ABSTRACT
Purpose: The primary purpose of this systematic review was to evaluate the anabolic effect of exercise intervention in adults with end-stage renal disease on hemodialysis (HD). The secondary objectives were to evaluate the influences of participant characteristics and exercise parameters on changes in muscle size. Methods: Electronic databases (Cochrane, CINAHL, EMBASE, PEDro, PubMed and SCOPUS) were searched from inception to November 2012. Randomized clinical trials published in English that included adults on HD undergoing an exercise intervention where muscle mass was measured as an outcome were included in this review. Two reviewers independently selected the studies, extracted data, and assessed risk of bias within the included studies. Results were then combined by meta-analysis. The effect of exercises was determined using a standardized mean difference (SMD), expressed as Hedges' g, computed using a random effects model. Results: Seven SMDs extracted from five studies were included for final analysis. Strength training was used in all studies; one study used aerobic and mixed strength and aerobic training with two subgroups of participants. The overall effect of exercise on muscle mass was statistically significant (SMD: 0.272; 95% CI, 0.020–0.525). Conclusions: Our results confirm a small but significant effect of strengthening exercise as an anabolic intervention to increase muscle mass. Exercise training should be included in routine management of people on maintenance HD. Although current results indicate that one in nine people on HD is likely to benefit from exercise intervention, parameters influencing these results require further research.
Key Words: bioelectrical impedance, computed tomography scan, dual energy x-ray absorptiometry, exercise, hemodialysis, magnetic resonance imaging, skeletal muscle
RÉSUMÉ
Objectif : Cette critique systématique visait principalement à évaluer l'effet anabolique de l'exercice chez des adultes vivant avec une insuffisance rénale chronique au stade ultime et suivant des traitements d'hémodialyse (HD). Les objectifs secondaires consistaient à évaluer l'influence des caractéristiques des participants et des paramètres de l'exercice sur les changements de la taille des muscles. Méthodes : On a effectué des recherches dans des bases de données électroniques (Cochrane, CINAHL, EMBASE, PEDro, PubMed et SCOPUS) depuis leur création jusqu'en novembre 2012. On a inclus des essais cliniques randomisés publiés en anglais et portant sur des adultes en HD suivant un programme d'exercice au cours duquel on a mesuré la masse musculaire comme résultat. Deux examinateurs agissant indépendamment ont choisi les études, extrait des données et évalué le risque de biais dans les études incluses. Les résultats ont ensuite été combinés par méta-analyse. On a déterminé l'effet de l'exercice au moyen d'une différence moyenne normalisée (DMN) exprimée par la mesure g de Hedges, calculé au moyen d'un modèle à effet aléatoire. Résultats : Sept DMV extraites de cinq études ont été incluses pour analyse finale. On a utilisé la musculation dans toutes les études, dont une a utilisé l'entraînement aérobie et mixte (musculation et aérobie) avec deux sous-groupes de participants. L'effet global de l'exercice sur la masse musculaire a été statistiquement significatif (DMN: 0,272; IC à 95%, 0,020 à 0,525). Conclusion : Nos résultats confirment un effet modeste mais significatif de l'exercice de renforcement comme intervention anabolique visant à accroître la masse musculaire. Il faudrait inclure l'exercice dans la prise en charge de routine des personnes en HD d'entretien. Même si les résultats courants indiquent qu'une personne sur neuf en HD est susceptible de bénéficier de l'exercice, une recherche plus poussée s'impose au sujet des paramètres qui jouent sur ces résultats.
Mots clés : Hémodialyse, exercice, muscle squelettique, examen DXA, impédance bioélectrique, IRM, TDM
Chronic kidney disease is the progressive failure of renal function over a period of years. In the end stage of renal disease, renal replacement therapies (RRT) such as haemodialysis (HD), peritoneal dialysis (PD), or kidney transplant are required to supplement the metabolic homeostatic functions of the kidney. According to the Canadian Organ Replacement Registry maintained by the Canadian Institute for Health Information, there were an estimated 39,352 people living with end-stage renal disease (ESRD) in Canada at the end of 2010, more than triple the number recorded in 1991.1 Of these, 23,188 were on HD/PD and 16,164 were living with a functioning kidney transplant. The aging of the Canadian population is reflected in the demographic profile of new ESRD patients: 53% of those who initiated RRT in 2010 were aged 65 years and older, compared to 39% in 1991.1 Haemodialysis is considered to have more pronounced deleterious effects on skeletal muscle than PD.2 One difference between the two dialysis techniques is the fluctuation in toxin levels2 (from very high pre-dialysis to very low post-dialysis): people on HD typically dialyze three times per week, whereas people on PD dialyze every 4 hours or daily.3
People with ESRD on HD experience multiple catabolic processes, including loss of albumin and amino acids during dialysis, metabolic derangements, and changes in skeletal muscle associated with conditions of muscle disuse.4 These changes result in muscle atrophy (loss of lean muscle mass). The presence of neurogenic (muscle atrophy or loss associated with nerve disorder), myogenic (damage intrinsic to the muscle), and mixed (neurogenic and myogenic) changes intrinsic to the skeletal muscle in people with ESRD on HD5 may further compromise the integrity of the motor-unit complex and contribute to muscle atrophy.6 Because muscle wasting is a significant predictor of morbidity and mortality in this population,7 prevention is clinically important to maintain or improve physical function and to prevent falls and related hospital visits, a critical issue for managing health care costs.8 Physical therapy interventions to counteract muscle atrophy and promote muscle anabolism have therefore been strongly recommended for this population.9
Exercise may promote an anabolic milieu, thus increasing muscle mass, improving muscle strength, and ameliorating muscle catabolism.10 Dong and Ikizler (2009)11 and Storer (2009)12 have recently reviewed anabolic interventions, including exercise, in people with ESRD on HD. They found that the studies using aerobic or strength training or mixed aerobic and strengthening exercise as anabolic interventions were unable to consistently demonstrate beneficial anabolic effects. Two recent systematic reviews and meta-analyses of exercise training in patients on HD, by Smart and Steele13 and Heiwe and colleagues,14 showed that exercise training is safe and is associated with sizeable improvements in peak oxygen consumption, sympathetico-adrenal activity, physical function, and health-related quality of life. Although Heiwe and colleagues14 reviewed outcomes evaluating muscle morphology and morphometrics, their results may not be relevant to our objectives for two reasons: first, a study that recruited participants with chronic kidney disease not on HD15 was included in their cumulative analysis; second, several studies that we have identified as relevant were not considered relevant by Heiwe and colleagues,14 which may have affected their evaluation of the effect of exercise on the mid-thigh muscle area.
