Abstract
Lymphocytes of the diffuse nasal-associated lymphoid tissue (d-NALT) are uniquely positioned to tackle respiratory pathogens at their point-of-entry, yet are rarely examined after intranasal (i.n.) vaccinations or infections. Here we evaluate an i.n. inoculation with Sendai virus (SeV) for elicitation of virus-specific antibody forming cells (AFCs) and CD8+ T cells in the d-NALT. Virus-specific AFCs and CD8+ T cells each appeared by day 7 after SeV inoculation and persisted for 8 months, explaining the long-sustained protection against respiratory virus challenge conferred by this vaccine. AFCs produced IgM, IgG1, IgG2a, IgG2b and IgA, while CD8+ T cells were cytolytic and produced low levels of cytokines. Phenotypic analyses of virus-specific T cells revealed striking similarities with pathogen-specific immune responses in the intestine, highlighting some key features of adaptive immunity at a mucosal site.
INTRODUCTION
Human parainfluenza virus type 1 (hPIV-1) is a serious pathogen of humans and causes infections which can range in severity from mild (e.g. rhinorrhoea and laryngitis) to severe (e.g. laryngotracheobronchitis). hPIV-1 infections result in approximately 50,000 pediatric hospitalizations per year in the US alone , with a rate of infection among immune compromised individuals exceeding that of healthy individuals by three-fold. Currently there is no licensed vaccine for hPIV-1.
Sendai virus (SeV), a natural pathogen of mice is endemic in many parts of the world, yet there have been no confirmed reports of SeV-mediated disease in humans. Based on sequence homology SeV is closely related to hPIV-1. The two viruses are also well related in terms of B and T cell cross-reactivities. SeV has been recently tested as a xenotropic vaccine for hPIV-1 and as a vector for expression of genes from other serious pathogens including respiratory syncytial virus (RSV). In the cotton rat model, recombinant SeVs have been shown to protect against RSV, hPIV-1, hPIV-2 and hPIV-3. The protection appears early and can persist for the lifetime of an animal. Clinical studies have also been conducted with unmodified SeV showing that the vaccine is well tolerated in adults and toddlers (data not shown).
The correlates of protection for respiratory infections are complex. In general, vaccine-induced antibody provides a first line of defense by neutralizing virus, opsonizing virus for attack by other effectors, and supporting antibody-dependent cell-mediated cytotoxicity (ADCC). CD8+ T cells also play a key role by recognizing and killing virally-infected targets. In the case of viral respiratory infections, the B and T cell responses of the d-NALT may be of particular importance as these cells are positioned as first defenders against virus at its point-of-entry. Despite their opportune location, d-NALT cells have been studied only rarely during vaccine evaluations. The current study was designed to examine both antibody forming cells (AFCs) and CD8+ T cells of the murine d-NALT following an i.n. inoculation with SeV. The results demonstrate that a single i.n. inoculation with SeV induced durable d-NALT-resident AFCs and CD8+ T cell activity. The characteristics of these responses were highly reminiscent of pathogen-specific immune responses of the gut.
MATERIALS AND METHODS
Animals and inoculations
Female C57BL/6J (B6; H2b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed under specific pathogen-free conditions in a biosafety level 2+ containment area at the St. Judes animal facility, as specified by the Association for Assessment and Accreditation for Laboratory Animal Care (AAALAC) guidelines. At the time of live virus challenge, mice anesthetized with Avertin were inoculated i.n. with 250 plaque forming units (PFU) of SeV, Enders strain. Mice were approximately 2 months of age at the initiation of the immunization protocols. Experiments were conducted in replicate with 4-10 animals per group in each experiment. Sentinel mice were routinely housed in racks with test mice to validate biocontainment practices and to ensure no inadvertent animal infections with SeV.
Preparation of samples
Immediately prior to sacrifice, mice were anesthetized with avertin and exsanguinated. Nasal wash samples were obtained from sacrificed animals by exposing the trachea and washing the upper trachea and nasal cavity with 200 μl of PBS. Bronchoalveolar lavage (BAL) samples were collected by inserting catheters into trachea and washing three times with 1 ml PBS (3 ml total). Wash samples were centrifuged to separate cellular material. d-NALT was harvested by removing skin, lower jaws, soft palates (including the attached o-NALT), muscles, cheek bones and incisors from the heads. Remaining snouts were cut into small pieces, after which cells were released by digestion with 4mg/ml collagenase in PBS at 37°C for 30 min (the collagenase treatment was omitted from studies in which panels of membrane markers were tested). Cells were first washed with PBS and then suspended in complete tumour medium (CTM), a Modified Eagles Medium (Invitrogen, Grand Island, NY) supplemented with dextrose (500 μg/ml), glutamine (2mM), 2-mercaptoethanol (3 × 10−5 M), essential and non-essential amino acids, sodium pyruvate, sodium bicarbonate and antibiotics , containing 10% heat inactivated fetal bovine serum (FBS) and layered onto a 40/75% discontinuous percoll gradient. After centrifugation at 600 × g for 30 min, cells were collected from the gradient interface. The cells were washed 2x in PBS and suspended in CTM containing 10% heat inactivated FBS. Lungs were suspended and similarly processed by collagenase digestion and purification on percoll gradients. For antibody ELISAs and free cytokine experiments, individual mouse samples were tested. For AFC, cytotoxic T cell and flow cytometry studies, mouse samples from each group were pooled prior to testing to ensure sufficient cell numbers for assay completion.
