.1007/s00395-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 86.Bellenguez C, Bevan S, Gschwendtner A , et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44(3):328–33. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tuttolomondo A, Di Raimondo D, Forte GI , et al. Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine. 2012;58(3):398–405. doi: 10.1016/j.cyto.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Jannes J, Hamilton-Bruce MA, Pilotto L , et al. Tissue plasminogen activator -7351C/T enhancer polymorphism is a risk factor for lacunar stroke. Stroke. 2004;35(5):1090–4. doi: 10.1161/01.STR.0000124123.76658.6c. [DOI] [PubMed] [Google Scholar]
- 89.Belfer I, Wu T, Hipp H , et al. Linkage of large-vessel carotid atherosclerotic stroke to inflammatory genes via a systematic screen. Int J Stroke. 2010;5(3):145–51. doi: 10.1111/j.1747-4949.2010.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kostulas N, Nikolaos K, Markaki I , et al. Common CCR 5 polymorphism in stroke: the CCR 5 delta32 polymorphism differentiates cardioembolism from other aetiologies of ischaemic cerebrovascular diseases. Scand J Immunol. 2009;70(5):475–80. doi: 10.1111/j.1365-3083.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 91.Dawson J, Walters M, Delles C, Mischak H, Mullen W. Urinary proteomics to support diagnosis of stroke. PLoS ONE. 2012;7(5):e35879. doi: 10.1371/journal.pone.0035879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dayon L, Turck N, Garcí-Berrocoso T , et al. Brain extracellular fluid protein changes in acute stroke patients. J Proteome Res. 2011;10(3):1043–51. doi: 10.1021/pr101123t. [DOI] [PubMed] [Google Scholar]
- 93.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 94.Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol. 2012;7(8):1355–64. doi: 10.2215/CJN.09590911. [DOI] [PubMed] [Google Scholar]