Abstract
Curcumin, a phytochemical isolated from curcuma plants which are used as coloring ingredient for the preparation of curry powder, has several activities which suggest that it might be an interesting drug for the treatment or prevention of cancer. Curcumin targets different pathways which are involved in the malignant phenotype of tumor cells, including the nuclear factor kappa B (NFKB) pathway. This pathway is deregulated in multiple tumor entities, including Hodgkin’s lymphoma (HL). Indeed, curcumin can inhibit growth of HL cell lines and increases the sensitivity of these cells for cisplatin. In this review we summarize curcumin activities with special focus on possible activities against HL cells.
Keywords: curcumin, cancer, Hodgkin’s lymphoma, vitamin D receptor, nuclear factor kappa B
Introduction
The name curcumin has been used for different unrelated dyestuffs. Today, the name is used more or less exclusively for 1,7-bis(4-hydroxy-3-methoxyphenyl)- 1,6-heptadiene-3,5-dione, the yellow dye from Curcuma longa (turmeric) and other curcuma plants.1,2 The name curcuma is based on the oriental name of the plant, which refers to its similarity with saffron (see the Hebrew word for saffron:
 ).3 Approximately 2000 years ago, Plinius described a plant with similar features which is considered to be turmeric (est et per se Indica herba, quae cypira vocatur, zingiberis effigie; commanducata croci vim reddit.4 [furthermore, an Indian plant exists which is called cypira and which has a similar appearance as ginger; while chewing it constitutes the effect of saffron]). Hybridization of C. longa with other species results in marked differences in the curcumin content between different plants.5C. longa is a member of the family Zingiberaceae (ginger family). This family contains several plants which are widely used as spices and/or officinal plants, for example Aframomum spec. (alligator pepper, false cardamom), Alpinia officinarum (Chinese ginger), Amomum subulatum (hill cardamom), Boesenbergia rotunda (Chinese keys), C. aromatica (wild turmeric), C. xanthorrhiza (temulawak), C. zedoaria (zedoary), Eletteria cardamomum (cardamom), Kaempferia galangal (galangal), Zingiber officinale (ginger), and Z. mioga (Japanese ginger).
).3 Approximately 2000 years ago, Plinius described a plant with similar features which is considered to be turmeric (est et per se Indica herba, quae cypira vocatur, zingiberis effigie; commanducata croci vim reddit.4 [furthermore, an Indian plant exists which is called cypira and which has a similar appearance as ginger; while chewing it constitutes the effect of saffron]). Hybridization of C. longa with other species results in marked differences in the curcumin content between different plants.5C. longa is a member of the family Zingiberaceae (ginger family). This family contains several plants which are widely used as spices and/or officinal plants, for example Aframomum spec. (alligator pepper, false cardamom), Alpinia officinarum (Chinese ginger), Amomum subulatum (hill cardamom), Boesenbergia rotunda (Chinese keys), C. aromatica (wild turmeric), C. xanthorrhiza (temulawak), C. zedoaria (zedoary), Eletteria cardamomum (cardamom), Kaempferia galangal (galangal), Zingiber officinale (ginger), and Z. mioga (Japanese ginger).
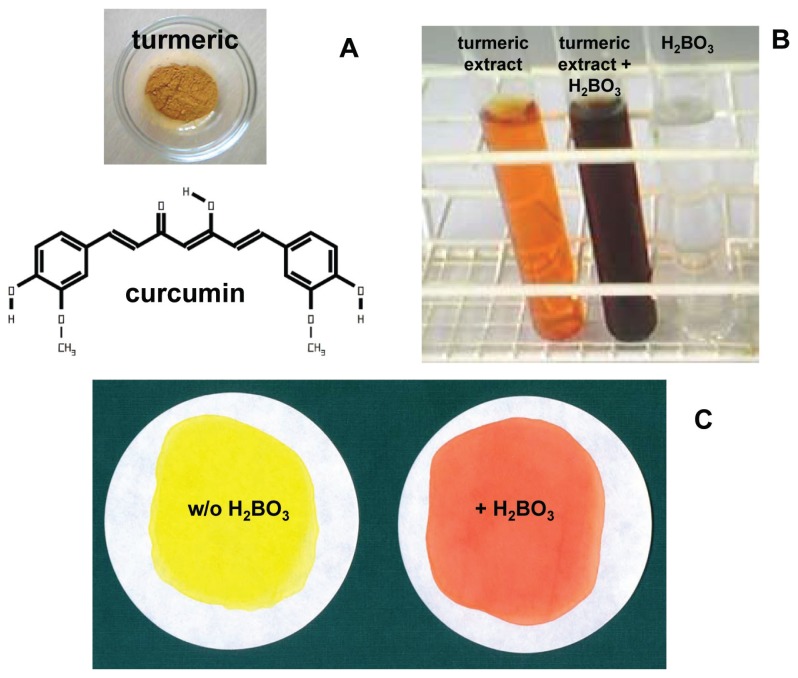
Recently, the complete transcriptome of C. longa rhizomes was analyzed.6 For a long time, the lipid-soluble yellow compound which can be isolated from the curcuma plants was used for cooking, cosmetics, and textile dying.7 In the 13th century, turmeric was introduced into Europe by Arab traders.8 Today, turmeric and the isolated curcumin are used as colorant in many food products. Turmeric is one of the important ingredients of curry powder. The color of curcumin depends on the pH and the presence or absence of other substances like boron (Fig. 1). With boron, curcumin forms rosocyanine, a reddish color (Fig. 1) which can be used for the detection and colorimetric quantification of boron.9
Figure 1. Curcumin and Curcuma.
A) Curcumin is the yellow dyestuff from turmeric (the structure of curcumin was drawn with C-Design 3.0f; http://www.ch.tum.de/oc1/EFontain/C-Design/). B) Curcumin forms colored complexes with boron. Turmeric powder (1 g) was extracted with 96% ethanol (10 mL, 37ºC, 2 h), centrifuged and steril filtered. In the presence of 0.1 M NaOH, a 1:20 dilution of this extract in water is colored orange. After adition of 0.05 M boric acid the colore turns into deep red. C) Turmeric powder (1.3 g) was extracted with 96% ethanol (18 mL, 37ºC, 4 h) in the absence or presence of boric acid (1 g), centrifuged, steril filtered, and dried on filter paper circles. In the presence of boron, the yellow color changed into red.
Multiple biological effects have been documented for curcumin. Curcumin has direct antibacterial10 and anti-inflammatory11 activity. The anti-inflammatory activity might be mediated in part by the strong antioxidant activity of its caffeic acid moiety.12 Curcumin influences multiple signaling pathways.13,14 In addition to the anti-cancer related activities described below, curcumin is used for the treatment of a plethora of other diseases including pulmonary, neurological, liver, metabolic, and autoimmune diseases.15
Anti-Cancer Related Activities of Curcumin
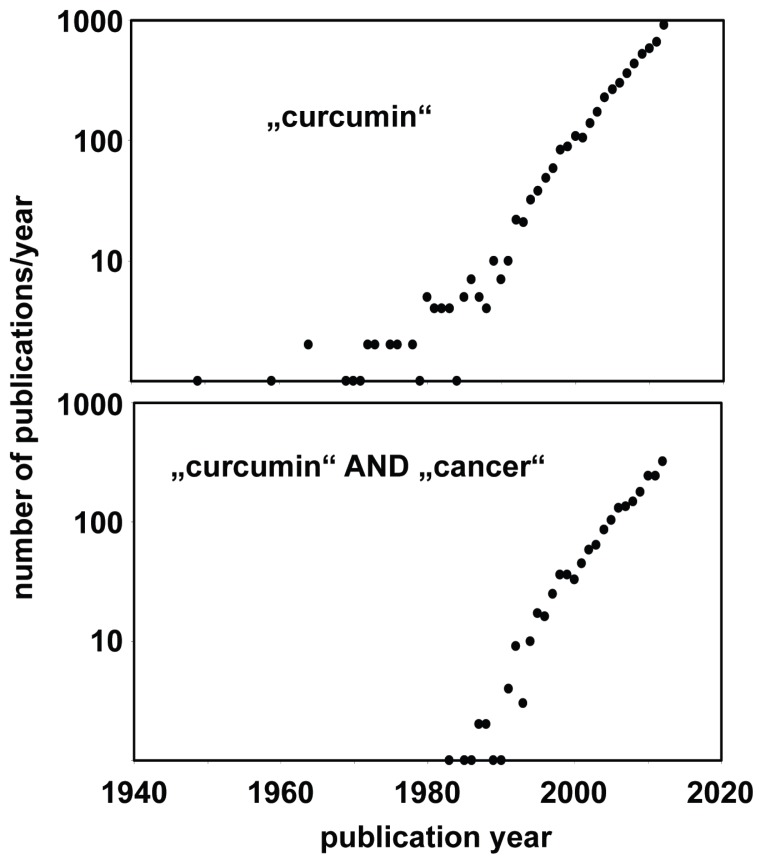
In the last decades, there has been growing interest in curcumin and curcumin derivatives for treatment or prevention of cancer as indicated by the increasing number of curcumin-related publications in the last years (Fig. 2). The basic biological activities of curcumin has been summarized in an excellent review.16
Figure 2. Increasing number of publications about curcumin and cancer.
The PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) was searched with the queries “curcumin” or “curcumin cancer”. Presented are the absolute numbers of found publications per puplication year.
Early observations suggested that curcumin can inhibit growth of tumor cells,17 can inhibit chemical carcinogenesis,18,19 and can be used for the treatment of patients with cancer.20 Since then, few clinical trials suggest that curcumin might be a drug of interest for treatment of cancer21,22 or for the prevention of different forms of cancer.23,24
It has been shown that curcurmin and curcumin derivatives alone or in combination with other drugs increase cell death in a wide variety of tumor cells, including brain tumors,25–28 sarcoma,29–35 breast cancer, 40–46 ovarian cancer,47–49 testicular cancer,50 prostate cancer,51–53 pancreatic cancer,54,55 liver cancer,56 biliary cancer,57 gastric cancer,58,59 colorectal cancer,60,61 lung cancer,62–65 mesothelioma,66 renal cancer,37 bladder cancer,67 esophageal cancer,68–71 head and neck cancer,72–75 and lymphoma/leukemia76–86 (Table 1). Curcumin induces death of cancer cells by activation of extrinsic and intrinsic apoptosis pathways. Activation of apoptosis by curcumin is accompanied by modulation of multiple signaling pathways (hedgehog pathway, extracellular signal-regulated kinase (ERK) pathway, wingless (WNT) pathway, Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, Notch pathway, nuclear factor of kappa light polypeptide gene enhancer in B-cells (NFKB) pathway; Table 1). Depending on the cell type investigated, activation or inhibition of certain signaling pathways can occur. The ERK pathway, for example, is activated by curcumin in monocytic leukemia cells77 whereas curcumin down-regulates ERK in breast cancer cells.43 In both cases, deregulation of this signaling pathway promotes cell death. Curcumin can up-regulate pro-apoptotic components of the extrinsic apoptosis pathway (FAS, FAS ligand, Tumor necrosis factor (TFR) receptors or TNF-related apoptosis inducing ligand (TRAIL) receptors).30,31 On the other hand, curcumin down-regulates anti-apoptotic factors (B cell leukemia/lymphoma 2 (BCL2), BCL2 like 1 (BCL2L1), X-linked inhibitor of apoptosis (XIAP), survivin).25,27,33,41,56,57,58,67,79 In addition, curcumin has been shown to inhibit telomerase which reverses immortalization of cancer cells.25 It seems that curcumin is able to induce apoptosis in cancer cells without pronounced cytotoxic effects on healthy cells. By inhibition of multi-drug resistance transporters, curcumin can overcome chemotherapy resistance of tumor cells.64,87 The inhibition of these transporters can explain the depletion of side populations in tumor cells after treatment with curcumin.88 If curcumin increases the chemotherapy sensitivity of this putative stem cell population, a combination with conventional chemotherapy might be able to kill the stem cell population. Antagonistic effects of curcumin and cytotoxic drugs have been observed, however.89–91 These observations indicate that the combination of curcumin with other drugs should be carefully evaluated in appropriate models in vitro or in vivo before these combinations are tested in patients.