ELISA
For the detection of SeV-specific antibodies, sucrose-gradient purified SeV was lysed in disruption buffer (0.05% TritonX-100, 60 mM KCl, 10 mM Tris pH7.8) and diluted with PBS to yield 10 μg/ml for the coating of 96-well ELISA plates. After overnight incubation with 50 μl of the diluted virus lysate per well at 4°C, plates were washed 3x with PBS and blocked with 200 μl of PBS containing 3% bovine serum albumin (BSA, Sigma, St Louis, MO). Nasal washes from vaccinated and control animals were serially diluted in PBS containing 1% BSA and 0.1% Tween 20. 50μl of the diluted samples were applied to the plates and incubated for 1h at 37°C. Plates were then washed 6x with PBS-Tween 20 (0.05%) and incubated with alkaline phosphatase-conjugated goat anti-mouse IgM (Cat #1020-04, Southern Biotechnologies Assoc, Birmingham, AL), IgG (Cat #1030-04) or IgA (Cat #1040-04) diluted 1:1000 in PBS containing 1% BSA and 0.1% Tween 20, for 1 hr at 37°C. Plates were washed 6x with PBS-Tween 20 (0.05%) and developed by addition of p-nitrophenyl phosphate substrate (0.5 mg/ml) in diethanolamine buffer (0.1 M TRIS, 0.1M NaCl. 5% Diethanolamine, 10 mM MgCl2, pH 9.8). The reaction was stopped after 20 min with 5 μl of 0.5 M EDTA (Cat# E-7889, Sigma). Assays were read at OD 405 nm (E max® precision microplate reader, Molecular Devices, Inc, Sunnyvale, CA). Titers were determined using non-linear regression, one-site binding software (0.12 OD cut-off, GraphPad Prism, San Diego, CA).
AFC ELISPOT
The ELISPOT plate (multiscreen™-IP, Millipore, Cat#MAIPS4510, Billerica, MA) was coated with sucrose gradient-purified disrupted SeV overnight at 1ug/100ul/well overnight at 4°C. Wells were washed 4x with PBS and blocked with CTM containing 10% FCS. 1×105 cells were then applied to the wells and incubated for 3 h at 37°C. After washing plates 3x with PBS and 3x with PBS-Tween 20, 100ul of alkaline phosphatase-conjugated goat anti mouse antibodies (see above; also anti-IgG1 (Cat #1070-04), IgG2a (Cat #1080-04), IgG2b (Cat #1090-04), and IgG3 (1100-04)) in 1% BSA were added to each well. After overnight incubation at 4°C, the antibody was removed and plates were developed with 100 microliters 1 mg/ml BCIP/NBT (Sigma Aldrich, Cat#B5655). The plates were incubated at room temperature (RT) and monitored for spot appearance. The exposure was stopped by rinsing plates with water. Plates were dried and spots were counted using a Nikon dissecting scope.
MHC tetrameric reagents and analyses
MHC class I (Kb) tetramers were generated by folding Sendai virus NP324-332 (FAPGNYPAL) with heavy and light chains as described previously. Tetramers were stored as aliquots at 4°C. Staining with PE-conjugated tetrameric reagent was for 1 h at RT, followed by staining with anti-CD8 PerCP Cy-5.5 and fluorescein isothiocyanate-conjugated anti-CD44, anti-CD62L, anti-CD69, anti-CD103, or anti-CD11a (Pharmingen, San Diego, CA) on ice for 20 min. Samples were analyzed on a FACSCalibur. Data were analyzed using Flowjo Version 7.5.5 Software (Tree Star Inc., Ashland, OR).
CTL assays
L929-Kb (L-Kb) target cells were loaded with peptide and Na51CrO4 as described previously. Briefly, monolayers of cells in 6-well plates were incubated with 75 μCi of Na51CrO4 with or without the NP324-332 peptide (75 micrograms) per 700 microliters serum free medium for one hour. FBS was then added to 10% final concentration and the targets were incubated overnight. Cells were washed and counted. Serially diluted effectors were added to 104 target cells in 96-well round-bottomed microtiter plates and incubated for approximately 5h after which supernatants were removed for measurement in a Cobra Quantum gamma counter. The percentage of specific release was calculated using the formula: (experimental – spontaneous)/(maximum – spontaneous)×100. Maximum release was counted from wells with Triton® X-100 (Sigma, Cat#T8787) diluted 1:20. Spontaneous release was typically <10% of maximum release.