The primary objective of this systematic review, therefore, was to evaluate the effectiveness of exercise training programs on increasing muscle mass in the adult population with ESRD on HD. Secondary objectives were to evaluate the influence of the following parameters on the cumulative results: (1) age; (2) day of exercise intervention (dialysis day vs. off-dialysis day); (3) mode of exercise (aerobic, strength-training, or a combination); (4) outcome measure used to reflect muscle mass, such as muscle cross-sectional area (CSA) or lean body mass (LBM); and (5) measurement techniques used, such as dual energy X-ray absorptiometry (DXA), bio-electrical impedance spectroscopy (BIS), computed tomography (CT), or magnetic resonance imaging (MRI).
Methods
Study design
Our systematic review used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout the literature search and reporting phases of development.16
Data sources and search strategy
We searched the online databases CINAHL, Cochrane Library, EMBASE, PEDro, PubMed, and SCOPUS from inception to the end of November 2012 to identify relevant studies using the following as MeSH search terms and keywords: kidney failure AND exercise AND muscle mass. We limited our searches to studies published in English and involving stable adult human participants on HD. Reference sections of the retrieved articles were hand-searched for citations missed by the electronic searches.
Study inclusion and exclusion criteria
We included studies that (1) were published in English; (2) used a randomized controlled trial or crossover trial design; (3) recruited participants with a diagnosis of ESRD requiring maintenance HD as renal replacement therapy and who were>18 years of age, were on HD for>3 months, and received exercise training for a minimum of 8 weeks, either during dialysis sessions (intra-dialytic) or on non-dialysis days, in a structured, supervised environment; and (4) included a measure of muscle mass. Studies that used biopsy or messenger RNA as the measure of muscle mass were excluded.
Outcome measures
Muscle mass has been measured in intervention and epidemiological studies at the cellular, molecular, and tissue level.17 Therefore, the primary outcome measures were validated surrogate measures of muscle mass: (1) fat-free-mass (FFM), measured using multi-frequency BIS and DXA; (2) LBM, measured using BIS or DXA; (3) muscle CSA, measured by CT or MRI; and (4) muscle attenuation, measured in Hounsfield unit values generated using CT scans.
Study quality
We assessed the internal validity of the included studies using statistical criteria of sample size and power required for establishing possible type I or type II errors. For randomized controlled trials (RCTs), we used the PEDro scale, a valid18 and reliable19 tool for evaluating study quality.
Study selection and data extraction
Following the primary search, two authors (AS, TO) independently reviewed titles, abstracts, and full text to determine whether each study met our inclusion and exclusion criteria. Discrepancies were resolved through discussion; all articles that were included were done so by consensus.
One author extracted required data for effect size calculations using a standardized form; a second author reviewed the forms for accuracy. We coded the data using categorical numerical codes based on theoretical constructs, including type of exercise (aerobic training, resistance training, or mixed aerobic and strength training), outcome measure (muscle attenuation, LBM, or muscle CSA), and measurement tool (CT, MRI, DXA, or BIS). We contacted the study authors for further information regarding published data that were presented in a format other than that accepted by the meta-analysis software or data that were not analyzed with intention to treat.
Statistical analyses
Comprehensive Meta-Analysis software, version 2.2.040 (Biostat, Englewood, NJ), was used to compute standardized mean differences (SMDs) expressed as Hedges' g.20 The effects on muscle mass of type of exercise, day of exercise intervention, and outcome measures used were analyzed using the Hedges' g values and their 95% CIs (CI). Hedges' g values reported here were calculated using the random effects model to account for methodological differences among studies. For the study by Dong and colleagues,21 data provided by the authors for participants who completed the study were included in the analysis.
The statistical significance of the differences in the moderator variables was computed by Page's L statistic22 using IBM SPSS V. 20.0 statistical software to calculate the between-groups and total sum of squares (SS). We then calculated the L statistic using the formula L=(N−1)r2, where N is the total number of effect sizes and r2 is the product of ; we then compared the computed value against the χ2 value for (k−1) degrees of freedom (df), where k is the total number of effect sizes included in the analysis. The significance of the L statistic was evaluated using a χ2 table.