Table 1.
Examples for growth inhibitory activities of curcumin and curcumin derivatives on tumor cells.
| Tumor types1 | Substances2 | Important observations3 | Ref. |
|---|---|---|---|
| brain tumors (glioblastoma, medulloblastoma) | curcumin; curcumin + paclitaxel |
cell cycle inhibition; apoptosis; inhibition of telomerase; inhibition of migration; inhibition of hedgehog signaling | 25–28 |
| MPNST | curcumin + TRAIL | enhanced TRAIL sensitivity; increased production of ROS | 29 |
| sarcoma (chondrosarcoma, liposarcoma, osteosarcoma, Ewing sarcoma) | curcumin; curcumin + JCTH-4; FLLL32 |
up-regulation of FAS, FAS ligand, and TRAILR2; enhanced JCTH-4 sensitivity; cell cycle inhibition; inhibition of ATP2A2; apoptosis; down-regulation of MMP2 | 30–35 |
| melanoma | curcumin; FLLL32; FLLL62; DM-1 + dacarbazine; D6 |
cell cycle inhibition; up-regulation of TNF receptor 1; apoptosis; inhibition of STAT3 signaling | 36–39 |
| neuroblastoma | curcumin; D6 |
apoptosis; inhibition of TNF induced NFKB signaling | 39 |
| breast cancer | curcumin; curcumin + fluoruracil; curcumin + tamoxifen; curcumin + vinblastin; RL66; RL71 |
cell cyle inhibition; inhibition of multiple signaling pathways; apoptosis; inhibition of ERK signaling; down-regulation of EZH2; inhibition of migration of endothelial cells; depolymerization of microtubules | 40–46 |
| ovarian cancer | curcumin + cisplatin; curcumin + oxaliplatin; B19; HO-3867 + cisplatin; |
inhibition of STAT3 signaling; apoptosis; up-regulation of TP53; increased production of ROS | 47–49 |
| testicular cancer | curcumin + bleomycin | apoptosis | 50 |
| prostate cancer | curcumin; dimethoxycurcumin |
cell cycle inhibition; apoptosis; inhibition of MMP2, inhibition of wingless signaling | 51–53 |
| pancreatic cancer | curcumin; curcumin + sulforaphane + aspirin |
cell cycle inhibition; apoptosis; inhibition of NFKB signaling | 54,55 |
| liver cancer | curcumin + adriamycin | apoptosis | 56 |
| biliary cancer | curcumin | apoptosis; inhibition of NFKB signaling; inhibition of STAT3 signaling; up-regulation of TRAIL receptors | 57 |
| gastric cancer | curcumin; curcumin + doxorubicin; curcumin + etoposide |
apoptosis; inhibition of NFKB signaling | 58,59 |
| colon cancer | bisdehydroxycurcumin | apoptosis; autophagy | 60,61 |
| lung cancer | curcumin; curcumin + cisplatin B63; T63; |
cell cycle arrest; apoptosis; enhanced production of ROS; down-regulation of MDR transporters; enhanced degradation of HIF1A | 62–65 |
| mesothelioma | curcumin; curcumin + cisplain |
apoptosis | 66 |
| renal cell carcinoma | curcumin; FLLL32; FLLL62 |
apoptosis; inhibition of STAT3 signaling | 37 |
| bladder cancer | curcumin + BCG | inhibition of NFKB signaling; up-regulation of TRAIL receptors; apoptosis | 67 |
| esophageal cancer | curcumin; curcumin + fluoruracil curcumin + cisplatin |
cell cycle inhibition; inhibition of NFKB signaling; apoptosis; non-apoptotic cell death; inhibition of Notch signaling; | 68–71 |
| head and neck cancer | curcumin; FLLL32 + cisplatin |
cell cycle inhibition; apoptosis; inhibition of STAT3 signaling; increased production of ROS | 72–75 |
| leukemia and lymphoma | curcumin; curcumin + daunorubicin; curcumin + IR; CA#12 + bortezomib |
cell cycle inhibition; increased production of ROS; apoptosis; inhibition of NFKB signaling; activation of ERK pathway; down-regulation of cyclin D1, down-regulation of MYC | 76–85 |
MPNST, malignant peripheral nerve sheath tumors.
B19, (1E, 4E)-1, 5-bis(2-methoxyphenyl)penta-1,4-dien-3-one; B63, 1,5-bis(2-methoxyphenyl)penta-1,4-dien-3-one; DM-1, sodium 4-[5-(4-hydroxy-3- methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2-methoxy-phenolate; BCG, Bacillus Calmette-Guerin; CA#12, (1E,6E)-1,7-Bis(4-valinoyl-3-methoxyphenyl) hepta-1,6-diene-3,5-dione hydrochloride; D6, (3E,3′E)-4,4′-(5,5′,6,6′-tetramethoxy-[1,1′-biphenyl]-3,3′-diyl)bis(but-3-en-2-one); FLLL32, (2E,2′E)-1,1′-(cyclohexane-1,1-diyl)bis(3-(3,4-dimethoxyphenyl)prop-2-en-1-one); FLLL62, (2E,2′E)-1,1′-(tetrahydropyran-4,4-diyl)bis(3-(3,4-dimethoxyphenyl)prop-2- en-1-one); HO-3867, 1-[(1-Oxyl-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl)methyl]-(3E,5E)-3,5-Bis(4-fluorobenzylidene)piperidin-4-one; IR, ionizing radiation; JCTH-4, synthetic pancratistatin analog; TRAIL, tumor necrosis factor-related apoptosis inducing ligand; RL66, 1-Methyl-3,5-bis[(E)-4-pyridyl) methylidene]-4-piperidone; RL71, 3,5-bis(3,4,5-trimethoxybenzylidene)-1-methylpiperidine-4-one; T63, (1E,6E)-1,7-Bis(3,4-dimethoxyphenyl)-4-(4-hy droxy- 3-methoxybenzylidene)hepta-1,6-diene-3,5-dione.
ATP2A2, sarcoplasmic/endoplasmic reticulum calcium ATPase; ERK, extracellular signal-regulated kinase; EZH2, enhancer of zeste homolog 2; HIF1A, hypoxia inducible factor 1, alpha subunit; NFKB, nuclear factor of kappa light polypeptide gene enhancer in B-cells; TNF, tumor necrosis factor; TP53, tumor protein 53; TRAILR2, TRAIL receptor 2 (death receptor 5); MDR, multi-drug resistance; MMP2, matrix metalloproteinase 2; MYC, myelocytomatosis viral oncogene homolog; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3.
Another interesting activity of curcumin is the inhibition of tumor cell motility, invasion, and metastasis.26,42,92–100 This activity is mediated in part by the down-regulation of matrix- metalloproteinases. A key step in tumor metastasis is the process of epithelial mesenchymal transition (EMT) usually accompanied by loss of epithelial cadherin (CDH1) expression. Inhibition of EMT and up- regulation of CDH1 in tumor cells by curcumin has been described.101,102 One inductor of cell motility in cancer cells is lysophosphatidic acid (LPA). LPA receptors are often expressed in tumor cells and co-expression of LPA receptors and LPA-producing phospholipases in tumor cells have been observed.103 Curcumin can also inhibit LPA-induced cancer cell motility.101 Taken together, these observations indicate that curcumin does not only inhibit tumor growth but also metastasis formation. However, enhanced metastasis has been described in a Lewis lung carcinoma model.104
In addition to the death inducing activities of curcumin on established tumor cells, curcurmin and curcumin derivatives can inhibit or delay tumor formation in different tumor models.92,105–113 Curcumin significantly inhibited the formation of preneoplastic lesions in animal models for chemical colon carcinogenesis. 92,102 The combination with fluorouracil or carnitine enhanced this effect. Curcumin inhibited ultraviolet radiation-induced or chemically induced skin carcinogenesis in mice.106,107 Furthermore, curcumin inhibited the development of gastrointestinal tumors,108,109 mammary tumors,110 and hepatocellular tumors111,112 in models for chemical carcinogenesis. The anti-inflammatory activity of curcumin seems to be responsible at least partially for such cancer preventive activities.113 In addition to the direct activities on tumor cells and pre-malignant cells, the inhibition of the growth of carcinogenic microorganisms such as Helicobacter pylori may partially account for tumor prevention by curcumin.114 Very infrequently, enhanced tumor formation in experimental models has been described.115 Such observations indicate that the biology of the used experimental models has not been fully elucidated and that further investigations are required in order to understand the effects of curcumin in the different models.
The Nuclear Factor Kappa B (NFKB) Pathway as Target for Curcumin
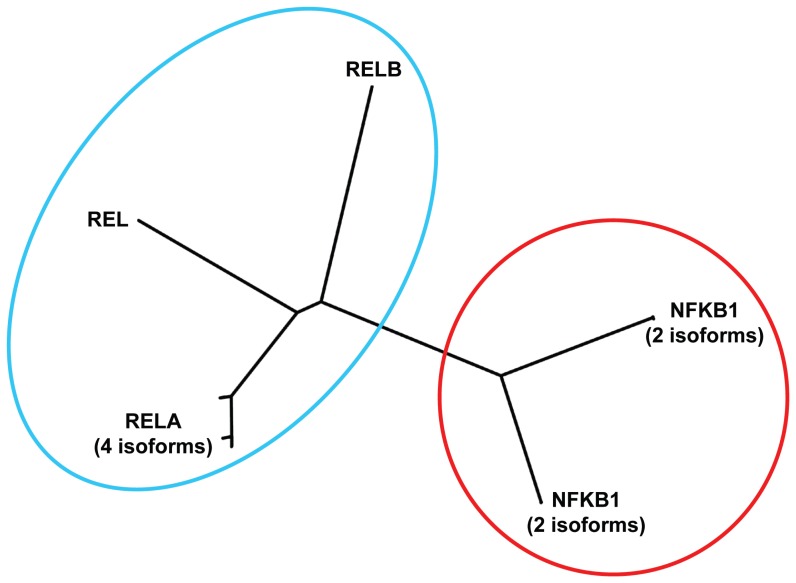
Avian reticuloendotheliosis defines a group of diseases that are observed in turkeys, broiler chickens, ducks, among other birds. One of the interesting features of reticuloendotheliosis is the development of lymphoid malignancies. The causative agent of reticuloendotheliosis is a gamma-retrovirus that transforms diverse avian cells. Analysis of the T strain of this virus (reticuloendotheliosis virus strain T; REV-T) led to the identification of the REL oncogene.116–118 Thereafter, the human REL homologue was found on chromosome 2.119 REL is a member of a gene family that includes in the human genome at least 5 members: REL, RELA,120,121 RELB,122 nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (NFKB1),123 and NFKB2 (Fig. 3).124 This family can be divided into two sub-families (Fig. 3) and usually one member of the NFKB1/2 subfamily forms a heterodimer with a member of the REL subfamily. The NFKB pathway is a highly conserved signaling pathway in eukaryotes. Members of the NFKB family are involved in chromosomal rearrangements that have been detected in cancer cells and gene fusions involving REL125,126 or NFKB2124,127 have been described.
Figure 3. Known human proteins of the REL/NFKB family.
Reference sequences of the indicated human proteins were used for a phylogenetic tree analysis using the clustalW algorithm (http://www.genome.jp/tools/clustalw/). For human RELA (protein accession numbers NP_001138610, NP_001230913, NP_001230914, and NP_068810), human NFKB1 (NP_001158884, NP_003989) and human NFKB2 (NP_001070962, NP_001248332) different protein isoforms with highly similar sequences have been identified. For REL (NP_002899) and RELB (NP_006500) no additional human isoforms are in the databases. The family can be separated into two sub-families. A complete NFKB transcription factor consists of a heterodimer of a member of the NFKB1/2 subfamily and a member of the REL subfamily.
The NFKB pathway is often deregulated in cancer cells. One example is Hodgkin’s lymphoma (HL), one of the most frequent lymphomas in Western countries. 128 The etiology of HL is unclear, but immunological and molecular properties suggest that in most cases HL cells are derived from B cells.129–131 HL cells have a characteristic gene expression profile that discriminates these cells from other normal and transformed hematopoietic cells.132 With the combination of radio- and chemotherapy the majority of patients, with HL can be cured. However, the established therapy is associated with the induction of secondary malignancies, cardiac toxicities, and treatment-related infertility.133–135 In addition, up to 10% of patients still cannot be cured with current therapy regimes, which represent a significant number.136,137 Thus, there is a clear demand to search for new treatment options and also for optimization of current treatment strategies by identifying potential treatment resistance mechanisms.