Intracellular cytokine staining following peptide stimulation
Cell populations (2–5 × 105) were suspended in 200 μl of CTM with 10 μg/ml Brefeldin A and were cultured for 6h in 96-well U-bottomed plates in the presence or absence of 1 μg/ml peptide, NP324-332. After culture, the cells were washed twice in PBS/Brefeldin A (10 μg/ml), blocked with monoclonal antibody to CD16/CD32 (FcγIII/II, Pharmingen) and stained with rat anti-mouse CD8-PerCp Cy5.5 monoclonal antibody (Pharmingen) for 20 minutes. They were then washed again in PBS/Brefeldin A, fixed in 1% formaldehyde in PBS for 20 min, washed in PBS, placed in PBS/0.5% Saponin (Sigma, St Louis, MO) for 10 min, and incubated with monoclonal antibodies specific for IFN-γ, IL-2, IL-4, IL-5, IL-17A (PE-conjugated, Pharmingen), TNF-α (APC-conjugated, Pharmingen), IL-10 (FITC-conjugated, Ebioscience) or IL-13 (PE-conjugated Ebioscience). Samples were run on a FACSCalibur. Data were analyzed with Flowjo Version 7.5.5 Software.
Cytokine measurements
Samples were tested for the presence of eight different cytokines (IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, IFN-γ, and TNF-α), using a Millipore 8-Plex Mouse Cytokine Assay Kit (Millipore Corporation). Cytokine samples of known concentrations were used to prepare standard curves. Individual samples were tested in duplicate. Assays were analyzed using the Luminex 100/200 System (Luminex Corporation, Austin, TX).
RESULTS
Rapid recruitment and long term persistence of SeV- specific AFCs in the d-NALT
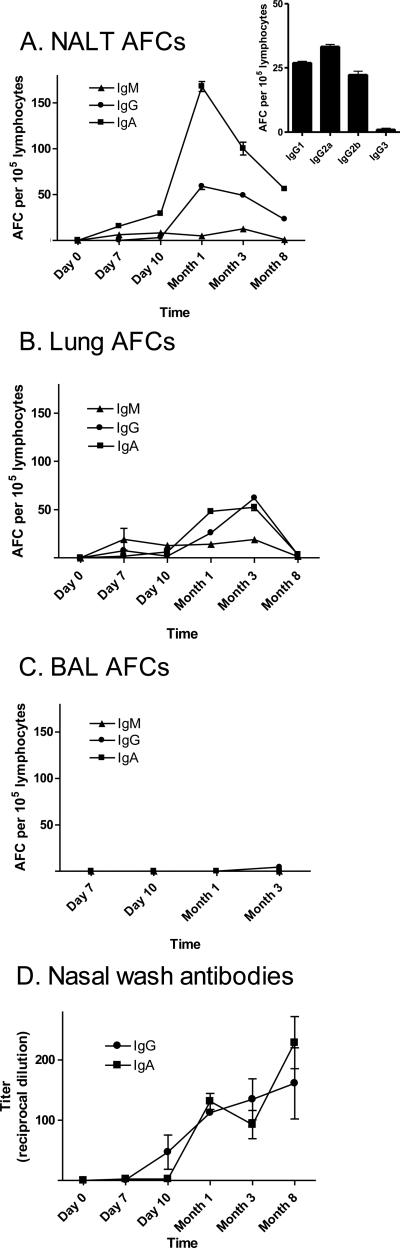
To evaluate the kinetics and persistence of respiratory tract immune responses toward SeV, we inoculated C57BL/6 mice with a single i.n. dose of virus (250 PFU). AFCs with reactivity toward SeV were then evaluated from 1 week to 8 months post-vaccination. Groups of mice were sacrificed at each time point for the collection of d-NALT, lungs and BAL. Significant numbers of virus-specific AFCs, inclusive of IgM, IgG and IgA-producing cells (IgA>IgG or IgM) were observed in the d-NALT as early as day 7 and throughout the course of analysis (Figure 1A). When a battery of IgG subclasses were examined at the peak of activity (Month 1), subtypes IgG1, IgG2a, and IgG2b were scored (Figure 1A insert). AFCs were more prevalent in the d-NALT than in the lungs (1B) and very few AFCs were observed in the BAL (Figure 1C) or in the nasal wash (data not shown).
1. AFCs are rapidly recruited and persistently maintained in the d-NALT following SeV vaccination.
AFCs were measured in C57BL/6 mice after a single i.n. inoculation with 250 PFU Sendai virus, on day 7, day 10, month 1, month 3 and month 8. IgM, IgG and IgA AFCs were monitored in the d-NALT, lungs and BAL (panels A, B, C, respectively). At peak activity, d-NALT cells were also assessed for IgG1, IgG2a, IgG2b and IgG3 production (panel A insert). For BAL samplings, due to limited cell numbers, AFCs were examined on day 7 (IgM and IgA only), day 10 (IgM, IgG and IgA), month 1 (IgG and IgA only), and month 3 (IgM, IgG and IgA). Nasal wash IgG and IgA isotypes were measured by ELISA (panel D).
To determine if the SeV-specific antibodies secreted by d-NALT AFCs might correlate with free antibodies in the upper airways, we examined SeV-specific IgG and IgA antibodies in the nasal wash. Both isotypes were evident (Figure 1D). Antibodies were present for as long as 8 months despite some reduction in AFC numbers at the end of the time course (Figure 1A). Experiments were curtailed at 8 months to avoid the morbidity and mortality associated with aged animals. IgG/IgA ratios were higher for free antibodies than for AFCs, perhaps reflecting the fact that IgA may be tethered to the airways by secretory component and may be difficult to capture by nasal wash.
