Abstract
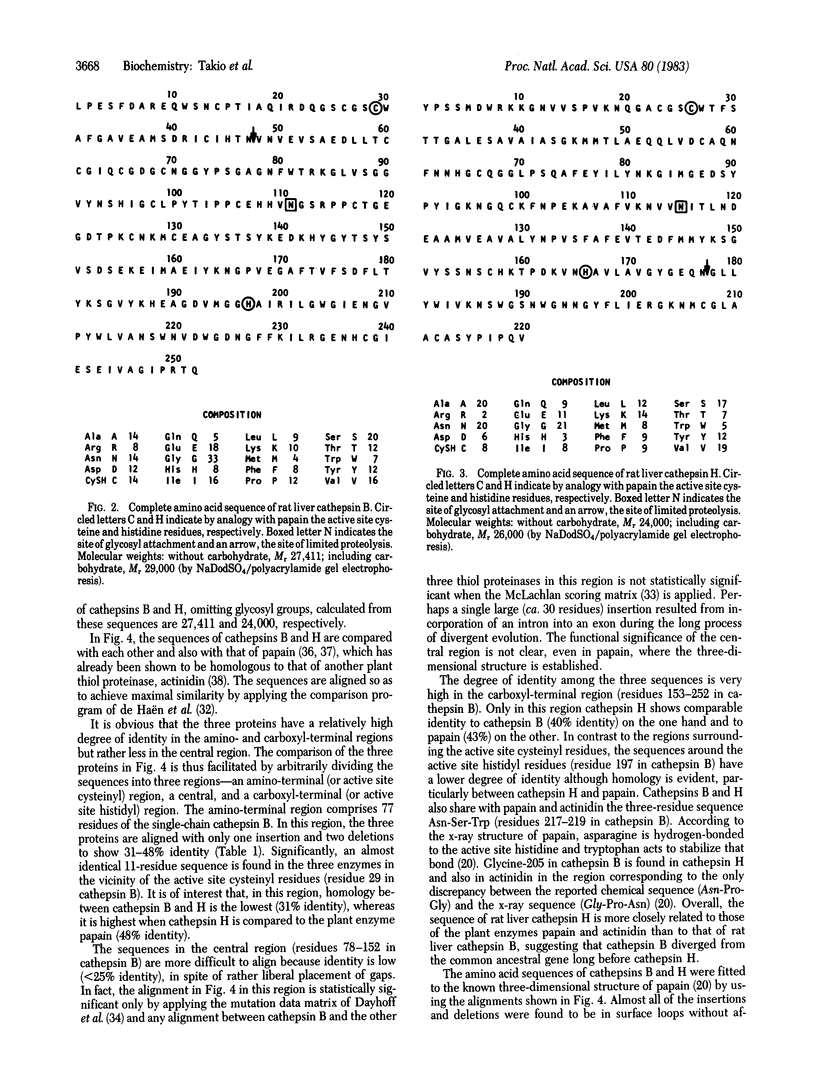
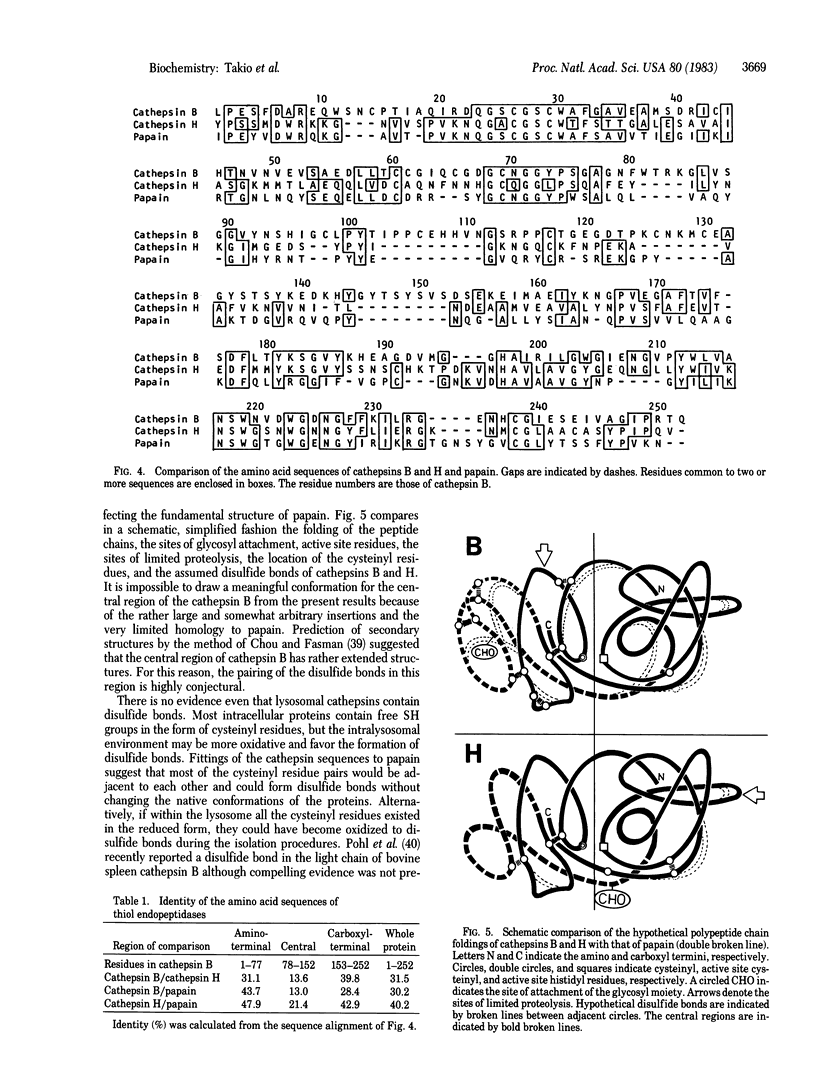
The amino acid sequences of rat liver lysosomal thiol endopeptidases, cathepsins B and H, are presented and compared with that of the plant thiol protease papain. The 252-residue sequence of cathepsin B and the 220-residue sequence of cathepsin H were determined largely by automated Edman degradation of their intact polypeptide chains and of the two chains of each enzyme generated by limited proteolysis. Subfragments of the chains were produced by enzymatic digestion and by chemical cleavage of methionyl and tryptophanyl bonds. Comparison of the amino acid sequences of cathepsins B and H with each other and with that of papain demonstrates a striking homology among their primary structures. Sequence identity is extremely high in regions which, according to the three-dimensional structure of papain, constitute the catalytic site. The results not only reveal the first structural features of mammalian thiol endopeptidases but also provide insight into the evolutionary relationships among plant and mammalian thiol proteases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Barrett A. J. The specificity of cathepsin B. Hydrolysis of glucagon at the C-terminus by a peptidyldipeptidase mechanism. Biochem J. 1978 Jun 1;171(3):759–765. doi: 10.1042/bj1710759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin: details of the polypeptide chain conformation and active site from an electron density map at 2-8 A resolution. J Mol Biol. 1977 Sep 25;115(3):263–277. doi: 10.1016/0022-2836(77)90154-1. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Wolthers B. G. The structure of papain. Adv Protein Chem. 1971;25:79–115. doi: 10.1016/s0065-3233(08)60279-x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Evans P., Etherington D. J. Characterisation of cathepsin B and collagenolytic cathepsin from human placenta. Eur J Biochem. 1978 Feb 1;83(1):87–97. doi: 10.1111/j.1432-1033.1978.tb12071.x. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gohda E., Pitot H. C. A new thiol proteinase from rat liver. J Biol Chem. 1981 Mar 10;256(5):2567–2572. [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Evaluation of several amino group reversible blocking reagents. Biochemistry. 1970 Dec 8;9(25):4939–4944. doi: 10.1021/bi00827a016. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. A reinvestigation of residues 64-68 and 175 in papain. Evidence that residues 64 and 175 are asparagine. Biochem J. 1970 Feb;116(4):689–692. doi: 10.1042/bj1160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunuma N., Towatari T., Kominami E., Hashida S., Takio K., Titani K. Rat liver thiol proteinases: cathepsin B, cathepsin H and cathepsin L. Acta Biol Med Ger. 1981;40(10-11):1419–1425. [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P., Broghammer U. Intrazellulärer Proteinabbau. VII. Kathepsin L und H: Zwei neue Proteinasen aus Rattenleberlysosomen. Acta Biol Med Ger. 1976;35(3-4):285–299. [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P., Hanson H. Cathepsin H: an endoaminopeptidase from rat liver lysosomes. Acta Biol Med Ger. 1977;36(2):185–199. [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Mitchel R. E., Chaiken I. M., Smith E. L. The complete amino acid sequence of papain. Additions and corrections. J Biol Chem. 1970 Jul 25;245(14):3485–3492. [PubMed] [Google Scholar]
- Omenn G. S., Fontana A., Anfinsen C. B. Modification of the single tryptophan residue of staphylococcal nuclease by a new mild oxidizing agent. J Biol Chem. 1970 Apr 25;245(8):1895–1902. [PubMed] [Google Scholar]
- Otto K., Bhakdi S. Zur Kenntnis des Kathepsins B': Spezifität und Eigenschaften. Hoppe Seylers Z Physiol Chem. 1969 Dec;350(12):1577–1588. [PubMed] [Google Scholar]
- Otto K. Uber ein neues Kathepsin. Reinigung aus Rindermilz, Eigenschaften, sowie Vergleich mit Kathepsin B. Hoppe Seylers Z Physiol Chem. 1967 Nov;348(11):1449–1460. [PubMed] [Google Scholar]