An involvement of the NFKB pathway in HL has been suggested by the high expression of NFKB family members in HL.138 Mutations in the NFKB inhibitors NFKB inhibitor alpha (NFKBIA) and tumor necros factor alpha-induced protein 3 (TNFAIP3) have been observed in a high percentage of HL.139,140 Curcumin inhibits NFKB activity in different cell types (Table 1) and the inhibition of the NFKB pathway can be seen by decreased expression of NFKB. The activity of NFKB is regulated by inhibitors that bind NFKB and release NFKB only after degradation. Curcumin can inhibit NFKB activity indirectly by stabilization of NFKBIA.40
In addition to constitutive activation of NFKB, in some tumors NFKB expression and activation is induced by treatment with cytotoxic drugs or cytokines. 40,141,142 Curcumin and curcumin derivatives can prevent such induced activation. Curcumin derivatives, for example, have been shown to decrease the TFR-induced expression of NFKB in melanoma and neuroblastoma cells.40 In some cases the anti-NFKB activity of curcumin requires the presence of additional treatment elements. In pancreatic tumor cells, for example, curcumin inhibits DNA binding-activity of NFKB only in combination with aspirin and sufuraphane.54 As the final consequence of NFKB inhibition, expression of NFKB target genes is suppressed. These target genes include anti-apoptotic factors and the suppression of these factors can increase tumor cell death. In addition, the crosstalk between NFKB and other signaling pathways can lead to complex alterations of the phenotype of tumor cells after inhibition of NFKB.
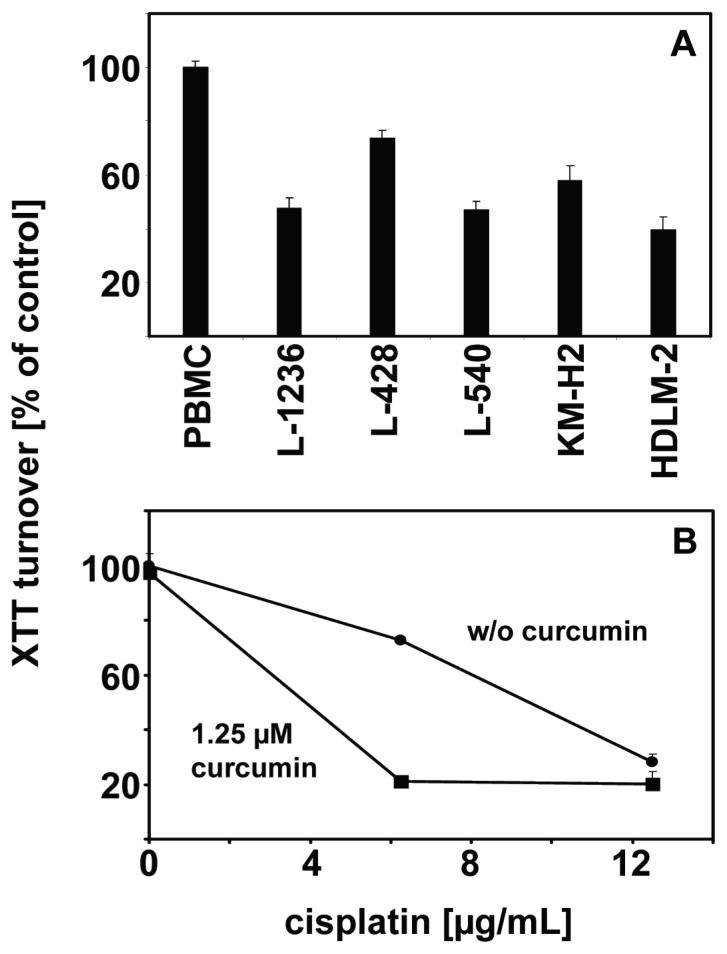
The activation of the NFKB pathway in HL makes curcumin an interesting drug for the treatment of HL. Indeed, curcumin induces cell death in HL cells. As shown in Figure 4A, incubation with curcumin leads to decreased cell viability in all HL cell lines tested whereas normal peripheral blood mononuclear cells (PBMC) are not affected by the used concentration of curcumin. Similar results were found by Mackenzie et al who showed that curcumin leads to cell cycle arrest in G2-M and reduced cell viability in Hodgkin and Reed-Sternberg cells.143
Figure 4. Curcumin inhibits growth of HL cells.
A) HL cells from the indicated cell lines and peripheral blood mononuclear cells (PBMC) were incubated for 24 hours with 25 μM curcumin (Sigma, Taufkirchen, Germany). Cell viability was assessed by 2,3-bis-(2-methoxy-4- nitro- 5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Roche, Mannheim, Germany). Shown are means and standard errors from 4 replicates. B) Cells of the HL cell line L-428 were incubated with 1.25 μM curcumin or with carrier. The cells were treated with 6.25 and 12.5 μg/mL cisplatin (Sigma) or with the same concentrations of carrier (dimethylformamide). After 24 hours the cell viability was assessed using XTT assay (Roche). Shown are means and standard errors from triplicates.
In order to determine whether the combination of curcumin with conventional chemotherapy might have synergistic effects, HL cells were incubated either without curcumin or with a low dose (1.25 μM) of curcumin and varying concentration of cisplatin. The results are shown in Figure 4B. With the used concentration of curcumin, only marginal effects on cell viability were observed. In contrast, the combination of curcumin with cisplatin had a strong cytotoxic effect on the cells. However, inhibition of the Fanconi anemia (FA) pathway might be an important factor for the synergistic effect of cisplatin and curcumin on HL cells. Ubiquitination of the FA complementation group D2 (FANCD2) protein in response to DNA damage is an important step during repair of cross-links in the DNA, caused by cisplatin. Chirnomas et al144 showed that curcumin inhibits the FA pathway by inhibition of ubiquituation of FANCD2. This inhibition sensitized ovarian and breast tumor cells for cisplatin.144 It remains to be shown whether similar mechanisms are responsible for increased cisplatin sensitivity of curcumin treated HL cells. The HL cell lines used in our study outlined above were established from patients with refractory disease and are not perfect models for the situation in most patients in vivo. However, the increased cell death of these highly chemotherapy resistant cell lines after treatment with curcurmin seems to be encouraging.
Curcumin shares the NFKB-inhibitory activity with another interesting dietary phytochemical: capsaicin.145 Curcumin can bind the vanilloid receptor TRPV1 (transient receptor potential cation channel, subfamily V, member 1), the same receptor that is activated by capsaicin.145 However, curcumin binding to TRPV1 did not result in the same signaling. TRPV1 is not the only receptor for curcumin and interestingly, curcumin can bind the aryl hydrocarbon receptor AHR.146 Many carcinogenic polycyclic aromatic hydrocarbons are carcinogens only after in vivo activation. Cytochrome P450 family member A1 (CYP1A1) is an enzyme which can catalyze this activation. Expression of CYP1A1 is induced after binding of AHR to polycyclic aromatic hydrocarbons. It was shown that curcumin can inhibit the CYP4A1 inducing activity of the AHR.147 Binding to AHR and competition with aryl hydrocarbon derivatives might explain the chemopreventive activity of curcumin.147 Probably more important for the cancer cell death inducing activity of curcumin is the third curcumin receptor, the vitamin D receptor.148,149
Vitamin D Receptors (VDR) and Curcumin
Anti-neoplastic activities of vitamin D have been described, but epidemiological data also suggests that for some cancer types sunlight can reduce cancer risk independent from vitamin D.150 Curcumin increases apoptosis and differentiation of vitamin D-treated tumor cells.151,152 Direct binding of curcumin to the vitamin D receptor (VDR) was demonstrated.148 This binding allows VDRs to heterodimerize with the retinoic X receptor and translocate to the nucleus. The complex can then activate gene transcription of vitamin D target genes.148
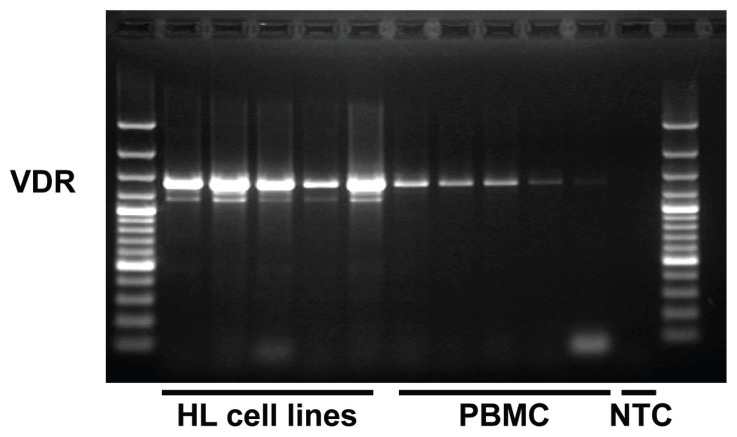
Recently, high expression of the VDR has been observed in HL biopsies.153 HL cell lines also express high levels of VDR (Fig. 5). Lymphoma cells can synthesize vitamin D154 and increased vitamin D levels have been repeatedly reported in HL patients.155 In addition, seasonal fluctuations of the prognosis of HL patients have been observed and discussed as being caused by fluctuations of endogenous vitamin D levels.156 Vitamin D can probably activate the NFKB pathway,157,158 but in most systems, vitamin D inhibits NFKB activation.159–162 In Jurkat cells with constitutive expression of VDR, no inhibition of NFKB by vitamin D was observed,163 suggesting that some tumor cells have inactivated this pathway. Interestingly, NFKB can inhibit VDR signaling.164 This cross-inhibition might explain the simultaneous high constitutive expression of VDR and activated NFKB in lymphoma cells. Stimulation of VDR by curcumin might shift this balance to the inhibitory activity of VDR.
Figure 5. Expression of VDR in HL cell lines.
RNA was isolated from HL cell lines and PBMC, reverse transcribed and used as template for polymerase chain reaction with specific primers for VDR (5′-gcc ttt ggg tct gaa gtg tc-3′ and 5′-cag gct gtc cta gtc agg aga t-3′). The used primers recognize all three transcript variants of the human VDR (accession numbers NM_000376, NM_001017535, NM_001017536). PCR products were sparated by agarose gel electrophoresis in the presence of ethidium bromide. NTC: no template control.
Indirect Effects of Curcumin: Immunomodulation
Curcumin can reduce the number of myeloid-derived suppressor cells,165 an important immuno-suppressive cell population in tumors. Tumor patients, especially HL patients, often have a tumor-mediated dysfunction of the immune system. Experimental data suggest that curcumin is able to restore the immune function in tumor-bearing hosts.166 On the other hand, induction of regulatory T cells might be increased in the presence of curcumin,167 and the direct induction of apoptosis in activated T cells has been described.168 As an anti- inflammatory agent, curcumin can suppress T cells.169,170
Such effects, however, might negatively affect anti-cancer immune responses. The balance between immunostimulation and -suppression might be dependent on the concentration of curcumin.171 In the case of HL, the immune system is a double-edged sword. On the one hand, cytotoxic T cells can kill HL cells; on the other hand, T cells can provide survival signals for HL cells. The very low number of tumor cells and the presence of a high background of “normal” cells in HL indicate the importance of the interaction between tumor cells and the stroma. Whether curcumin can shift the balance between tumor promoting activities and anti-tumor activities of T cells in HL into the direction of tumor destruction must be analyzed. HL is a disease with features of chronic inflammation and the accumulation of stroma cells by HL derived cytokines is an important factor for HL biology.172,173 The anti-inflammatory activities of curcumin may be able to counteract this inflammatory stimulus.