Antigen-specific CD8+ cells appear in the d-NALT in response to SeV
The T cell response to SeV inoculation in C57BL/6 mice is dominated by CD8+ T cells specific for an immunodominant epitope in the viral nucleoprotein (NP324-332/Kb). Analysis of SeV-inoculated mice revealed the rapid recruitment of the CD8+NP324-332/Kb-specific T cells in the upper and lower respiratory tract (URT and LRT) by day 7 (Figure 2). The percentages of CD8+NP324-332/Kb-specific T cells were much higher than SeV-specific AFCs. While AFCs were detected at levels of <1% among total lymphocytes in the d-NALT (Figure 1), CD8+NP324-332/Kb-specific T cells exceeded 10% on day 10. Lungs and BAL harbored even greater percentages of CD8+NP324-332/Kb-specific T cells on day 10. The T cells were also detected in the nasal wash, reaching percentages as high as 25% among lymphocytes at peak activity (data not shown; total lymphocyte numbers in the nasal wash were too small for comprehensive analyses). Examination of the organized NALT (o-NALT) was precluded by the very small size of o-NALT tissues in the adult mouse. Cervical lymph nodes (CLN) were examined, but the frequencies of CD8+NP324-332/Kb-specific T cells in the CLN were less than 1% on day 10, suggesting that these tissues may not be the primary inductive sites for effector cells in the d-NALT. Of interest, the percentages of CD8+NP324-332/Kb-specific T cells in the d-NALT exceeded those of other samples (BAL, lungs, nasal wash) at the latest tested time point (Figure 2). Clearly, persistence typified lymphocytes of the d-NALT for both AFC and T cell populations.
2. Rapid and durable appearance of CD8+NP324-332/Kb-specific T cells in the d-NALT after SeV inoculation.
The percentages of CD8+ NP324-332/Kb-specific T cells in the d-NALT, lungs and BAL of C57BL/6 mice inoculated with 250 PFU SeV were measured on day 7, day 10, month 1, month 3 and month 8.
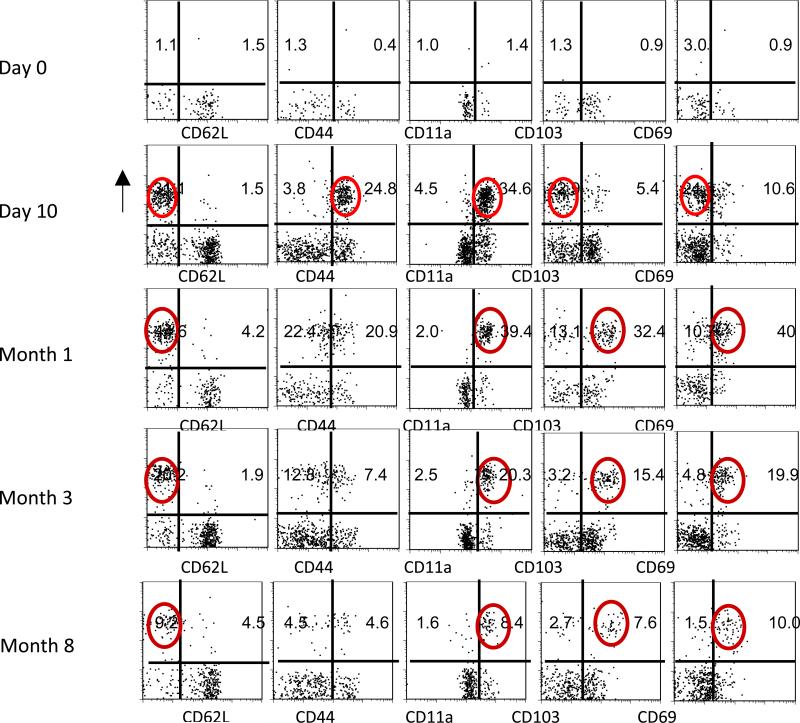
A change in phenotype among NP324-332/Kb-specific T cells in the d-NALT between day 10 and month 1
The expression of CD62L, CD44, CD11a, CD103 and CD69 were evaluated on CD8+NP324-332/Kb-specific T cells in the d-NALT between day 10 and month 3. As shown in Figure 3, Day 10 CD8+NP324-332/Kb-specific T cells were predominantly CD62LLoCD44HiCD11aHiCD103LoCD69Int. By month 1 there was an increase in CD103 and CD69 expression and a marginal decrease in CD44 expression, rendering most cells CD62LLoCD44IntCD11aHiCD103HiCD69Hi. Cells maintained this phenotype throughout the 8 month time course of analyses.
3. Phenotypic analyses of d-NALT cells.
After inoculation of C57BL/6 mice with 250 PFU SeV, d-NALT cells were collected for T cell phenotype analyses. Cells were gated on lymphocytes and on CD8+ cells. NP324-332/Kb-specific T cells were then analyzed for markers CD62L, CD44, CD11a, CD103 and CD69. Test days included day 0, day 10, month 1, month 3 and month 8. Circles highlight the major CD8+ NP324-332/Kb-specific T cell populations at month 1, month 3, and month 8, being of phenotype CD62LLoCD11aHiCD103HiCD69Hi.