The presence of heterogeneity among the moderator variables was evaluated by the Q statistic, using a random-effects model. Studies were considered heterogeneous if p<0.05 for the Q statistic. The SMDs (Hedges' g) were interpreted as small, medium, and large for values ≤0.2, ≤0.5, and ≤0.8 respectively.23 We assessed publication bias using a funnel plot of the standard error versus the SMD. A 95% CI around the point estimates of the effect sizes and the number of null studies required to change a statistically significant result to a non-significant finding (Fail Safe N) were used to assess robustness of the findings. The critical number of studies (Fail Safe N) was calculated using Hedges and Olkin's24 formula:
, where K0=number of new studies needed to produce a trivial effect size, K=number of studies in the meta-analysis, mean d=mean effect size from all studies, and d-trivial=estimate of a trivial effect size, assumed at 0.05.
Significance was set at p<0.05 for all statistical tests.
Results
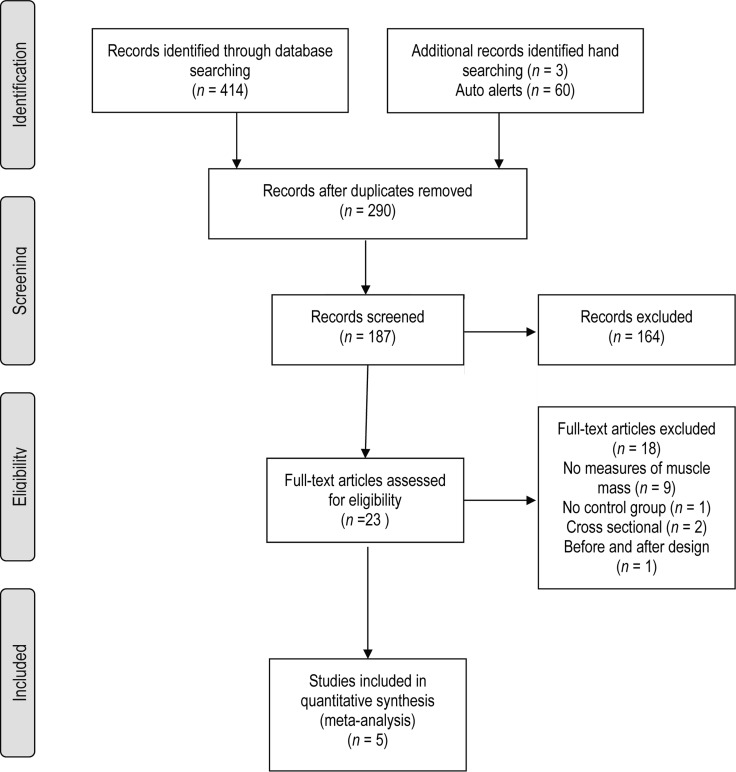
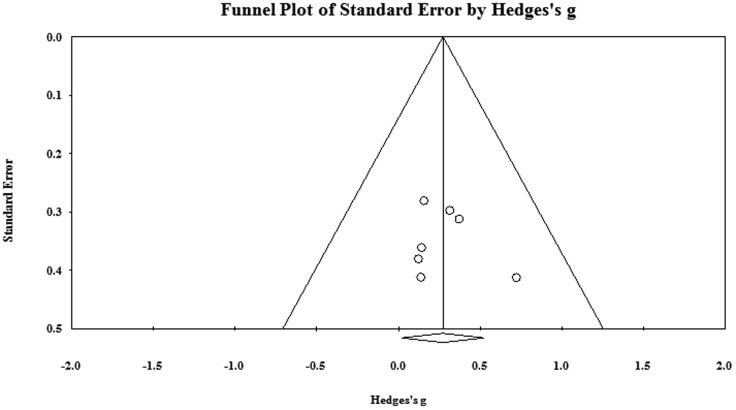
Five7,21,25–27 of the 477 citations retrieved following the primary search were included for analysis (see Figure 1). A total of 10 SMDs were extracted, seven of which were analyzed to evaluate the efficacy of exercise training on muscle mass (i.e., one effect size per group receiving exercise intervention; see Figure 2). Kopple and colleagues27 investigated the effect of strength, aerobic, and mixed training in three separate groups of participants; three SMDs extracted from this study were therefore included in the analysis. Characteristics of studies included in the SMD analysis are shown in Table 1; Table 2 presents characteristics of participants included in these studies. As Figure 3 indicates, there was no evidence of publication bias in the pool of included studies; however, the absence of studies in the two-tailed areas indicates a lack of publications demonstrating large significant or non-significant effects of exercise.
Figure 1.
Flowchart of study selection.
Figure 2.
Effect of exercises on muscle mass.
Heterogeneity Q6=1.86; p=0.93; I2=0
Table 1.