- Pohl J., Baudys M., Tomásek V., Kostka V. Identification of the active site cysteine and of the disulfide bonds in the N-terminal part of the molecule of bovine spleen cathepsin B. FEBS Lett. 1982 Jun 1;142(1):23–26. doi: 10.1016/0014-5793(82)80210-x. [DOI] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Kalnitsky G. alpha-N-benzoylarginine-beta-naphthylamide hydrolase, an aminoendopeptidase from rabbit lung. J Biol Chem. 1980 Jan 25;255(2):369–374. [PubMed] [Google Scholar]
- Suhar A., Marks N. Purification and properties of brain cathepsin B. Evidence for cleavage of pituitary lipotropins. Eur J Biochem. 1979 Nov 1;101(1):23–20. doi: 10.1111/j.1432-1033.1979.tb04211.x. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H., JONES M. E., FRUTON J. S. On the proteolytic enzymes of animal tissues. X. Beef spleen cathepsin C. J Biol Chem. 1952 Feb;194(2):793–805. [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Titani K. Primary structure study of rat liver cathepsin B -- a striking resemblance to papain. Biochem Biophys Res Commun. 1980 Nov 17;97(1):340–346. doi: 10.1016/s0006-291x(80)80173-2. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Towatari T., Kawabata Y., Katunuma N. Crystallization and properties of cathepsin B from rat liver. Eur J Biochem. 1979 Dec;102(1):279–289. doi: 10.1111/j.1432-1033.1979.tb06290.x. [DOI] [PubMed] [Google Scholar]
- Towatari T., Tanaka K., Yoshikawa D., Katunuma N. Purification and properties of a new cathepsin from rat liver. J Biochem. 1978 Sep;84(3):659–672. doi: 10.1093/oxfordjournals.jbchem.a132171. [DOI] [PubMed] [Google Scholar]
- Towatari T., Tanaka K., Yoshikawa D., Katunuma N. Separation of a new protease from cathepsin B1 of rat liver lysosomes. FEBS Lett. 1976 Sep 1;67(3):284–288. doi: 10.1016/0014-5793(76)80548-0. [DOI] [PubMed] [Google Scholar]
- Turnsek T., Kregar I., Lebez D. Acid sulphydryl protease from calf lymph nodes. Biochim Biophys Acta. 1975 Oct 22;403(2):514–520. doi: 10.1016/0005-2744(75)90079-0. [DOI] [PubMed] [Google Scholar]
- de Haën C., Swanson E., Teller D. C. The evolutionary origin of proinsulin. Amino acid sequence homology with the trypsin-related serine proteases detected and evaluated by new statistical methods. J Mol Biol. 1976 Sep 25;106(3):639–661. doi: 10.1016/0022-2836(76)90256-4. [DOI] [PubMed] [Google Scholar]