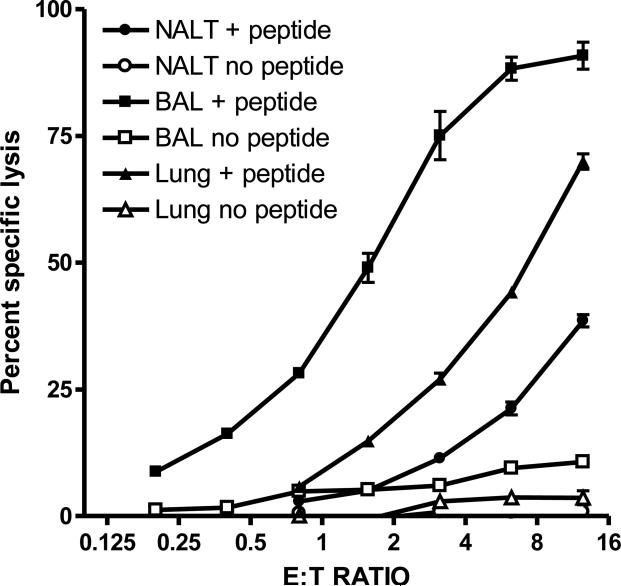
Virus-specific CTLs in the d-NALT of SeV primed mice
To investigate the functional capacity of the d-NALT T lymphocytes, we examined cytolytic activity by 51Cr-release assay with NP324-332 pulsed L929-Kb target cells. Assays were conducted on day 10, the time of peak NP324-332/Kb-specific T cell percentages. Cells from the d-NALT were clearly cytotoxic as demonstrated in Figure 4, although responses were slightly weaker than those of the lower respiratory tract. By month 1, cytolytic activity was much reduced and was detected inconsistently between experiments (data not shown).
4. Cells of the d-NALT are cytolytic.
Lymphocytes from d-NALT, lungs and BAL were tested for cytotoxicity on day 10. Results from assays with NP324-332-pulsed and unpulsed cells are shown. Effectors were serially diluted (1:2) to test six E:T ratios beginning at 12.5:1.
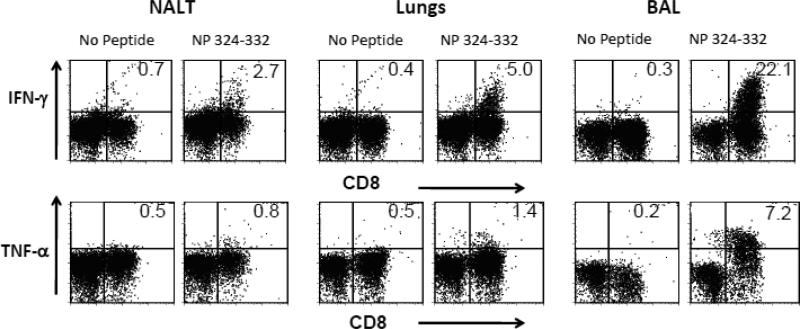
Cytokine responses following SeV inoculations
Intracellular cytokine assays were conducted to define the capacity of NP324-332/Kb-specific T cells to produce cytokines on day 10. These studies were performed with and without the addition of the NP324-332 peptide. As shown in Figure 5, IFN-γ and TNF-α were produced predominantly by CD8+ T cells of the BAL. Responses were weaker in the lung and again weaker in the d-NALT. For example, approximately 22% of the peptide-stimulated CD8+ T cells of the BAL produced IFN-γ, whereas the percentage was approximately 5% in the lung and 2% in the d-NALT. Activities did not improve when antigen presenting cells were added to cultures or when collagenase was omitted from the cell isolation method (data not shown). Other cytokines (IL-2, IL-4, IL-5 IL-10, IL-13, and IL-17) were also tested, but for these, there was little difference between test and control wells (with and without NP324-332 peptide).
5. Cytokine expression by d-NALT, lung and BAL cells following peptide stimulation.
Ten days post-SeV inoculation of C57BL/6 mice, d-NALT, lungs and BAL cells were isolated for measurement of intracellular cytokine production. Results for cytokines IFN-γ and TNF-α are shown, as these were detected at significant levels.