Other Curcumin Activities Related to HL Biology
Another interesting activity of curcumin is the suppression of histone deacetylases (HDAC).174,175 Reports describe the inhibition of histon acetyltrasferases by curcumin without HDAC inhibition.176,177 In addition, curcumin has DNA methylation inhibiting activity.178 Epigenetic regulation of gene expression by DNA methylation or histone deacetylation plays an important role in HL biology. Treatment of HL cells with HDAC inhibitors can increase the sensitivity of HL cells to cytotoxic drugs.179 Treatment of HL cells with DNA methylation inhibitors can increase the expression of tumor antigens which might increase the recognition by the immune system.180,181 However, increased expression of factors involved in chemotherapy resistance might also occur after the treatment of HL cells with such drugs.181
Interestingly, curcumin has been shown to inhibit transcription initiated by human immunodeficiency virus (HIV) type 1 long terminal repeats (LTR).182 The activation of LTRs from endogenous retroviruses (ERV) in HL has been demonstrated. This activation can lead to the aberrant expression of oncogenes in HL.183 Whether curcumin can modulate the activity of ERV promoters in tumor cells is under investigation. If ERV activity is essentially involved in the pathogenesis of HL, such ERV inhibition might reverse the malignant phenotype of the tumor cells.
A high percentage of HL carries copies of the Epstein Barr virus (EBV) in the tumor cells. Activation of the NFKB pathway by EBV seems to be involved in the pathogenesis of EBV positive HL. Curcumin can inhibit B cell immortalization by EBV.184,185 EBV-immortalized B cells undergo pronounced apoptosis in the presence of curcumin which may inhibit the outgrowth of cell lines.185 In addition, it seems that oxidative stress promotes B cell immortalization by EBV.186 The anti-oxidant activity of curcumin might inhibit this effect in a similar way as it was shown for vitamin E.186 Whether this activity can interfere with the EBV driven HL pathogenesis requires further investigations.
Additional HL related activities of curcumin have been described. For example, HL cells express the anti-apoptotic BCL-XL isoform of the B cell leukemia/ lymphoma 2 (BCL2)-like 1 (BCL2L1) oncogene.187,188 Recently, a link between expression of BCL-XL and survival of HL patients has been suggested.189 Curcumin can down-regulate BCL-XL.190 Furthermore, curcumin is a topoisomerase II inhibitor with a similar activity as etoposide, a drug which is used for treatment of HL.191 In addition to the NFKB pathway, curcumin inhibits the STAT pathway (Table 1). This pathway is similarly important for HL cell proliferation and survival as the NFKB pathway and inhibitors of this pathway can induce apoptosis in HL cells.143,189,192–200 Constitutive activation of the STAT pathway by rearrangements of the JAK2 have been described in HL.201 Taken together, curcumin targets several pathways in HL cells. The scarcity of the tumor cells in the tumors hampers the analysis of larger numbers of ex vivo isolated living cells. The number of established HL cell lines is very low and these cell lines are not perfectly representative for the tumor cells in vivo. Whether curcumin or curcumin derivatives with better bio-availability can be used for the treatment of HL patients requires further investigation.
A number of microarray experiments of curcumin treated cells and animals have been published (Table 2). These experiments indicate that the specific effects of curcumin on gene expression are dependent on the investigated model. However, a model-independent common schema of curcumin activities can be seen. These activities include the shift from pro- inflammatory to anti-inflammatory gene expression signatures, the shift from anti-apoptotic to pro- apoptotic signatures, cell cycle inhibition, and down-regulation of pro-invasive factors (e.g., metal-loproteinases). Such experiments might help to identify the relevant pathways which are involved in the biological effects of curcumin in the different models and may lead to the identification of new therapeutic targets.
Table 2.
Examples for DNA microarray experiments investigating the gene expression after treatment of cells or animals with curcumin or curcumin derivatives.
| Models | Substances1,2 | Important observations3 | Ref. |
|---|---|---|---|
| head and neck cancer cells | curcumin | decreased expression of MMP10 | 97 |
| colon cancer cells | curcumin | regulation of cell-cycle-related genes; down-regulation of RELA and MMP2 | 202,203 |
| bladder cancer4 | curcumin | regulation of cell-cycle-related genes | 205 |
| endothelial cells | demethoxycurcumin | decreased expression of MMP9 and other angiogenesis-related genes | 206 |
| lung cancer cells | curcumin; CLEFMA |
decreased expression of MMP14 and other invasionrelated genes; down-regulation of microRNA-186*; up-regulation of genes related to cellular redox status | 207–209 |
| smooth muscle cells, peritoneal phagocytes | curcumin | up-regulation of pro-apoptotic genes, cell adhesion molecules, and anti-inflammatory factors | 210 |
| breast cancer cells | curcumin | up-regulation of cell cycle inhibitors; complex regulation of apoptosis-related genes; down-regulation of pro-inflammatory chemokines; down-regulation of EGF pathway | 211–214 |
| hepatic stellate cell line | Curcuma oil | down-regulation of interleukin 6 and TIMP2 | 215 |
| colon5 | curcumin | strain-depended differences in regulated genes; reduced expression of pro-inflammatory genes | 216–218 |
| Ewing sarcoma cells6 | curcumin | down-regulation of radiation-induced anti-apoptotic factors | 220 |
| pancreatic cancer cells | curcumin | regulation of microRNAs and microRNA-target genes | 221 |
| liver7 | curcumin | weak peroxisomal proliferator activity (in rats) | 222 |
| leukemia cells | curcumin | down-regulation of cell cycle regulators and JAK/STAT signaling | 223 |
| heart8 | curcumin | down-regulation of pro-inflammatory factors | 224 |
| liver cancer cells | curcumin | down-regulation of protein kinase C | 225 |
| microglia cell line | curcumin | up-regulation of anti-inflammatory factors | 226 |
| retinoblastoma cells | curcumin | regulation of multiple genes involved in apoptosis, cell cycle regulation, and angiogenesis; regulation of microRNAs and microRNA-target genes | 227,228 |
| blood cells9 | curcumin | down-regulation of pro-inflammatory factors | 229 |
CLEFMA, 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid].
In some investigations curcumin or curcumin derivatives were compared with other substances.
EGF, epidermal growth factor; JAK/STAT, janus kinase/signal transducer and activator of transcription MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9; MMP10, matrix metalloproteinase 10; MMP14, matrix metalloproteinase 14; TIMP2, tissue inhibitor of metalloproteinases 2.
In the cited publication the investigated cell line (ECV304) was used as a model for endothelial cells, but there is evidence that this cell line is identical to the bladder cancer cell line T24.204
In-vivo models for inflammatory bowel diseases.
In the cited publication the investigated cell line (SK-N-MC) was used as a model for neuroblastoma, but there is evidence that this cell line is derived from Ewing sarcoma (Askin tumor).219
In-vivo toxicology study.
In-vivo model for myocardial infarction.
In-vivo model for arthritis.
Future Directions
The bio-availability of curcumin is limited by the rapid metabolism and the low water solubility of this phytoceutical. Curcumin is rapidly conjugated with sulfate or glucuronic acid in the gut and liver.230 In addition, reduction to tetrahydrocurcumin and hexahydrocurcumin occurs.230 The resulting curcumin sulfate, curcumin glucuronide, and reduced curcumin derivatives can be found in the feces of curcumin treated patients.231 The curcumin metabolites have some biologic activities, for example tetrahydrocurcumin can inhibit the activity of multi-drug resistance (MDR) transporters.232 However, enhanced excretion of curcumin metabolites and the shortening of the half life of curcumin after conjugation are not desired. Inhibition of glucuronidation by piperine (the alkaloid from black pepper) has been shown to increase the bio-availability of curcumin.233
In addition to inhibition of glucuronidation, piperine can inhibit the activity of MDR transporters.234 On the other hand, increased expression of these trans porters after exposure to piperine has been observed.235 Together with the inhibitory effect of curcumin on MDR transporter expression and function,88,236 inhibition of MDR transporter function by piperine might counteract the effect of piperine on MDR transporter expression. In order to increase the solubility of curcumin, conjugation of curcumin with proteins or cyclodextrins have been tested.236,237 In addition, different types of nanoparticles, liposomes, and self- assembling polymers have been used for encapsulation of curcumin.238–240 Such formulations might finally overcome the bio-availability problem of curcumin.
Another interesting question that remains to be addressed is whether the effects of turmeric are only mediated by curcumin or whether additional turmeric ingredients are involved. Early studies suggest that curcumin-free turmeric extracts also have cancer preventing activities.241,242 Indeed, differences between turmeric and curcumin in the regulation of pro-inflammatory genes have been described.243 The characterization of the factors that are responsible for biological activities of curcumin-free turmeric requires further investigations.
Acknowledgements
We thank Vera Marks (communication skills) for critically reading and copy editing the manuscript.
Footnotes
Author Contributions
Conceived and designed the experiments: MSS, SK. Analyzed the data: SK, IV, MSS. Wrote the first draft of the manuscript: SK, MSS. Contributed to the writing of the manuscript: SK, IV, MSS. Agree with manuscript results and conclusions: SK, IV, MSS. Jointly devel oped the structure and arguments for the paper: SK, IV, MSS. Made critical revisions and approved final version: SK, IV, MSS. All authors reviewed and approved of the final manuscript.
Competing Interests
Authors disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
Funding
This work was supported by a fellowship from the Konrad-Adenauer-Stiftung (SK).