Characteristics of Individual Studies
| Study and PEDro Score |
Sample Size |
Experimental Intervention |
Duration, timing, and frequency of intervention |
|||||
|---|---|---|---|---|---|---|---|---|
| Exp | Con | Control Intervention | Outcome tool | Results and p values | Hedge's g | |||
| Cheema 20077 8 |
24 | 25 | Strength training | No intervention | 12 wk, during dialysis, 3×/wk | CT | No change in thigh muscle CSA; p=0.40 | 0.078 |
| Chen 201026 6 |
22 | 22 | Strength training | Stretching exercises with light resistance bands | 24 wk, during dialysis, 2×/wk | DXA | Leg lean mass changed; p<0.001 | 0.323 |
| Dong 201121 5 |
10 | 12 | Strength training and nutritional supplement | Nutritional supplement alone | 6 mo, 3 sets of 12 reps of 1 RM; just before dialysis on dialysis day | DXA | Leg lean mass did not change significantly; p=0.33 | 0.146 |
| Johansen 200625 7 |
20 | 20 | Strength training | Placebo nandrolone | 12 wk, 2 sets of 10 reps of each exercise during dialysis | MRI | Change in quadriceps muscle area was significant; p=0.02 | 0.362 |
| Kopple 200627 4 |
ET-10 ST-15 EST-12 |
14 | Endurance training, Strength training Endurance and strength training | No exercises | 21 wk, ET-during first 60 min of dialysis ST—just before dialysis EST—as above, 3×/wk | DXA, BIS | Significant change in FFM between pre and post measurements in the ET, ST, EST and controls as measured with DXA, p<0.05 Non-significant when measured with BIS for the three exercising groups combined |
BIS: 0.724; DXA: 0.527 BIS: 0.144; DXA: 0.059 BIS: 0.124; DXA: 0.235 |
Exp=experimental; Con=control; CT=computerized tomography; CSA=cross-sectional area; DXA=dual energy X-ray absorptiometry; RM=repetition maximum; MRI=magnetic resonance imaging; BIS=bioelectrical impedance spectroscopy; FFM=fat-free mass; ET=endurance training; ST=strength training; EST=endurance and strength training.
Table 2.
Characteristics of Participants Included in Individual Studies
| Study | Mean (SD) age of participants, y |
Mean (SD) no. of comorbidities; duration of dialysis, mean (SD/range) |
Inclusion criteria |
|---|---|---|---|
| Cheema 20077 | Exp: 60.0 (14.2) Con: 65.0 (15.3) |
Exp: 4.9 (1.6); 3.3 (0.3–16.7) y Con: 5.2 (2.0); 1.6 (0.6–10.3) y |
Age >18 y; >3 mo on HD; without acute or chronic medical condition that could preclude PRT of data collection; able to ambulate without assistive devices >50m; Kt/V ≥1.2; stable during dialysis. |
| Chen 201026 | Exp: 71.1 (12.6) Con: 66.9 (13.4) |
Exp: 7.3 (2.9); 1.54 (0.14) Con: 6.3 (2.4); 1.65 (0.38) |
Age >30 y; serum albumin <4.2 g/dl; on HD 3×/week and 80% compliance. |
| Dong 201121 | Exp: 46.5 (12.1) Con: 40.2 (13.5) |
Co-morbidities and duration of dialysis not indicated | Age >18 y; >3 mo on HD; Kt/V ≥1.2; on HD 3×/week using bio-compatible HD membrane. |
| Johansen 200625 | Exp: 54.4 (13.6) Con: 56.8 (13.8) |
% of participants with DM, CAD, HTN, PAD and CVA in both groups has been reported. Total number of comorbidities not reported. Exp: 33.0 (3.5–108) mo Con: 25.5 (3–156) mo |
Kt/V ≥1.2; on HD 3×/week; free of HIV and malignancy; did not have infection in the past 3 mo. |
| Kopple 200627 | Exp: (a) ET 45.9 (4.1) (b) ST46.0 (2.7) (c) EST 42.7 (3.8) |
No. of comorbidities not indicated; Exp: 45.9(4.1) mo Con: 51.4(21.0) mo Exp: 46.0(2.7) mo Con: 51.4(21.0) mo Exp: 42.7(3.8) mo Con: 51.4(21.0) mo |
Age 25–65 y; Clinically stable HD; on HD 3×/week. |
Exp=experimental; Con=control; HD=haemodialysis; PRT=progressive resistance training; DM=diabetes mellitus; CAD=coronary artery disease; HTN=hypertension; PAD=peripheral arterial disease; CVA=cerebro-vascular accident; ET=endurance training; ST=strength training; EST=endurance and strength training.
Figure 3.
Funnel plot for publication bias.
Study quality
All studies reported protection against type I error by convention; however, only one study7 also reported sample size calculations to protect against type II error. Four7,21,25,26 of the five studies suggest adequate opportunity of inclusion in any one group by ensuring allocation concealment. None of the studies blinded participants, therapists, or outcome assessors; however, use of objective quantitative measures such as MRI and CT reduces the risk of potential observer bias (see Table 3).
Table 3.
Risk of Bias: PEDro Scores of Included Papers
| Study ID | Cheema (2007)7 | Chen (2010)26 | Dong (2011)21 | Johansen (2006)25 | Kopple (2006)27 |
|---|---|---|---|---|---|
| Allocation | |||||
| Random | Yes | Yes | Yes | Yes | Yes |
| Concealed | Yes | Yes | Yes | Yes | No |
| Subjects | No | No | No | No | No |
| Blinding | |||||
| Therapists | No | No | No | No | No |
| Assessors | Yes | No | No | No | No |
| Adequate | Yes | Yes | No | Yes | No |
| Follow-up | |||||
| Intention-to-treat | Yes | No | No | Yes | No |
| Baseline comparability | Yes | Yes | Yes | Yes | Yes |
| Between-group comparison | Yes | Yes | Yes | Yes | Yes |
| Point estimates and variability | Yes | Yes | Yes | Yes | Yes |
Effect of exercise on muscle mass
The SMD (Hedges' g value) for exercise intervention on muscle mass under the random effects model was statistically significant (0.272; 95% CI, 0.020–0.579; p= 0.034) (see Figure 2). More than 30 studies showing a null effect would be required to negate the significance of our findings (trivial difference=0.05, Fail safe N= 31.08). Interestingly, the Q statistic assessing the heterogeneity of the studies was not statistically significant (Q6=1.864; p=0.93). This lack of heterogeneity is likely due to the small number of included studies.