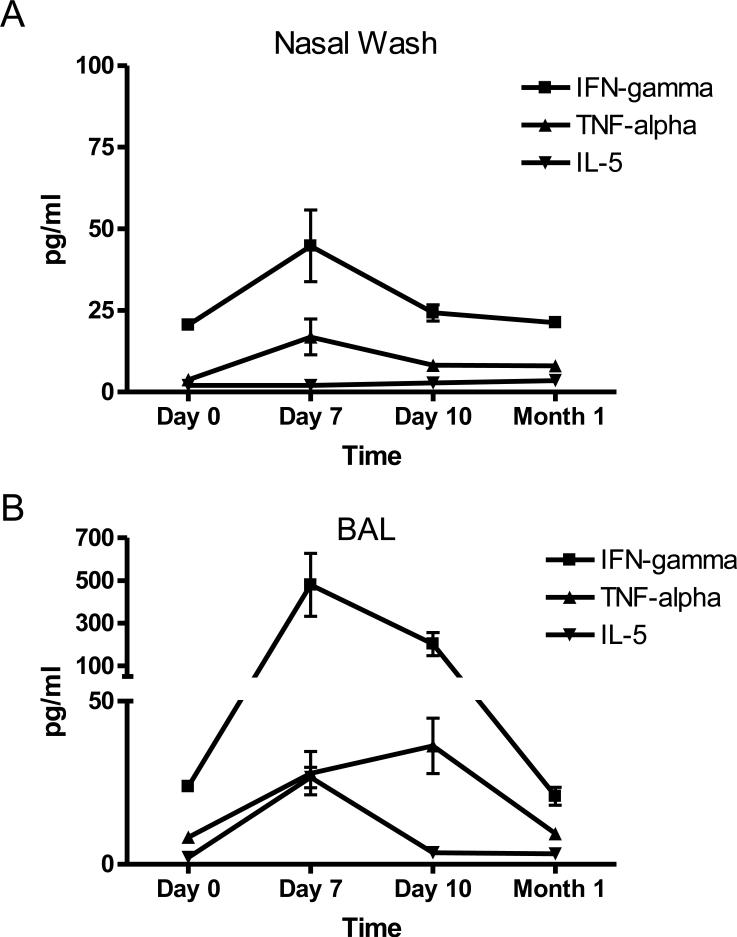
We also measured the concentrations of free cytokines in the nasal wash and BAL. Low levels of IFN-γ and TNF-α were measured in the nasal wash as compared to the BAL (Figure 6), consistent with data from the intracellular cytokine assays (Figure 5). There was also weak IL-5 activity in the BAL while levels of IL-2, IL-4, IL-10, IL-13 and IL17a were below detection.
6. Free cytokine in the respiratory tract of animals exposed to SeV.
Nasal wash and BAL samples were tested for the presence of free cytokines over the course of a 1 month period. Results for cytokines IFN-γ, TNF-α and IL-5 are shown, as these were detected at significant levels.
DISCUSSION
Preclinical studies have previously demonstrated the protective efficacy of SeV against hPIV-1 in both cotton rats and African green monkeys. Research has also indicated the safety of SeV in clinical trials. To better understand the immune correlates of protection for this vaccine, we have employed a well-characterized murine system for evaluation of the AFCs and T cell activities in the d-NALT. Data show that after a single i.n. inoculation of C57BL/6 mice with SeV, the d-NALT is quickly populated with virus-specific AFCs and CD8+NP324-332/Kb-specific T cells. Both of these lymphocyte populations appear by day 7 and persist for the lifetime of the animals, and both are found at greater percentages in the d-NALT than in the lungs and BAL at 8 months post-vaccination. These d-NALT lymphocytes are well positioned to inhibit virus at its point-of-entry and may therefore be the most influential gatekeepers for long-term virus control.
Our study of d-NALT lymphocytes responsive to a respiratory pathogen revealed a striking similarity between immune responses of the d-NALT and gut. Indeed, a common mucosal immune system has been previously described which predicts some of these similarities. An obvious similarity is the relatively high numbers of IgA-producing AFCs in the d-NALT and significant IgA titers in nasal washes. We note that in the d-NALT, IgGs of multiple sub-isotypes were also produced inclusive of IgG1, IgG2a and IgG2b. While IgA is best suited for deposition at mucosal surfaces via poly-Ig assisted transcytosis, IgGs may also facilitate viral clearance by mediating a spectrum of functions including complement fixation, neutralization, and ADCC , all of which may reduce the spread of virus and virus-infected cells.
Our analysis of the CD8+ T cell population revealed that virus-specific T cells expressed a predominantly CD62LLoCD103Lo and CD44Hi phenotype on day 10 and transitioned to a predominantly CD62LLoCD103HiCD44Int phenotype after 1 month. The shift in phenotype revealed an additional similarity between d-NALT and gut-associated immune activities. As an example, a study by Kim et. al. examined intestinal intraepithelial lymphocytes (IELs) following the activation of adoptively transferred transgenic Ova-specific CD8+ T cells with Vesicular stomatitis virus (VSV)-Ova. Four days post- challenge, the CD8+ T cells that had trafficked to the intestinal epithelium were predominantly CD62LLoCD103Lo and CD44Hi. By day 38, cells maintained the CD62LLo phenotype, but had shifted to CD103HiCD44Int expression. The relevance of CD103 to mucosal immunity relates to its binding to the epithelial cell-specific marker e-cathedrin. In CD103 KO mice there is a deficiency in mucosal T cell numbers in the intestinal epithelium, yet the requirement for CD103 as a mediator of T cell migration and retention in the intestine is not absolute. Takamura et al. have further shown that when SeV-specific memory cells are adoptively transferred into a naïve mouse and re-challenged, they traffic to the lower respiratory tract and upregulate CD103. Notwithstanding, the maintenance of the CD103Hi phenotype is more striking in the d-NALT and intestinal IEL than in the lungs or BAL. The CD44Int phenotype is also more pronounced in the d-NALT and intestinal IEL than in the LRT , possibly defining a cell with an attenuated contraction phase but poised for immediate recall upon virus challenge.
Another feature shared between d-NALT and intestine was the CD69Hi phenotype. This marker is often upregulated upon acute activation, but then subsides. In our study, virtually all d-NALT cells were CD69Hi at the 1 and 3 month time points. Masopust et. al. have found that intestinal IELs are also close to 100% CD69Hi three months following a VSV infection. This marker is more pronounced in intestinal IEL than in lung-resident VSV-specific cells or in populations of NP324-332/Kb-specific T cells in lungs and BAL 1 month after an SeV inoculation (the latter are CD69Hi at the 70% level). Possibly, the maintenance of the CD69Hi phenotype among virtually all d-NALT and intestinal IEL CD8+ T cells reflects long-term antigen deposition in affected tissues, which in turn assists the maintenance of virus-specific lymphocytes in the mucosa.