References
- 1.Lillie RD, editor. HJ Conn’s biological stains. 9th edition. St. Louis, MO: Sigma Chemical Company; 1991. pp. 474–476. 2nd reprint. [Google Scholar]
- 2.Neil OM, editor. The Merck index. 13th edition. Whitehouse Station, NJ: Merck & Co; 2001. p. 2707. [Google Scholar]
- 3.Buhl F, editor. Wilhelm Gesenius’ Hebräisches und Aramäisches Handwörter-buch über das Alte Testament. 17th edition. Berlin: Springer; 1962. p. 362. reprint. [Google Scholar]
- 4.König RC, Winkler G, editors. C Plinii Secundi naturalis historiae libri XXXVII. XXI/XXII. Munich: Artemis; 1985. p. 92. [Google Scholar]
- 5.Hayakawa H, Minaniya Y, Ito K, Yamamoto, Fukuda T. Difference of curcumin content in Curcuma longa L. (Zingiberaceae) caused by hybridization with other Curcuma species. Am J Plant Sci. 2011;2(2):111–119. [Google Scholar]
- 6.Annadurai RS, Neethiraj R, Jayakumar V, et al. De Novo Transcriptome Assembly (NGS) of Curcuma longa L. Rhizome Reveals Novel Transcripts Related to Anticancer and Antimalarial Terpenoids. PLoS One. 2013;8:e56217. doi: 10.1371/journal.pone.0056217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammon H, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57(1):1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 9.Tolk A, Tap WA, Lingerak WA. Direct spectrophotometric method for determination of traces of boron in iron, steels and other compounds. Talanta. 1969;16(1):111–115. doi: 10.1016/0039-9140(69)80248-1. [DOI] [PubMed] [Google Scholar]
- 10.Schraufstätter E, Bernt H. Antibacterial action of curcumin and related compounds. Nature. 1949;164(4167):456. doi: 10.1038/164456a0. [DOI] [PubMed] [Google Scholar]
- 11.Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25(6):447–452. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25(15):1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 13.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: From ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: Signal transduction. Biofactors. 2013;39(1):27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 15.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors. 2013;39(1):2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6(3):259–70. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 17.Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29(2):197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 18.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12–O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48(21):5941–5946. [PubMed] [Google Scholar]
- 19.Polasa K, Sesikaran B, Krishna TP, Krishnaswamy K. Turmeric (Curcuma longa)-induced reduction in urinary mutagens. Food Chem Toxicol. 1991;29(10):699–706. doi: 10.1016/0278-6915(91)90128-t. [DOI] [PubMed] [Google Scholar]
- 20.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73(1):29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 21.Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9(1):8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 22.Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled cross-over 4 g study and an open-label 8g extension study. Am J Hematol. 2012;87(5):455–460. doi: 10.1002/ajh.23159. [DOI] [PubMed] [Google Scholar]
- 23.Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4(3):354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 25.Khaw AK, Hande MP, Kalthur G, Hande MP. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J Cell Biochem. 2013;114(6):1257–1270. doi: 10.1002/jcb.24466. [DOI] [PubMed] [Google Scholar]
- 26.Hossain M, Banik NL, Ray SK. Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem Int. 2012;61(7):1102–1113. doi: 10.1016/j.neuint.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangaru ML, Chen S, Woodliff J, Kansra S. Curcumin (diferuloylmethane) induces apoptosis and blocks migration of human medulloblastoma cells. Anticancer Res. 2010;30(2):499–504. [PubMed] [Google Scholar]
- 28.Elamin MH, Shinwari Z, Hendrayani SF, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 2010;49(3):302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 29.Reuss DE, Mucha J, Hagenlocher C, et al. Sensitivity of Malignant Peripheral Nerve Sheath Tumor Cells to TRAIL Is Augmented by Loss of NF1 through Modulation of MYC/MAD and Is Potentiated by Curcumin through Induction of ROS. PLoS One. 2013;8(2):e57152. doi: 10.1371/journal.pone.0057152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HP, Li TM, Tsao JY, Fong YC, Tang CH. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int Immunopharmacol. 2012;13(2):163–169. doi: 10.1016/j.intimp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Wang L, Song R, et al. Targeting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2 by curcumin induces ER stress-associated apoptosis for treating human liposarcoma. Mol Cancer Ther. 2011;10(3):461–471. doi: 10.1158/1535-7163.MCT-10-0812. [DOI] [PubMed] [Google Scholar]
- 32.Ma D, Tremblay P, Mahngar K, Collins J, Hudlicky T, Pandey S. Selective cytotoxicity against human osteosarcoma cells by a novel synthetic C-1 analogue of 7-deoxypancratistatin is potentiated by curcumin. PLoS One. 2011;6(12):e28780. doi: 10.1371/journal.pone.0028780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fossey SL, Bear MD, Lin J, et al. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer. 2011;11:112. doi: 10.1186/1471-2407-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29(12):5039–5044. [PubMed] [Google Scholar]
- 35.Singh M, Pandey A, Karikari CA, Singh G, Rakheja D. Cell cycle inhibition and apoptosis induced by curcumin in Ewing sarcoma cell line SK-NEP-1. Med Oncol. 2010;27(4):1096–1101. doi: 10.1007/s12032-009-9341-6. [DOI] [PubMed] [Google Scholar]
- 36.Faião-Flores F, Suarez JA, Maria-Engler SS, Soto-Cerrato V, Pérez-Tomás R, Maria DA. The curcumin analog DM-1 induces apoptotic cell death in melanoma. Tumour Biol. 2013;34(2):1119–1129. doi: 10.1007/s13277-013-0653-y. [DOI] [PubMed] [Google Scholar]
- 37.Bill MA, Nicholas C, Mace TA, et al. Structurally modified curcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS One. 2012;7(8):e40724. doi: 10.1371/journal.pone.0040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bill MA, Fuchs JR, Li C, et al. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol Cancer. 2010;9:165. doi: 10.1186/1476-4598-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisano M, Pagnan G, Dettori MA, et al. Enhanced anti-tumor activity of a new curcumin-related compound against melanoma and neuroblastoma cells. Mol Cancer. 2010;9:137. doi: 10.1186/1476-4598-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinod BS, Antony J, Nair HH, et al. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4:e505. doi: 10.1038/cddis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang M, Huang O, Zhang X, et al. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules. 2013;18(1):701–720. doi: 10.3390/molecules18010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Sun L, Wu Q, et al. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int J Pharm. 2013;443(1–2):175–182. doi: 10.1016/j.ijpharm.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Sun XD, Liu XE, Huang DS. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol Med Rep. 2012;6(6):1267–1270. doi: 10.3892/mmr.2012.1103. [DOI] [PubMed] [Google Scholar]
- 44.Yadav B, Taurin S, Larsen L, Rosengren RJ. RL66 a second-generation curcumin analog has potent in vivo and in vitro anticancer activity in ER-negative breast cancer models. Int J Oncol. 2012;41(5):1723–1732. doi: 10.3892/ijo.2012.1625. [DOI] [PubMed] [Google Scholar]
- 45.Yadav B, Taurin S, Larsen L, Rosengren RJ. RL71, a second-generation curcumin analog, induces apoptosis and downregulates Akt in ER-negative breast cancer cells. Int J Oncol. 2012;41(3):1119–1127. doi: 10.3892/ijo.2012.1521. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee M, Singh P, Panda D. Curcumin suppresses the dynamic instability of microtubules, activates the mitotic checkpoint and induces apoptosis in MCF-7 cells. FEBS J. 2010;277(16):3437–3448. doi: 10.1111/j.1742-4658.2010.07750.x. [DOI] [PubMed] [Google Scholar]
- 47.Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer Res. 2012;32(11):4843–4850. [PubMed] [Google Scholar]
- 48.Zhang X, Zhang HQ, Zhu GH, et al. A novel mono-carbonyl analogue of curcumin induces apoptosis in ovarian carcinoma cells via endoplasmic reticulum stress and reactive oxygen species production. Mol Med Rep. 2012;5(3):739–744. doi: 10.3892/mmr.2011.700. [DOI] [PubMed] [Google Scholar]
- 49.Selvendiran K, Ahmed S, Dayton A, et al. HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol Ther. 2011;12(9):837–845. doi: 10.4161/cbt.12.9.17713. [DOI] [PubMed] [Google Scholar]
- 50.Cort A, Timur M, Ozdemir E, Kucuksayan E, Ozben T. Synergistic anticancer activity of curcumin and bleomycin: an in vitro study using human malignant testicular germ cells. Mol Med Rep. 2012;5(6):1481–146. doi: 10.3892/mmr.2012.848. [DOI] [PubMed] [Google Scholar]
- 51.Ni X, Zhang A, Zhao Z, Shen Y, Wang S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncol Rep. 2012;28(1):85–90. doi: 10.3892/or.2012.1783. [DOI] [PubMed] [Google Scholar]
- 52.Teiten MH, Gaascht F, Cronauer M, Henry E, Dicato M, Diederich M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int J Oncol. 2011;38(3):603–611. doi: 10.3892/ijo.2011.905. [DOI] [PubMed] [Google Scholar]
- 53.Hilchie AL, Furlong SJ, Sutton K, et al. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr Cancer. 2010;62(3):379–389. doi: 10.1080/01635580903441238. [DOI] [PubMed] [Google Scholar]
- 54.Thakkar A, Sutaria D, Grandhi BK, Wang J, Prabhu S. The molecular mechanism of action of aspirin, curcumin and sulforaphane combinations in the chemoprevention of pancreatic cancer. Oncol Rep. 2013;29(4):1671–1677. doi: 10.3892/or.2013.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100(9):1425–1433. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian H, Yang Y, Wang X. Curcumin enhanced adriamycin-induced human liver-derived Hepatoma G2 cell death through activation of mitochondria-mediated apoptosis and autophagy. Eur J Pharm Sci. 2011;43(3):125–131. doi: 10.1016/j.ejps.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Prakobwong S, Gupta SC, Kim JH, et al. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32(9):1372–1380. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai XZ, Huang WY, Qiao Y, et al. Inhibitory effects of curcumin on gastric cancer cells: A proteomic study of molecular targets. Phytomedicine. 2013;20(6):495–505. doi: 10.1016/j.phymed.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Yu LL, Wu JG, Dai N, Yu HG, Si JM. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol Rep. 2011;26(5):1197–1203. doi: 10.3892/or.2011.1410. [DOI] [PubMed] [Google Scholar]
- 60.Basile V, Belluti S, Ferrari E, et al. bis-dehydroxy-curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy. PLoS One. 2013;8(1):e53664. doi: 10.1371/journal.pone.0053664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo LD, Chen XJ, Hu YH, Yu ZJ, Wang D, Liu JZ. Curcumin Inhibits Proliferation and Induces Apoptosis of Human Colorectal Cancer Cells by Activating the Mitochondria Apoptotic Pathway. Phytother Res. 2013;27(3):422–430. doi: 10.1002/ptr.4731. [DOI] [PubMed] [Google Scholar]
- 62.Datta R, Halder SK, Zhang B. Role of TGF-βsignaling in curcumin-mediated inhibition of tumorigenicity of human lung cancer cells. J Cancer Res Clin Oncol. 2013;139(4):563–572. doi: 10.1007/s00432-012-1352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H, Zhou BH, Qiu X, et al. T63, a new 4-arylidene curcumin analogue, induces cell cycle arrest and apoptosis through activation of the reactive oxygen species-FOXO3a pathway in lung cancer cells. Free Radic Biol Med. 2012;53(12):2204–2217. doi: 10.1016/j.freeradbiomed.2012.10.537. [DOI] [PubMed] [Google Scholar]
- 64.Ye MX, Zhao YL, Li Y, et al. Curcumin reverses cis-platin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine. 2012;19(8–9):779–787. doi: 10.1016/j.phymed.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Xiao J, Wang Y, Peng J, et al. A synthetic compound, 1,5-bis(2-methoxyphenyl) penta-1,4-dien-3-one (B63), induces apoptosis and activates endoplasmic reticulum stress in non-small cell lung cancer cells. Int J Cancer. 2012;131(6):1455–1465. doi: 10.1002/ijc.27406. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Rishi AK, Wu W, et al. Curcumin suppresses growth of mesothelioma cells in vitro and in vivo, in part, by stimulating apoptosis. Mol Cell Biochem. 2011;357(1–2):83–94. doi: 10.1007/s11010-011-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamat AM, Tharakan ST, Sung B, Aggarwal BB. Curcumin potentiates the antitumor effects of Bacillus Calmette-Guerin against bladder cancer through the downregulation of NF-kappa B and upregulation of TRAIL receptors. Cancer Res. 2009;69(23):8958–8966. doi: 10.1158/0008-5472.CAN-09-2045. [DOI] [PubMed] [Google Scholar]