Effect of type of exercise intervention
The influence of type of exercise intervention on estimates of muscle mass following exercise was not statistically significant (L9=4.05, p>0.05). Five SMDs were extracted from studies that provided a strength training intervention.7,21,25–27 The effect of strength training on muscle mass was not statistically significant (Hedges' g=0.238; 95% CI, −0.046 to 0. 522; p=0.10). Only one study27 evaluated the effect of aerobic and mixed aerobic and strength training; in neither case was there a statistically significant effect (aerobic training: Hedges' g= 0.0.724; 95% CI, −0.09 to 1.53; p=0.80; mixed training: Hedges' g=0.12; 95% CI, −0.62 to 0.87; p=0.74).
Effect of differences in outcome measure
The SMDs evaluating the influence of exercise (aerobic and/or strength training) on muscle mass using FFM (Hedges' g=0.304; 95% CI, −0.130 to 0.739; p=0.17), leg lean mass (LLM) (Hedges' g=0.257; 95% CI, −0.217 to 0.730; p=0.29), thigh/quadriceps muscle CSA (Hedges' g=0.210; 95% CI, −0.2 to 0.62; p=0.32), or muscle attenuation (Hounsfield units) (Hedges' g= 0.159; 95%CI, −0.393 to 0.711; p=0.57) as outcome measures were not statistically significant (L9=2.059, p>0.05). Kopple and colleagues27 reported estimates of muscle mass as FFM using DXA and BIS. Our analysis used measures of FFM acquired using BIS. Two studies21,26 used LLM as an outcome measure to estimate muscle mass. However, only one study7 measured muscle attenuation using CT, and one25 used MRI to measure quadriceps muscle CSA.
Effect of differences in the outcome tool
The Hedges' g values for the effect sizes evaluating the influence of exercise (aerobic and/or strength training) on muscle mass using DXA (Hedges' g=0.258; 95% CI, −0.064 to 0.579; p=0.12), BIS (Hedges' g=0.304; 95% CI, −0.130 to 0.739); p=0.17), CT (Hedges' g=0.159; 95% CI, −0.393 to 0.711; p=0.57), or MRI (Hedges' g=0.374; 95% CI, −0.239 to 0.987; p=0.23) as outcome tools were not statistically significant (L9=1.418, p> 0.05). Three studies21,26,27 used DXA to estimate LLM or FFM, providing five SMDs. Only Kopple and colleagues27 reported estimates of FFM using BIS, providing three SMDs; one study used CT,7 and one used MRI.25
We were unable to evaluate the effect of age because none of the included studies categorically recruited participants over age 60 years (using 60 years as the cut-off age to form groups of “old” participants has been suggested elsewhere).28 However, the mean age of the participants recruited in two studies7,26 was>60 years, while participants in the other three studies21,25,27 had a mean age of<60 years. An exploratory evaluation of the effect of exercise on muscle mass (Hedges' g) in the two studies with “older” participants7,26 was smaller (Hedges' g= 0.234; 95%CI, −0.168 to 0.635; p=0.25) compared to the studies21,25,27 in which the mean age of the participants was less than 60 years of age; the Hedges' g value approached significance in the latter group of studies (Hedges' g=0.298; 95% CI, −0.027 to 0.622; p=0.07).
We were also unable to evaluate the influence of day of exercise intervention (on- or off-dialysis) on muscle mass, as all included studies administered exercise interventions on dialysis days, either during or just before dialysis.
Discussion
The results of our systematic review and mathematical combination of SMDs indicate a small but statistically significant positive effect of strength training on muscle mass in people with ESRD on HD. This indicates that one in nine participants is likely to show increased muscle mass following exercise intervention.29
All the included studies employed strength training interventions; only one reported results of aerobic and mixed exercise interventions in two separate groups of participants.27 However, factors such as differences in participants' mean age, participants' level of physical activity, male to female participant ratio,30 or magnitude of effort31 may have influenced our findings. The participants in the included studies performed strength training at various levels of intensity; participants in the studies by Chen and colleagues26 and Johansen and colleagues25 performed 3 sets of 10 repetitions of leg exercises at 60% of 1 and 3 repetition maximum (RM), respectively, whereas participants in the Cheema7 study performed 2 sets of 8 repetitions of 10 exercises targeting the major muscle groups of the upper and lower extremities at a rating of perceived exertion of “hard” to “very hard.” Thus, the intensity of strength training adjusted to 60% of 1 or 3 RM may influence its effectiveness as an anabolic intervention. Interestingly, participants in the study by Dong and colleagues21 worked slightly harder (70% of 1 RM), but no increase in lean muscle mass was observed following 24 weeks of training. Whether differences in the intensity of exercise truly influence the effectiveness of exercise intervention for muscle anabolism requires further investigation.
Häkkinen and colleagues30 observed a difference in training effects in men and women over age 70 years. They reported a small (2%) and non-significant increase in quadriceps muscle CSA in older men and a significant 6% increase in quadriceps muscle CSA in elderly women following 6 months of strength training. These results suggest greater gains in muscle mass in healthy older women than in men following strength training exercises. Thus, the training effects observed by Cheema and colleagues7 (adjusted mean difference of −0.4 in muscle attenuation; male:female ratio of 17:7) and Johansen and colleagues25 (mean change of 1.2% in muscle CSA; male:female ratio of 12:8) may have been influenced by the sex of the participants; the larger training effects observed in women may have been nullified by smaller training effects in male participants. Since the studies included in this review did not report results by sex, the evidence on how the anabolic effect of exercise differs by sex remains inconclusive.