Questions concerning antigen deposition and lymphocyte persistence have been a topic of much debate. Some experiments suggest that long-term antigen deposition is instrumental in lymphocyte persistence, while other experiments reveal that lymphocyte persistence can be maintained even when receptor-antigen interactions are blocked. Experiments are warranted to further dissect mechanisms responsible for the impressive durability of SeV-induced lymphocyte responses. A future analysis of immunodominant CD4+ T cell populations is also warranted to determine if/how d-NALT B and CD8+ T cells are affected by CD4+ T cell activities. Results may better explain how SeV-based vaccines, like some other live viral vaccines, can confer B cell function, T cell function and protective immunity for the lifetime of the animal.
The CD11aHi T cell phenotype was one additional characteristic shared by the majority of d-NALT and intestinal IELs. CD11a is both an adhesion and activation marker that is involved in the extravasation of cells from the circulation. The blockade of CD11a can impair T-cell adhesion to endothelial cells and retention of cells in the lungs, as demonstrated by inhibition studies with CD11a-specific antibodies. In the LRT, CD11a expression is mixed; one month following SeV inoculation, cells in the BAL and lungs are 30-40% and 90-100% CD11aHi, respectively. Cells which have down-regulated CD11a are not terminally differentiated, as upon transfer to naïve mice and re-challenge, they return to the airways, and regain CD11aHi expression levels.
A final comparison revealed similarities between T cell functions of the d-NALT and intestinal lymphocytes in terms of cytotoxicity and IFN-γ cytokine expression (albeit IFN-γ was expressed at low levels among d-NALT T cells ). In each case, cell numbers and their associated functional capacities dropped with time. Masopust et. al. and Kim et. al. have suggested that the overall phenotype/function of the intestinal memory T cell depicts a cell that: (i) remains in the mucosa for long periods, (ii) modifies its phenotype while tissue-resident, and (iii) may not be part of the recirculating CD8+ T memory pool. Despite the gradual drop in numbers and function with time , intestinal T cells are capable of rapid recall.
In conclusion, data from AFC and CD8+ T cell analyses identify the d-NALT as an important site for immune surveillance following a single inoculation with SeV. Unique AFC and T cell features typify immune responses of the intestinal mucosa. Possibly, these features assist the long-term-maintenance of d-NALT lymphocytes in a position opportune for tackling respiratory virus at its point-of-entry.
ACKNOWLEDGEMENTS
This work was supported in part by NIH NIAID grants P01 AI054955 and R01 AI088729, NIH NCI grant P30-CA21765 and the American-Lebanese Syrian Associated Charities (ALSAC). We thank Dr. Mark Sangster for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol.Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J.Immunol.Methods. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- Cauley LS, Cookenham T, Miller TB, Adams PS, Vignali KM, Vignali DA, Woodland DL. Cutting edge: virus-specific CD4+ memory T cells in nonlymphoid tissues express a highly activated phenotype. J.Immunol. 2002;169:6655–6658. doi: 10.4049/jimmunol.169.12.6655. [DOI] [PubMed] [Google Scholar]
- Cole GA, Cole GA, Clements VK, Garcia EP, Ostrand-Rosenberg S. Allogeneic H-2 antigen expression is insufficient for tumor rejection. Proc.Natl.Acad.Sci.U.S.A. 1987;84:8613–8617. doi: 10.1073/pnas.84.23.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GA, Hogg TL, Coppola MA, Woodland DL. Efficient priming of CD8+ memory T cells specific for a subdominant epitope following Sendai virus infection. J.Immunol. 1997;158:4301–4309. [PubMed] [Google Scholar]
- Cole GA, Hogg TL, Woodland DL. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. International Immunology. 1994;6:1767–1775. doi: 10.1093/intimm/6.11.1767. [DOI] [PubMed] [Google Scholar]
- Cole GA, Katz JM, Hogg TL, Ryan KW, Portner A, Woodland DL. Analysis of the primary T-cell response to Sendai virus infection in C57BL/6 mice: CD4+ T-cell recognition is directed predominantly to the hemagglutinin-neuraminidase glycoprotein. Journal of Virology. 1994;68:6863–6870. doi: 10.1128/jvi.68.11.6863-6870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Dave VP, Allan JE, Slobod KS, Smith SF, Ryan K, Powell U, Portner A, Hurwitz JL. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology. 1994;199:376–383. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- Davis SS. Nasal vaccines. Adv.Drug Deliv.Rev. 2001;51:21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J.Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J.Immunol. 2003;171:3338–3342. doi: 10.4049/jimmunol.171.7.3338. [DOI] [PubMed] [Google Scholar]
- Faisca P, Desmecht D. Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res Vet.Sci. 2007;82:115–125. doi: 10.1016/j.rvsc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr.Mol.Med. 2009;9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman WL, Gill DS, Scroggs RA, Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology. 1990;175:211–223. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- Gray D. Immunological memory: a function of antigen persistence. Trends Microbiol. 1993;1:39–41. doi: 10.1016/0966-842x(93)90026-n. [DOI] [PubMed] [Google Scholar]