- 68.Tian F, Fan T, Zhang Y, Jiang Y, Zhang X. Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 2012;44(10):847–855. doi: 10.1093/abbs/gms074. [DOI] [PubMed] [Google Scholar]
- 69.Subramaniam D, Ponnurangam S, Ramamoorthy P, et al. Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One. 2012;7(2):e30590. doi: 10.1371/journal.pone.0030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartojo W, Silvers AL, Thomas DG, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappa B in esophageal adenocarcinoma. Transl Oncol. 2010;3(2):99–108. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Sullivan-Coyne G, O’Sullivan GC, O’Donovan TR, Piwocka K, McKenna SL. Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br J Cancer. 2009;101(9):1585–1595. doi: 10.1038/sj.bjc.6605308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ip SW, Wu SY, Yu CC, et al. Induction of apoptotic death by curcumin in human tongue squamous cell carcinoma SCC-4 cells is mediated through endoplasmic reticulum stress and mitochondria-dependent pathways. Cell Biochem Funct. 2011;29(8):641–650. doi: 10.1002/cbf.1800. [DOI] [PubMed] [Google Scholar]
- 73.Abuzeid WM, Davis S, Tang AL, et al. Sensitization of head and neck cancer to cisplatin through the use of a novel curcumin analog. Arch Otolaryngol Head Neck Surg. 2011;137(5):499–507. doi: 10.1001/archoto.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuo CL, Wu SY, Ip SW, et al. Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int J Oncol. 2011;39(2):319–328. doi: 10.3892/ijo.2011.1057. [DOI] [PubMed] [Google Scholar]
- 75.Lin YT, Wang LF, Hsu YC. Curcuminoids suppress the growth of pharynx and nasopharyngeal carcinoma cells through induced apoptosis. J Agric Food Chem. 2009;57(9):3765–3770. doi: 10.1021/jf803758x. [DOI] [PubMed] [Google Scholar]
- 76.Khan MA, Gahlot S, Majumdar S. Oxidative stress induced by curcumin promotes the death of cutaneous T-cell lymphoma (HuT-78) by disrupting the function of several molecular targets. Mol Cancer Ther. 2012;11(9):1873–1883. doi: 10.1158/1535-7163.MCT-12-0141. [DOI] [PubMed] [Google Scholar]
- 77.Yang CW, Chang CL, Lee HC, Chi CW, Pan JP, Yang WC. Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK pathways. BMC Complement Altern Med. 2012;12:22. doi: 10.1186/1472-6882-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kizhakkayil J, Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Glutathione regulates caspase-dependent ceramide production and curcumin- induced apoptosis in human leukemic cells. Free Radic Biol Med. 2012;52(9):1854–1864. doi: 10.1016/j.freeradbiomed.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 79.Qiao Q, Jiang Y, Li G. Curcumin improves the antitumor effect of X-ray irradiation by blocking the NF-κB pathway: an in vitro study of lymphoma. Anticancer Drugs. 2012;23(6):597–605. doi: 10.1097/CAD.0b013e3283503fbc. [DOI] [PubMed] [Google Scholar]
- 80.Mujtaba T, Kanwar J, Wan SB, Chan TH, Dou QP. Sensitizing human multiple myeloma cells to the proteasome inhibitor bortezomib by novel curcumin analogs. Int J Mol Med. 2012;29(1):102–106. doi: 10.3892/ijmm.2011.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh AT, Ghosh M, Forte TM, Ryan RO, Gordon LI. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk Lymphoma. 2011;52(8):1537–1543. doi: 10.3109/10428194.2011.584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao J, Xu DR, Zheng FM, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9:71. doi: 10.1186/1479-5876-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ng AP, Chng WJ, Khan M. Curcumin sensitizes acute promyelocytic leukemia cells to unfolded protein response-induced apoptosis by blocking the loss of misfolded N-CoR protein. Mol Cancer Res. 2011;9(7):878–888. doi: 10.1158/1541-7786.MCR-10-0545. [DOI] [PubMed] [Google Scholar]
- 84.Kong Y, Ma W, Liu X, et al. Cytotoxic activity of curcumin towards CCRF-CEM leukemia cells and its effect on DNA damage. Molecules. 2009;14(12):5328–5338. doi: 10.3390/molecules14125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayun R, Okun E, Berrebi A, et al. Rapamycin and curcumin induce apoptosis in primary resting B chronic lymphocytic leukemia cells. Leuk Lymphoma. 2009;50(4):625–632. doi: 10.1080/10428190902789181. [DOI] [PubMed] [Google Scholar]
- 86.Li ZX, Ouyang KQ, Jiang X, Wang D, Hu Y. Curcumin induces apoptosis and inhibits growth of human Burkitt’s lymphoma in xenograft mouse model. Mol Cells. 2009;27(3):283–289. doi: 10.1007/s10059-009-0036-9. [DOI] [PubMed] [Google Scholar]
- 87.Xu D, Tian W, Shen H. P-gp upregulation may be blocked by natural curcuminoids, a novel class of chemoresistance-preventing agent. Mol Med Rep. 2012 Sep 27; doi: 10.3892/mmr.2012.1106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM. Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett. 2010;293(1):65–72. doi: 10.1016/j.canlet.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saleh EM, El-awady RA, Eissa NA, Abdel-Rahman WM. Antagonism between curcumin and the topoisomerase II inhibitor etoposide: a study of DNA damage, cell cycle regulation and death pathways. Cancer Biol Ther. 2012;13(11):1058–1071. doi: 10.4161/cbt.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cort A, Timur M, Ozdemir E, Ozben T. Effects of curcumin on bleomycin-induced apoptosis in human malignant testicular germ cells. J Physiol Biochem. 2013;69(2):289–296. doi: 10.1007/s13105-012-0211-x. [DOI] [PubMed] [Google Scholar]
- 91.Cort A, Ozdemir E, Timur M, Ozben T. Effects of curcumin on bleomycin-induced oxidative stress in malignant testicular germ cell tumors. Mol Med Rep. 2012;6(4):860–866. doi: 10.3892/mmr.2012.991. [DOI] [PubMed] [Google Scholar]
- 92.Srimuangwong K, Tocharus C, Tocharus J, Suksamrarn A, Chintana PY. Effects of hexahydrocurcumin in combination with 5-fluorouracil on dimethylhydrazine-induced colon cancer in rats. World J Gastroenterol. 2012;18(47):6951–6959. doi: 10.3748/wjg.v18.i47.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mo N, Li ZQ, Li J, Cao YD. Curcumin Inhibits TGF-β1-Induced MMP-9 and Invasion through ERK and Smad Signaling in Breast Cancer MDAMB- 231 Cells. Asian Pac J Cancer Prev. 2012;13(11):5709–5714. doi: 10.7314/apjcp.2012.13.11.5709. [DOI] [PubMed] [Google Scholar]
- 94.Killian PH, Kronski E, Michalik KM, et al. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis. 2012;33(12):2507–2519. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- 95.Hassan ZK, Daghestani MH. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(7):3259–3264. doi: 10.7314/apjcp.2012.13.7.3259. [DOI] [PubMed] [Google Scholar]
- 96.Kim JM, Noh EM, Kwon KB, et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP- expression in MCF-7 human breast cancer cells. Phytomedicine. 2012;19(12):1085–1092. doi: 10.1016/j.phymed.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Tsang RK, Tang WW, Gao W, et al. Curcumin inhibits tongue carcinoma cells migration and invasion through downregulation of matrix metallopeptidase 10. Cancer Invest. 2012;30(7):503–512. doi: 10.3109/07357907.2012.691192. [DOI] [PubMed] [Google Scholar]
- 98.Mudduluru G, George-William JN, Muppala S, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31(3):185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 99.Seo JH, Jeong KJ, Oh WJ, et al. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: their inhibition by curcumin. Cancer Lett. 2010;288(1):50–56. doi: 10.1016/j.canlet.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 100.Lin SS, Lai KC, Hsu SC, et al. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett. 2009;285(2):127–133. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 101.Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT through downregulation of NF-κB-Snail signaling in breast cancer cells. Oncol Rep. 2013;29(1):117–124. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- 102.Wong TS, Chan WS, Li CH, et al. Curcumin alters the migratory phenotype of nasopharyngeal carcinoma cells through up-regulation of E- cadherin. Anticancer Res. 2010;30(7):2851–286. [PubMed] [Google Scholar]
- 103.Schmiedel BJ, Hutter C, Hesse M, Staege MS. Expression of multiple membrane-associated phospholipase A1 beta transcript variants and lysophosphatidic acid receptors in Ewing tumor cells. Mol Biol Rep. 2011;38(7):4619–4628. doi: 10.1007/s11033-010-0595-z. [DOI] [PubMed] [Google Scholar]
- 104.Yan L. Dietary supplementation with curcumin enhances metastatic growth of Lewis lung carcinoma in mice. Int J Cancer. 2013;132(2):269–275. doi: 10.1002/ijc.27683. [DOI] [PubMed] [Google Scholar]
- 105.Roscilli G, Marra E, Mori F, et al. Carnitines slow down tumor development of colon cancer in the DMH-chemical carcinogenesis mouse model. J Cell Biochem. 2013;114(7):1665–1673. doi: 10.1002/jcb.24508. [DOI] [PubMed] [Google Scholar]
- 106.Phillips J, Moore-Medlin T, Sonavane K, Ekshyyan O, McLarty J, Nathan CA. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 Mice. Otolaryngol Head Neck Surg. 2013;148(5):797–803. doi: 10.1177/0194599813476845. [DOI] [PubMed] [Google Scholar]
- 107.Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997;116(2):197–203. doi: 10.1016/s0304-3835(97)00187-0. [DOI] [PubMed] [Google Scholar]
- 108.Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N. Curcumin attenuates gastric cancer induced by N-methyl-N-nitrosourea and saturated sodium chloride in rats. J Biomed Biotechnol. 2012;2012:915380. doi: 10.1155/2012/915380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubota M, Shimizu M, Sakai H, et al. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer. 2012;64(1):72–79. doi: 10.1080/01635581.2012.630554. [DOI] [PubMed] [Google Scholar]
- 110.Carroll CE, Benakanakere I, Besch-Williford C, Ellersieck MR, Hyder SM. Curcumin delays development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz[a]anthracene-induced mammary tumors. Menopause. 2010;17(1):178–184. doi: 10.1097/gme.0b013e3181afcce5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000;21(2):331–335. doi: 10.1093/carcin/21.2.331. [DOI] [PubMed] [Google Scholar]
- 112.Ghosh D, Choudhury ST, Ghosh S, Mandal AK, Sarkar S, et al. Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem Biol Interact. 2012;195(3):206–214. doi: 10.1016/j.cbi.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 113.Prakobwong S, Khoontawad J, Yongvanit P, et al. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer. 2011;129(1):88–100. doi: 10.1002/ijc.25656. [DOI] [PubMed] [Google Scholar]
- 114.Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22(6C):4179–4181. [PubMed] [Google Scholar]
- 115.Dance-Barnes ST, Kock ND, Moore JE, et al. Lung tumor promotion by curcumin. Carcinogenesis. 2009;30(6):1016–1023. doi: 10.1093/carcin/bgp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonda MA, Rice NR, Gilden RV. Avian reticuloendotheliosis virus: characterization of the high-molecular-weight viral RNA in transforming and helper virus populations. J Virol. 1980;34(3):743–751. doi: 10.1128/jvi.34.3.743-751.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen IS, Mak TW, O’Rear JJ, Temin HM. Characterization of reticuloendotheliosis virus strain T DNA and isolation of a novel variant of reticuloendotheliosis virus strain T by molecular cloning. J Virol. 1981;40(3):800–811. doi: 10.1128/jvi.40.3.800-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen IS, Temin HM. Substitution of 5′ helper virus sequences into non-rel portion of reticuloendotheliosis virus strain T suppresses transformation of chicken spleen cells. Cell. 1982;31(1):111–120. doi: 10.1016/0092-8674(82)90410-x. [DOI] [PubMed] [Google Scholar]
- 119.Brownell E, O’Brien SJ, Nash WG, Rice N. Genetic characterization of human c-rel sequences. Mol Cell Biol. 1985;5:2826–2831. doi: 10.1128/mcb.5.10.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baeuerle PA, Baltimore D. A 65-kappa D subunit of active NF-kappa B is required for inhibition of NF-kappa B by I kappa B. Genes Dev. 1989;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- 121.Deloukas P, Dauwerse JG, van Ommen GJ, van Loon AP. The human NFKB3 gene encoding the p65 subunit of transcription factor NF-kappa B is located on chromosome 11q12. Genomics. 1994;19:592–594. doi: 10.1006/geno.1994.1115. [DOI] [PubMed] [Google Scholar]
- 122.Bours V, Burd PR, Brown K, et al. A novel mitogen-inducible gene product related to p50/p105-NF-kappa B participates in transactivation through a kappa B site. Mol Cell Biol. 1992;12(2):685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 124.Neri A, Chang CC, Lombardi L, et al. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- 125.Lu D, Thompson JD, Gorski GK, Rice NR, Mayer MG, Yunis JJ. Alterations at the rel locus in human lymphoma. Oncogene. 1991;6(7):1235–1241. [PubMed] [Google Scholar]