Reporting of participant demographics requires further attention for adequate comparison of factors limiting outcomes of exercise training, such as the presence of peripheral neuropathies or participants' physical activity levels. Since neuropathic and myopathic changes have been reported in the skeletal muscle of people with ESRD on HD,5 unequal proportions of participants with neuropathies in the different groups may have influenced the outcomes.32 However, the included studies did not report the incidence of polyneuropathy among participants. Further, Cupisti and colleagues33 reported a positive relationship between the mean daily metabolic equivalent of tasks and the phase angle measured using bio-electrical impedance vector analysis, a proxy measure for muscle mass and, hence, for strength.34 It is intuitive to assume that level of physical activity may augment the effects of strength training in this population, particularly for people with ESRD on HD with physical activity levels 20%–50% lower than age- and sex-matched sedentary population controls.35,36 However, because none of the included studies reported participants' level of physical activity, we were unable to confirm whether it varied significantly to affect the outcomes.
The lack of significant difference by type of exercise (aerobic or strength training) in our analysis should also be interpreted with caution. Sakkas and colleagues37 were able to demonstrate an increase in muscle fibre areas following 6 months of aerobic training in nine participants, but further research in this area is required to confirm the differences in anabolic effects that result from aerobic versus strengthening exercises.
Another factor requiring further investigation is the impact of accrued extracellular fluid (ECF) between dialysis treatments. During the interdialytic period, fluid accumulates within tissues,38 particularly in the lower extremities, as a result of gravitational forces.39 According to Kushner and colleagues,40 mean ECF in people with ESRD on HD is ∼107% (SD 19%) of control values, and intracellular fluid is ∼93% (SD 18%) of controls, suggesting that extra body water accruing during interdialytic periods tends to accumulate mainly in the extracellular spaces. This suggests the possibility of a higher percentage of ECF volume in people on dialysis when compared to controls. This “excess ECF” may tend to confound the measurement of true muscle size. Further research is required to establish techniques for accurately estimating ECF between dialysis treatments and its impact on measurements of muscle mass and size.
Several observational studies have shown improvements in aerobic capacity following exercise training on non-dialysis days.41 Although we set out to evaluate whether participant age and day of exercise intervention (dialysis vs. non-dialysis) affect the anabolic effect of exercises, we found no RCT that categorically recruited “older” participants or described training that took place on non-dialysis days. Therefore, our results are applicable for only exercise administered during dialysis in an adult population.
One of the major limitations of this review is that the included studies used different estimates of skeletal muscle mass. Johansen and colleagues25 showed improvement in the quadriceps muscle area following 12 weeks of progressive resistance training as measured by MRI, but Cheema and colleagues7 were unable to demonstrate any increase in thigh muscle CSA using CT scans following a resistance training program of similar duration. However, Cheema and colleagues7 did demonstrate an increase in the attenuation of the CT signal, which suggests an increase in lean muscle mass. These results, expressed in Hounsfield units, were included in our analysis. We included data on muscle attenuation to reduce the bias of suggesting there was no improvement (as demonstrated by non-significant change in thigh-muscle CSA) associated with exercise intervention, when in fact there was an increase in thigh muscle attenuation, reflective of possible muscle hypertrophy without “true” increase in size. Five effect sizes included in the analysis were calculated from the three studies21,26,27 that used DXA to measure LBM or FFM; we also included two effect sizes from the one study that used BIS to measure FFM.27 Body composition analysis using DXA is considered to be at the molecular17 level and is dependent on several factors, including the software used to analyze the acquired images.42 Measurement of body composition using BIS is considered to be at a “whole body”17 level and is also dependent on several factors, including cellular resistance, amount of subcutaneous fat, and equations used to calculate body composition.42 While it is reasonable to question the wisdom and validity of combining the SMDs obtained for such heterogeneous outcomes, we contend that this effort was warranted, at the very least, to provide a crude estimate of the likely effect of exercise on skeletal muscle mass. Furthermore, our review emphasizes the need for further research to evaluate the anabolic effects of exercise using uniform, gold standard measurements of muscle mass for appropriate comparisons.
Conclusion
Our study evaluated the anabolic effect of exercise training in people with ESRD on HD using data from five studies, representing a total of 206 participants. Our results support the use of exercise to promote anabolism; one in nine participants is likely to benefit from such an intervention.28 We did not find a study providing direct evidence of a relationship between increments in muscle size and reduction of relative risk of death from all causes. However, among people on HD43 or PD,44 those with normal BMI and high muscle mass had a lower relative risk than those with high BMI and lower muscle mass.
Key messages
What is already known on this topic
Exercise interventions are beneficial for maintaining and/or improving skeletal muscle strength and mass in people with end-stage kidney disease on haemodialysis. Exercise training should be routinely included in management of this population.
What this study adds
This study found that one in nine participants with end-stage kidney disease on haemodialysis is likely to benefit from exercise interventions. Those most likely to improve are people <60 years of age who perform strength and/or aerobic exercise training during haemodialysis sessions three times a week.