- Gray D. A role for antigen in the maintenance of immunological memory. Nat.Rev.Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr.Infect.DIs.J. 2004;23:S11–S18. doi: 10.1097/01.inf.0000108188.37237.48. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J.Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Hurwitz JL, Soike KF, Sangster MY, Portner A, Sealy RE, Dawson DH, Coleclough C. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, Portner A, Hurwitz JL. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009;27:1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J.Exp.Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast WM, Roux L, Curren J, Blom HJ, Voordouw AC, Meloen RH, Kolakofsky D, Melief CJ. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc.Natl.Acad.Sci.U.S.A. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi H, Saltzman LE, Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J.Exp.Med. 1983;157:433. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J.Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J.Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: Antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J.Immunol. 2007;178:4721–4725. doi: 10.4049/jimmunol.178.8.4721. [DOI] [PubMed] [Google Scholar]
- Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J.Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Parker CM, Olson S, Muller W, Wagner N, Schon MP, Puddington L. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J.Exp.Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Hyland L, Hou S. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. Journal of Virology. 2001;75:5416–5420. doi: 10.1128/JVI.75.11.5416-5420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyn D, Gill DS, Scroggs RA, Portner A. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. Journal of General Virology. 1991;72:983–987. doi: 10.1099/0022-1317-72-4-983. [DOI] [PubMed] [Google Scholar]
- MacDonald TT. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr.Top.Microbiol.Immunol. 1999;236:113–135. doi: 10.1007/978-3-642-59951-4_7. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- Masopust D, Jiang J, Shen H, Lefrancois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J.Immunol. 2001;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J.Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- McCarthy AJ, Goodman SJ. Reassessing conflicting evolutionary histories of the Paramyxoviridae and the origins of respiroviruses with Bayesian multigene phylogenies. Infect.Genet.Evol. 2010;10:97–107. doi: 10.1016/j.meegid.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, Von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mora JR, Von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal.Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J.Exp.Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M. Janeway's Immunobiology. Garland Science; New York, NY: 2008. [Google Scholar]
- Ostrand-Rosenberg S, Roby C, Clements VK, Cole GA. Tumor-specific immunity can be enhanced by transfection of tumor cells with syngeneic MHC-class-II genes or allogeneic MHC-class-I genes. Int.J.Cancer Suppl. 1991;6:61–68. doi: 10.1002/ijc.2910470714. [DOI] [PubMed] [Google Scholar]
- Power UF, Ryan KW, Portner A. Sequence characterization and expression of the matrix protein gene of human parainfluenza virus type 1. Virology. 1992;191:947–952. doi: 10.1016/0042-6822(92)90270-y. [DOI] [PubMed] [Google Scholar]
- Sato M, Wright PF. Current status of vaccines for parainfluenza virus infections. Pediatr.Infect.DIs.J. 2008;27:S123–S125. doi: 10.1097/INF.0b013e318168b76f. [DOI] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J.Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Singleton R, Etchart N, Hou S, Hyland L. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. Journal of Virology. 2003;77:11303–11311. doi: 10.1128/JVI.77.21.11303-11311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Ahmed R. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 1996;4:394–400. doi: 10.1016/0966-842X(96)10059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, Portner A, Coleclough C, Hurwitz JL. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22:3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Smith FS, Portner A, Leggiadro RJ, Turner EV, Hurwitz JL. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology. 1994;205:453–461. doi: 10.1006/viro.1994.1665. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Nochi T, Yuki Y, Kiyono H. New horizon of mucosal immunity and vaccines. Curr.Opin.Immunol. 2009;21:352–358. doi: 10.1016/j.coi.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J.Exp.Med. 2010;207:1153–1160. doi: 10.1084/jem.20090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T, Hurwitz JL, Coleclough C, Prouser C, Krishnamurthy S, Zhan X, Boyd K, Scroggs RA, Brown B, Nagai Y, Portner A, Slobod KS. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78:6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T, Hurwitz JL, Zhan X, Krishnamurthy S, Prouser C, Brown B, Coleclough C, Boyd K, Scroggs RA, Portner A, Slobod KS. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunology. 2005;18:255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood. 2003;101:4916–4922. doi: 10.1182/blood-2002-10-3159. [DOI] [PubMed] [Google Scholar]
- Vainionpaa R, Hyypia T. Biology of parainfluenza viruses. Clin.Microbiol.Rev. 1994;7:265–275. doi: 10.1128/cmr.7.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J.Immunol. 2001;167:3293–3299. doi: 10.4049/jimmunol.167.6.3293. [DOI] [PubMed] [Google Scholar]
- Woodland DL, Happ MP, Bill J, Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive cells. Science. 1990;247:964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, Surman S, Portner A, Slobod KS. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Slobod KS, Krishnamurthy S, Luque LE, Takimoto T, Jones B, Surman S, Russell CJ, Portner A, Hurwitz JL. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 2008;26:3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Marshall D, Coleclough C, Woodland DL. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J.Immunol. 2000;164:3274–3282. doi: 10.4049/jimmunol.164.6.3274. [DOI] [PubMed] [Google Scholar]