- 126.Kalaitzidis D, Gilmore TD. Genomic organization and expression of the rearranged REL proto-oncogene in the human B-cell lymphoma cell line RC-K8. Genes Chromosomes Cancer. 2002;34(1):129–135. doi: 10.1002/gcc.10051. [DOI] [PubMed] [Google Scholar]
- 127.Fracchiolla NS, Lombardi L, Salina M, et al. Structural alterations of the NF-kappa B transcription factor lyt-10 in lymphoid malignancies. Oncogene. 1993;8(10):2839–2845. [PubMed] [Google Scholar]
- 128.Novelli S, Briones J, Sierra J. Epidemiology of lymphoid malignancies: last decade update. Springerplus. 2013;2(1):70. doi: 10.1186/2193-1801-2-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tzankov A, Dirnhofer S. Pathobiology of classical Hodgkin lymphoma. Pathobiology. 2006;73(3):107–125. doi: 10.1159/000095558. [DOI] [PubMed] [Google Scholar]
- 130.Drexler HG, Minowada J. Hodgkin’s disease derived cell lines: a review. Hum Cell. 2009;5:42–53. [PubMed] [Google Scholar]
- 131.Sahin U, Neumann F, Tureci O, Schmits R, Perez F, Pfreundschuh M. Hodgkin and Reed-Sternberg cell-associated autoantigen CLIP-170/ restin is a marker for dendritic cells and is involved in the trafficking of macropinosomes to the cytoskeleton, supporting a function-based concept of Hodgkin and Reed-Sternberg cells. Blood. 2002;100(12):4139–4145. doi: 10.1182/blood.V100.12.4139. [DOI] [PubMed] [Google Scholar]
- 132.Schwering I, Bräuninger A, Klein U, et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101(4):1505–1512. doi: 10.1182/blood-2002-03-0839. [DOI] [PubMed] [Google Scholar]
- 133.Lin HM, Teitell MA. Second malignancy after treatment of pediatric Hodgkin disease. J Ped Hematol Oncol. 2005;27(1):28–36. doi: 10.1097/01.mph.0000150740.80690.d4. [DOI] [PubMed] [Google Scholar]
- 134.Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol. 2011;154(1):23–31. doi: 10.1111/j.1365-2141.2011.08713.x. [DOI] [PubMed] [Google Scholar]
- 135.van der Kaaij MA, van Echten-Arends J, Simons AH, Kluin-Nelemans HC. Fertility preservation after chemotherapy for Hodgkin lymphoma. Hematol Oncol. 2010;28(4):168–179. doi: 10.1002/hon.939. [DOI] [PubMed] [Google Scholar]
- 136.Körholz D, Claviez A, Hasenclever D, et al. The concept of the GPOH-HD 2003 therapy study for pediatric Hodgkin’s disease: evolution in the tradition of the DAL/GPOH studies. Klin Padiatr. 2004;216(3):150–156. doi: 10.1055/s-2004-822627. [DOI] [PubMed] [Google Scholar]
- 137.Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]
- 138.Barth TF, Martin-Subero JI, Joos S, et al. Gains of 2p involving the REL locus correlate with nuclear c-Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood. 2003;101(9):3681–3686. doi: 10.1182/blood-2002-08-2577. [DOI] [PubMed] [Google Scholar]
- 139.Schmitz R, Hansmann ML, Bohle V, et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J Exp Med. 2009;206(5):981–989. doi: 10.1084/jem.20090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lake A, Shield LA, Cordano P, et al. Mutations of NFKBIA, encoding Ikappa B alpha, are a recurrent finding in classical Hodgkin lymphoma but are not a unifying feature of non-EBV-associated cases. Int J Cancer. 2009;125(6):1334–1342. doi: 10.1002/ijc.24502. [DOI] [PubMed] [Google Scholar]
- 141.Sandur SK, Deorukhkar A, Pandey MK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappa B activity. Int J Radiat Oncol Biol Phys. 2009;75(2):534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Surh YJ, Han SS, Keum YS, Seo HJ, Lee SS. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-kappa B and AP-1. Biofactors. 2000;12(1–4):107–112. doi: 10.1002/biof.5520120117. [DOI] [PubMed] [Google Scholar]
- 143.Mackenzie GG, Queisser N, Wolfson ML, Fraga CG, Adamo AM, Oteiza PI. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappa B and STAT3 pathways in Hodgkin’s lymphoma cells. Int J Cancer. 2008;123(1):56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 144.Chirnomas D, Taniguchi T, de la Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5(4):952–961. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 145.Yeon KY, Kim SA, Kim YH, et al. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res. 2010;89(2):170–174. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- 146.Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56(2):197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 147.Rinaldi AL, Morse MA, Fields HW, et al. Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits (-)-benzo(a)pyrene-7R-trans- 7,8-dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Res. 2002;62(19):5451–5456. [PubMed] [Google Scholar]
- 148.Bartik L, Whitfield GK, Kaczmarska M, et al. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21(12):1153–1161. doi: 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Batie S, Lee JH, Jama RA, et al. Synthesis and biological evaluation of halogenated curcumin analogs as potential nuclear receptor selective agonists. Bioorg Med Chem. 2013;21(3):693–702. doi: 10.1016/j.bmc.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 150.van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer. 2013;49(6):1422–1436. doi: 10.1016/j.ejca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 151.Sokoloski JA, Shyam K, Sartorelli AC. Induction of the differentiation of HL-60 promyelocytic leukemia cells by curcumin in combination with low levels of vitamin D3. Oncol Res. 1997;9(1):31–39. [PubMed] [Google Scholar]
- 152.Mosieniak G, Sliwinska M, Piwocka K, Sikora E. Curcumin abolishes apoptosis resistance of calcitriol-differentiated HL-60 cells. FEBS Lett. 2006;580(19):4653–4660. doi: 10.1016/j.febslet.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 153.Renné C, Benz AH, Hansmann ML. Vitamin D3 receptor is highly expressed in Hodgkin’s lymphoma. BMC Cancer. 2012;12:215. doi: 10.1186/1471-2407-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davies M, Hayes ME, Yin JA, Berry JL, Mawer EB. Abnormal synthesis of 1,25-dihydroxyvitamin D in patients with malignant lymphoma. J Clin Endocrinol Metab. 1994;78(5):1202–1207. doi: 10.1210/jcem.78.5.8175979. [DOI] [PubMed] [Google Scholar]
- 155.Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood. 1993;82(5):1383–1394. [PubMed] [Google Scholar]
- 156.Porojnicu AC, Robsahm TE, Ree AH, Moan J. Season of diagnosis is a prognostic factor in Hodgkin’s lymphoma: a possible role of sun-induced vitamin D. Br J Cancer. 2005;93(5):571–574. doi: 10.1038/sj.bjc.6602722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tse AK, Wan CK, Shen XL, et al. 1,25-dihydroxyvitamin D3 induces biphasic NF-kappa B responses during HL-60 leukemia cells differentiation through protein induction and PI3K/Akt-dependent phosphorylation/ degradation of I kappa B. Exp Cell Res. 2007;313(8):1722–1734. doi: 10.1016/j.yexcr.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 158.Adams LS, Teegarden D. 1,25-dihydroxycholecalciferol inhibits apoptosis in C3H10T1/2 murine fibroblast cells through activation of nuclear factor kappa B. J Nutr. 2004;134(11):2948–2952. doi: 10.1093/jn/134.11.2948. [DOI] [PubMed] [Google Scholar]
- 159.Geldmeyer-Hilt K, Heine G, Hartmann B, Baumgrass R, Radbruch A, Worm M. 1,25-dihydroxyvitamin D3 impairs NF-κB activation in human naïve B cells. Biochem Biophys Res Commun. 2011;407(4):699–702. doi: 10.1016/j.bbrc.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 160.Chen Y, Kong J, Sun T, et al. 1,25-Dihydroxyvitamin D3 suppresses inflammation- induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch Biochem Biophys. 2011;507(2):241–247. doi: 10.1016/j.abb.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harant H, Wolff B, Lindley IJ. 1Alpha,25-dihydroxyvitamin D3 decreases DNA binding of nuclear factor-kappa B in human fibroblasts. FEBS Lett. 1998;436(3):329–334. doi: 10.1016/s0014-5793(98)01153-3. [DOI] [PubMed] [Google Scholar]
- 162.Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1995;92(24):10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shifera AS, Leong D, Hardin JA. Vitamin D does not modulate NF-kappa B activity in Jurkat T cells. Immunol Lett. 2010;131(2):151–158. doi: 10.1016/j.imlet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Farmer PK, He X, Schmitz ML, Rubin J, Nanes MS. Inhibitory effect of NF-kappa B on 1,25-dihydroxyvitamin D(3) and retinoid X receptor function. Am J Physiol Endocrinol Metab. 2000;279(1):E213–20. doi: 10.1152/ajpendo.2000.279.1.E213. [DOI] [PubMed] [Google Scholar]
- 165.Tu SP, Jin H, Shi JD, et al. Curcumin induces the differentiation of myeloidderived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012;5(2):205–215. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bhattacharyya S, Md Sakib Hossain D, Mohanty S, et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7(4):306–315. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cong Y, Wang L, Konrad A, Schoeb T, Elson CO. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol. 2009;39(11):3134–3146. doi: 10.1002/eji.200939052. [DOI] [PubMed] [Google Scholar]
- 168.Zheng M, Zhang Q, Joe Y, et al. Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. Int Immunopharmacol. 2013;15(3):517–523. doi: 10.1016/j.intimp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 169.Kliem C, Merling A, Giaisi M, Köhler R, Krammer PH, Li-Weber M. Curcumin suppresses T cell activation by blocking Ca2+ mobilization and nuclear factor of activated T cells (NFAT) activation. J Biol Chem. 2012;287(13):10200–10209. doi: 10.1074/jbc.M111.318733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim W, Fan YY, Smith R, et al. Dietary curcumin and limonin suppress CD4+ T-cell proliferation and interleukin-2 production in mice. J Nutr. 2009;139(5):1042–1048. doi: 10.3945/jn.108.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luo F, Song X, Zhang Y, Chu Y. Low-dose curcumin leads to the inhibition of tumor growth via enhancing CTL-mediated antitumor immunity. Int Immunopharmacol. 2011;11(9):1234–1240. doi: 10.1016/j.intimp.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 172.Khan G. Epstein-Barr virus, cytokines, and inflammation: a cocktail for the pathogenesis of Hodgkin’s lymphoma? Exp Hematol. 2006;34(4):399–406. doi: 10.1016/j.exphem.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 173.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 174.Chen CQ, Yu K, Yan QX, et al. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis. 2013;34(7):1442–1449. doi: 10.1093/carcin/bgt070. [DOI] [PubMed] [Google Scholar]
- 175.Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol. 2007;101(6):427–433. doi: 10.1111/j.1742-7843.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 176.Kang J, Chen J, Shi Y, Jia J, Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem Pharmacol. 2005;69(8):1205–1213. doi: 10.1016/j.bcp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 177.Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15(2):165–174. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- 178.Liu Z, Xie Z, Jones W, et al. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009;19(3):706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 179.Kewitz S, Bernig T, Staege MS. Histone deacetylase inhibition restores cisplatin sensitivity of Hodgkin’s lymphoma cells. Leuk Res. 2012;36(6):773–778. doi: 10.1016/j.leukres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 180.Winkler C, Steingrube DS, Altermann W, et al. Hodgkin’s lymphoma RNA-transfected dendritic cells induce cancer/testis antigen-specific immune responses. Cancer Immunol Immunother. 2012;61(10):1769–1779. doi: 10.1007/s00262-012-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kewitz S, Staege MS. Knock-Down of PRAME increases retinoic acid signaling and cytotoxic drug sensitivity of Hodgkin lymphoma cells. PLoS One. 2013;8(2):e55897. doi: 10.1371/journal.pone.0055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li CJ, Zhang LJ, Dezube BJ, Crumpacker CS, Pardee AB. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc Natl Acad Sci U S A. 1993;90(5):1839–1842. doi: 10.1073/pnas.90.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lamprecht B, Walter K, Kreher S, et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16(5):571–579. doi: 10.1038/nm.2129. [DOI] [PubMed] [Google Scholar]
- 184.Ranjan D, Siquijor A, Johnston TD, Wu G, Nagabhuskahn M. The effect of curcumin on human B-cell immortalization by Epstein-Barr virus. Am Surg. 1998;64(1):47–51. [PubMed] [Google Scholar]
- 185.Ranjan D, Johnston TD, Reddy KS, Wu G, Bondada S, Chen C. Enhanced apoptosis mediates inhibition of EBV-transformed lymphoblastoid cell line proliferation by curcumin. J Surg Res. 1999;87(1):1–5. doi: 10.1006/jsre.1999.5719. [DOI] [PubMed] [Google Scholar]