Physiotherapy Canada 2014; 66(1);44–53; doi:10.3138/ptc.2012-59
References
- 1.Canadian Institute of Health Information. Canadian Organ Replacement Register annual report: treatment of end-stage organ failure in Canada, 2001 to 2010. [Internet] Ottawa: The Institute; 2012. [cited 2012 Nov 15]. [updated 2011]. Available from: https://secure.cihi.ca/free_products/2011_CORR_Annua_Report_EN.pdf. [Google Scholar]
- 2.Raj DS, Sun Y, Tzamaloukas AH. Hypercatabolism in dialysis patients. Curr Opin Nephrol Hypertens. 2008;17(6):589–94. doi: 10.1097/MNH.0b013e32830d5bfa. http://dx.doi.org/10.1097/MNH.0b013e32830d5bfa. Medline:18941351. [DOI] [PubMed] [Google Scholar]
- 3.Sakkas GK, Ball D, Sargeant AJ, et al. Skeletal muscle morphology and capillarization of renal failure patients receiving different dialysis therapies. Clin Sci (Lond) 2004;107(6):617–23. doi: 10.1042/CS20030282. http://dx.doi.org/10.1042/CS20030282. Medline:15253690. [DOI] [PubMed] [Google Scholar]
- 4.Ikizler TA, Himmelfarb J. Muscle wasting in kidney disease: let's get physical. J Am Soc Nephrol. 2006;17(8):2097–8. doi: 10.1681/ASN.2006060629. http://dx.doi.org/10.1681/ASN.2006060629. Medline:16837638. [DOI] [PubMed] [Google Scholar]
- 5.Sawant A, Garland SJ, House AA, et al. Morphological, electrophysiological, and metabolic characteristics of skeletal muscle in people with end-stage renal disease: a critical review. Physiother Can. 2011;63(3):355–76. doi: 10.3138/ptc.2010-18. http://dx.doi.org/10.3138/ptc.2010-18. Medline:22654242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appell HJ. Muscular atrophy following immobilisation. A review. Sports Med. 1990;10(1):42–58. doi: 10.2165/00007256-199010010-00005. http://dx.doi.org/10.2165/00007256-199010010-00005. Medline:2197699. [DOI] [PubMed] [Google Scholar]
- 7.Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–601. doi: 10.1681/ASN.2006121329. http://dx.doi.org/10.1681/ASN.2006121329. Medline:17409306. [DOI] [PubMed] [Google Scholar]
- 8.Lecker SH. Given the science on malnutrition, how does the clinician respond? Practical lessons for and application to the dialysis patient. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S64–70. doi: 10.2215/CJN.02650409. http://dx.doi.org/10.2215/CJN.02650409. Medline:19996008. [DOI] [PubMed] [Google Scholar]
- 9.Gray PJ. Management of patients with chronic renal failure. Role of physical therapy. Phys Ther. 1982;62(2):173–6. doi: 10.1093/ptj/62.2.173. Medline:7036195. [DOI] [PubMed] [Google Scholar]
- 10.Pupim LB, Flakoll PJ, Levenhagen DK, et al. Exercise augments the acute anabolic effects of intradialytic parenteral nutrition in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2004;286(4):E589–97. doi: 10.1152/ajpendo.00384.2003. http://dx.doi.org/10.1152/ajpendo.00384.2003. Medline:14678952. [DOI] [PubMed] [Google Scholar]
- 11.Dong J, Ikizler TA. New insights into the role of anabolic interventions in dialysis patients with protein energy wasting. Curr Opin Nephrol Hypertens. 2009;18(6):469–75. doi: 10.1097/MNH.0b013e328331489d. http://dx.doi.org/10.1097/MNH.0b013e328331489d. Medline:19713839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Storer TW. Anabolic interventions in ESRD. Adv Chronic Kidney Dis. 2009;16(6):511–28. doi: 10.1053/j.ackd.2009.08.008. http://dx.doi.org/10.1053/j.ackd.2009.08.008. Medline:19801139. [DOI] [PubMed] [Google Scholar]
- 13.Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 2011;16(7):626–32. doi: 10.1111/j.1440-1797.2011.01471.x. Medline:21557787. [DOI] [PubMed] [Google Scholar]
- 14.Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;10(10):CD003236. doi: 10.1002/14651858.CD003236.pub2. http://dx.doi.org/10.1002/14651858.CD003236.pub2. Medline:21975737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castaneda C, Gordon PL, Uhlin KL, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001;135(11):965–76. doi: 10.7326/0003-4819-135-11-200112040-00008. http://dx.doi.org/10.7326/0003-4819-135-11-200112040-00008. Medline:11730397. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. http://dx.doi.org/10.1371/journal.pmed.1000097. Medline:19621072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heymsfield SB, Gallagher D, Visser M, et al. Measurement of skeletal muscle: laboratory and epidemiological methods. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):23–9. doi: 10.1093/gerona/50a.special_issue.23. Medline:7493213. [DOI] [PubMed] [Google Scholar]
- 18.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–33. doi: 10.1016/s0004-9514(09)70043-1. http://dx.doi.org/10.1016/S0004-9514(09)70043-1. Medline:19463084. [DOI] [PubMed] [Google Scholar]
- 19.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21. Medline:12882612. [PubMed] [Google Scholar]
- 20.Borenstein M. Introduction to meta-analysis. Chichester, U.K.: John Wiley & Sons; 2009. http://dx.doi.org/10.1002/9780470743386. [Google Scholar]