- 186.Chen C, Johnston TD, Reddy KS, Merrick JC, Mastrangelo M, Ranjan D. Cyclosporine directly causes oxidative stress and promotes Epstein-Barr virus transformation of human B cells. J Surg Res. 2001;100(2):166–170. doi: 10.1006/jsre.2001.6233. [DOI] [PubMed] [Google Scholar]
- 187.Bai M, Papoudou-Bai A, Horianopoulos N, Grepi C, Agnantis NJ, Kanavaros P. Expression of bcl2 family proteins and active caspase 3 in classical Hodgkin’s lymphomas. Hum Pathol. 2007;38(1):103–113. doi: 10.1016/j.humpath.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 188.Staege MS, Banning-Eichenseer U, Weissflog G, et al. Gene expression profiles of Hodgkin’s lymphoma cell lines with different sensitivity to cytotoxic drugs. Exp Hematol. 2008;36(7):886–896. doi: 10.1016/j.exphem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 189.Navarro A, Diaz T, Martinez A, et al. Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood. 2009;114(14):2945–2951. doi: 10.1182/blood-2009-02-204842. [DOI] [PubMed] [Google Scholar]
- 190.Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumininduced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 191.Martín-Cordero C, López-Lázaro M, Gálvez M, Ayuso MJ. Curcumin as a DNA topoisomerase II poison. J Enzyme Inhib Med Chem. 2003;18(6):505–509. doi: 10.1080/14756360310001613085. [DOI] [PubMed] [Google Scholar]
- 192.Diaz T, Navarro A, Ferrer G, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One. 2011;6(4):e18856. doi: 10.1371/journal.pone.0018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6- mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112(4):1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martini M, Hohaus S, Petrucci G, et al. Phosphorylated STAT5 represents a new possible prognostic marker in Hodgkin lymphoma. Am J Clin Pathol. 2008;129(3):472–477. doi: 10.1309/63H1A83HRTBQ07DB. [DOI] [PubMed] [Google Scholar]
- 195.Weniger MA, Melzner I, Menz CK, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25(18):2679–2684. doi: 10.1038/sj.onc.1209151. [DOI] [PubMed] [Google Scholar]
- 196.Baus D, Pfitzner E. Specific function of STAT3, SOCS1, and SOCS3 in the regulation of proliferation and survival of classical Hodgkin lymphoma cells. Int J Cancer. 2006;118(6):1404–1413. doi: 10.1002/ijc.21539. [DOI] [PubMed] [Google Scholar]
- 197.Cochet O, Frelin C, Peyron JF, Imbert V. Constitutive activation of STAT proteins in the HDLM-2 and L540 Hodgkin lymphoma-derived cell lines supports cell survival. Cell Signal. 2006;18(4):449–455. doi: 10.1016/j.cellsig.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 198.Hinz M, Lemke P, Anagnostopoulos I, et al. Nuclear factor kappa B-dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med. 2002;196(5):605–617. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Skinnider BF, Elia AJ, Gascoyne RD, et al. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2002;99(2):618–626. doi: 10.1182/blood.v99.2.618. [DOI] [PubMed] [Google Scholar]
- 200.Kube D, Holtick U, Vockerodt M, et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98(3):762–770. doi: 10.1182/blood.v98.3.762. [DOI] [PubMed] [Google Scholar]
- 201.Van Roosbroeck K, Cox L, Tousseyn T, et al. JAK2 rearrangements, including the novel SEC31A-JAK2 fusion, are recurrent in classical Hodgkin lymphoma. Blood. 2011;117(15):4056–4064. doi: 10.1182/blood-2010-06-291310. [DOI] [PubMed] [Google Scholar]
- 202.Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60(16):4561–4572. [PubMed] [Google Scholar]
- 203.Su CC, Chen GW, Lin JG, Wu LT, Chung JG. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B/p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase- 2 expressions. Anticancer Res. 2006;26(2A):1281–1288. [PubMed] [Google Scholar]
- 204.Brown J, Reading SJ, Jones S, Fitchett CJ, Howl J, et al. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab Invest. 2000;80(1):37–45. doi: 10.1038/labinvest.3780006. [DOI] [PubMed] [Google Scholar]
- 205.Park MJ, Kim EH, Park IC, et al. Curcumin inhibits cell cycle progression of immortalized human umbilical vein endothelial (ECV304) cells by upregulating cyclin-dependent kinase inhibitor, p21WAF1/CIP1, p27KIP1 and p53. Int J Oncol. 2002;21(2):379–383. [PubMed] [Google Scholar]
- 206.Kim JH, Shim JS, Lee SK, et al. Microarray-based analysis of antiangiogenic activity of demethoxycurcumin on human umbilical vein endothelial cells: crucial involvement of the down-regulation of matrix metalloproteinase. Jpn J Cancer Res. 2002;93(12):1378–1385. doi: 10.1111/j.1349-7006.2002.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen HW, Yu SL, Chen JJ, et al. Anti-invasive gene expression profile of curcumin in lung adenocarcinoma based on a high throughput microarray analysis. Mol Pharmacol. 2004;65(1):99–110. doi: 10.1124/mol.65.1.99. [DOI] [PubMed] [Google Scholar]
- 208.Zhang J, Du Y, Wu C, et al. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep. 2010;24(5):1217–1223. doi: 10.3892/or_00000975. [DOI] [PubMed] [Google Scholar]
- 209.Sahoo K, Dozmorov MG, Anant S, Awasthi V. The curcuminoid CLEFMA selectively induces cell death in H441 lung adenocarcinoma cells via oxidative stress. Invest New Drugs. 2012;30(2):558–567. doi: 10.1007/s10637-010-9610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nguyen KT, Shaikh N, Shukla KP, Su SH, Eberhart RC, Tang L. Molecular responses of vascular smooth muscle cells and phagocytes to curcumin- eluting bioresorbable stent materials. Biomaterials. 2004;25(23):5333–5346. doi: 10.1016/j.biomaterials.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 211.Ramachandran C, Rodriguez S, Ramachandran R, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25(5):3293–3302. [PubMed] [Google Scholar]
- 212.Bachmeier BE, Mohrenz IV, Mirisola V, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NF kappa B. Carcinogenesis. 2008;29(4):779–789. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- 213.Altenburg JD, Bieberich AA, Terry C, et al. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cine N, Limtrakul P, Sunnetci D, Nagy B, Savli H. Effects of curcumin on global gene expression profiles in the highly invasive human breast carcinoma cell line MDA-MB 231: A gene network-based microarray analysis. Exp Ther Med. 2013;5(1):23–27. doi: 10.3892/etm.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang Y, Li ZS, Jiang FS, Deng X, Yao CS, Nie G. Effects of different ingredients of zedoary on gene expression of HSC-T6 cells. World J Gastroenterol. 2005;11(43):6780–6786. doi: 10.3748/wjg.v11.i43.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Billerey-Larmonier C, Uno JK, Larmonier N, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14(6):780–793. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nones K, Dommels YE, Martell S, et al. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a-/-) mice, a model of inflammatory bowel diseases. Br J Nutr. 2009;101(2):169–181. doi: 10.1017/S0007114508009847. [DOI] [PubMed] [Google Scholar]
- 218.Jia Q, Ivanov I, Zlatev ZZ, et al. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br J Nutr. 2011;106(4):519–529. doi: 10.1017/S0007114511000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Staege MS, Hutter C, Neumann I, Foja S, Hattenhorst UE, et al. DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res. 2004;64(22):8213–8221. doi: 10.1158/0008-5472.CAN-03-4059. [DOI] [PubMed] [Google Scholar]
- 220.Aravindan N, Madhusoodhanan R, Ahmad S, Johnson D, Herman TS. Curcumin inhibits NF kappa B mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther. 2008;7(4):569–576. doi: 10.4161/cbt.7.4.5534. [DOI] [PubMed] [Google Scholar]
- 221.Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008;7(3):464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 222.Stierum R, Conesa A, Heijne W, et al. Transcriptome analysis provides new insights into liver changes induced in the rat upon dietary administration of the food additives butylated hydroxytoluene, curcumin, propyl gallate and thiabendazole. Food Chem Toxicol. 2008;46(8):2616–2628. doi: 10.1016/j.fct.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 223.Teiten MH, Eifes S, Reuter S, Duvoix A, Dicato M, Diederich M. Gene expression profiling related to anti-inflammatory properties of curcumin in K562 leukemia cells. Ann N Y Acad Sci. 2009;1171:391–398. doi: 10.1111/j.1749-6632.2009.04890.x. [DOI] [PubMed] [Google Scholar]
- 224.Hong D, Zeng X, Xu W, Ma J, Tong Y, Chen Y. Altered profiles of gene expression in curcumin-treated rats with experimentally induced myocardial infarction. Pharmacol Res. 2010;61(2):142–148. doi: 10.1016/j.phrs.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 225.Kao HH, Wu CJ, Won SJ, Shin JW, Liu HS, Su CL. Kinase gene expression and subcellular protein expression pattern of protein kinase C isoforms in curcumin-treated human hepatocellular carcinoma Hep 3B cells. Plant Foods Hum Nutr. 2011;66(2):136–142. doi: 10.1007/s11130-011-0228-2. [DOI] [PubMed] [Google Scholar]
- 226.Karlstetter M, Lippe E, Walczak Y, et al. Curcumin is a potent modulator of microglial gene expression and migration. J Neuroinflammation. 2011;8:125. doi: 10.1186/1742-2094-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sreenivasan S, Thirumalai K, Krishnakumar S. Expression profile of genes regulated by curcumin in Y79 retinoblastoma cells. Nutr Cancer. 2012;64(4):607–6016. doi: 10.1080/01635581.2012.669875. [DOI] [PubMed] [Google Scholar]
- 228.Sreenivasan S, Thirumalai K, Danda R, Krishnakumar S. Effect of curcumin on miRNA expression in human Y79 retinoblastoma cells. Curr Eye Res. 2012;37(5):421–428. doi: 10.3109/02713683.2011.647224. [DOI] [PubMed] [Google Scholar]
- 229.Colitti M, Gaspardo B, Della Pria A, Scaini C, Stefanon B. Transcriptome modification of white blood cells after dietary administration of curcumin and non-steroidal anti-inflammatory drug in osteoarthritic affected dogs. Vet Immunol Immunopathol. 2012;147(3–4):136–146. doi: 10.1016/j.vetimm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 230.Ireson CR, Jones DJ, Orr S, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11(1):105–111. [PubMed] [Google Scholar]
- 231.Sharma RA, Ireson CR, Verschoyle RD, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. 2001;7(5):1452–1458. [PubMed] [Google Scholar]
- 232.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 233.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296(1–2):85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 234.Singh DV, Godbole MM, Misra K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: simulation for next generation of P-gp inhibitors. J Mol Model. 2013;19(1):227–238. doi: 10.1007/s00894-012-1535-8. [DOI] [PubMed] [Google Scholar]
- 235.Qiang F, Kang KW, Han HK. Repeated dosing of piperine induced gene expression of P-glycoprotein via stimulated pregnane-X-receptor activity and altered pharmacokinetics of diltiazem in rats. Biopharm Drug Dispos. 2012;33(8):446–454. doi: 10.1002/bdd.1811. [DOI] [PubMed] [Google Scholar]
- 236.Derochette S, Franck T, Mouithys-Mickalad A, Deby-Dupont G, Neven P, Serteyn D. Intra- and extracellular antioxidant capacities of the new watersoluble form of curcumin (NDS27) on stimulated neutrophils and HL-60 cells. Chem Biol Interact. 2013;201(1–3):49–57. doi: 10.1016/j.cbi.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 237.Aziz MT, El Ibrashy IN, Mikhailidis DP, et al. Signaling mechanisms of a water soluble curcumin derivative in experimental type 1 diabetes with cardiomyopathy. Diabetol Metab Syndr. 2013;5(1):13. doi: 10.1186/1758-5996-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mohanty C, Das M, Sahoo SK. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin Drug Deliv. 2012;9(11):1347–1364. doi: 10.1517/17425247.2012.724676. [DOI] [PubMed] [Google Scholar]
- 239.Li C, Zhang Y, Su T, Feng L, Long Y, Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int J Nanomedicine. 2012;7:5995–6002. doi: 10.2147/IJN.S38043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gong C, Deng S, Wu Q, et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials. 2013;34(4):1413–1432. doi: 10.1016/j.biomaterials.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 241.Deshpande SS, Ingle AD, Maru GB. Chemopreventive efficacy of curcumin-free aqueous turmeric extract in 7,12-dimethylbenz[a]anthracene-induced rat mammary tumorigenesis. Cancer Lett. 1998;123(1):35–40. doi: 10.1016/s0304-3835(97)00400-x. [DOI] [PubMed] [Google Scholar]
- 242.Deshpande SS, Ingle AD, Maru GB. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997;118(1):79–85. doi: 10.1016/s0304-3835(97)00238-3. [DOI] [PubMed] [Google Scholar]
- 243.Martin RC, Aiyer HS, Malik D, Li Y. Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects. Food Chem Toxicol. 2012;50(2):227–231. doi: 10.1016/j.fct.2011.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]