- 21.Dong J, Sundell MB, Pupim LB, et al. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr. 2011;21(2):149–59. doi: 10.1053/j.jrn.2010.03.004. http://dx.doi.org/10.1053/j.jrn.2010.03.004. Medline:20580251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page EB. Ordered hypotheses for multiple treatments: a significance test for linear ranks. J Am Stat Assoc. 1963;301(58):216–30. http://dx.doi.org/10.1080/01621459.1963.10500843. [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. Rev ed. New York: Academic Press; 1977. [Google Scholar]
- 24.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 25.Johansen KL, Painter PL, Sakkas GK, et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17(8):2307–14. doi: 10.1681/ASN.2006010034. http://dx.doi.org/10.1681/ASN.2006010034. Medline:16825332. [DOI] [PubMed] [Google Scholar]
- 26.Chen JLT, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25(6):1936–43. doi: 10.1093/ndt/gfp739. http://dx.doi.org/10.1093/ndt/gfp739. Medline:20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopple JD, Cohen AH, Wang H, et al. Effect of exercise on mRNA levels for growth factors in skeletal muscle of hemodialysis patients. J Ren Nutr. 2006;16(4):312–24. doi: 10.1053/j.jrn.2006.04.028. http://dx.doi.org/10.1053/j.jrn.2006.04.028. Medline:17046615. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Definition of an older or elderly person. [Internet] Geneva: The Organization; 2013. [cited 2012 Jun 13]. [updated 2012]. Available from: http://www.who.int/healthinfo/survey/ageingdefnolder/en/index.html. [Google Scholar]
- 29.Kraemer HC, Morgan GA, Leech NL, et al. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1524–9. doi: 10.1097/00004583-200312000-00022. http://dx.doi.org/10.1097/00004583-200312000-00022. Medline:14627890. [DOI] [PubMed] [Google Scholar]
- 30.Häkkinen K, Kallinen M, Izquierdo M, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 1998;84(4):1341–9. doi: 10.1152/jappl.1998.84.4.1341. Medline:9516202. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674–88. doi: 10.1249/01.mss.0000121945.36635.61. http://dx.doi.org/10.1249/01.MSS.0000121945.36635.61. Medline:15064596. [DOI] [PubMed] [Google Scholar]
- 32.Chetlin RD, Gutmann L, Tarnopolsky MA, et al. Resistance training exercise and creatine in patients with Charcot-Marie-Tooth disease. Muscle Nerve. 2004;30(1):69–76. doi: 10.1002/mus.20078. http://dx.doi.org/10.1002/mus.20078. Medline:15221881. [DOI] [PubMed] [Google Scholar]
- 33.Cupisti A, Capitanini A, Betti G, et al. Assessment of habitual physical activity and energy expenditure in dialysis patients and relationships to nutritional parameters. Clin Nephrol. 2011;75(3):218–25. doi: 10.5414/cnp75218. http://dx.doi.org/10.5414/CNP75218. Medline:21329632. [DOI] [PubMed] [Google Scholar]
- 34.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86(6):509–16. doi: 10.1007/s00421-001-0570-4. http://dx.doi.org/10.1007/s00421-001-0570-4. Medline:11944099. [DOI] [PubMed] [Google Scholar]
- 35.Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6):2564–70. doi: 10.1046/j.1523-1755.2000.00116.x. http://dx.doi.org/10.1046/j.1523-1755.2000.00116.x. Medline:10844626. [DOI] [PubMed] [Google Scholar]
- 36.O'Hare AM, Tawney K, Bacchetti P, et al. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis. 2003;41(2):447–54. doi: 10.1053/ajkd.2003.50055. http://dx.doi.org/10.1053/ajkd.2003.50055. Medline:12552509. [DOI] [PubMed] [Google Scholar]
- 37.Sakkas GK, Sargeant AJ, Mercer TH, et al. Changes in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol Dial Transplant. 2003;18(9):1854–61. doi: 10.1093/ndt/gfg237. http://dx.doi.org/10.1093/ndt/gfg237. Medline:12937235. [DOI] [PubMed] [Google Scholar]
- 38.Kaysen GA, Zhu F, Sarkar S, et al. Estimation of total-body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy. Am J Clin Nutr. 2005;82(5):988–95. doi: 10.1093/ajcn/82.5.988. Medline:16280429. [DOI] [PubMed] [Google Scholar]
- 39.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. 1993;148(4):379–85. doi: 10.1111/j.1748-1716.1993.tb09573.x. http://dx.doi.org/10.1111/j.1748-1716.1993.tb09573.x. Medline:8213193. [DOI] [PubMed] [Google Scholar]
- 40.Kushner RF, de Vries PM, Gudivaka R. Use of bioelectrical impedance analysis measurements in the clinical management of patients undergoing dialysis. Am J Clin Nutr. 1996;64(3 Suppl):503S–9S. doi: 10.1093/ajcn/64.3.503S. Medline:8780371. [DOI] [PubMed] [Google Scholar]
- 41.Kouidi EJ. Central and peripheral adaptations to physical training in patients with end-stage renal disease. Sports Med. 2001;31(9):651–65. doi: 10.2165/00007256-200131090-00002. http://dx.doi.org/10.2165/00007256-200131090-00002. Medline:11508521. [DOI] [PubMed] [Google Scholar]
- 42.Oates MK. The use of DXA for total body composition analysis. SCAN. 2007;13:6–7. [Google Scholar]
- 43.Beddhu S, Pappas LM, Ramkumar N, et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14(9):2366–72. doi: 10.1097/01.asn.0000083905.72794.e6. http://dx.doi.org/10.1097/01.ASN.0000083905.72794.E6. Medline:12937315. [DOI] [PubMed] [Google Scholar]
- 44.Ramkumar N, Pappas LM, Beddhu S. Effect of body size and body composition on survival in peritoneal dialysis patients. Perit Dial Int. 2005;25(5):461–9. Medline:16178479. [PubMed] [Google Scholar]