Abstract
Objective
Liver X Receptor (LXR) activators decrease atherosclerosis in mice. LXR activators (1) directly up-regulate genes involved in reverse cholesterol transport (RCT) and (2) exert anti-inflammatory effects mediated by transrepression of NFκB target genes. We investigated whether myeloid cell deficiency of ATP-binding cassette transporters A1 and G1 (ABCA1/G1), principal targets of LXR that promote macrophage cholesterol efflux and initiate RCT, would abolish the beneficial effects of LXR activation on atherosclerosis.
Approach and Results
LXR activator T0901317 (T0) substantially reduced inflammatory gene expression in macrophages lacking ABCA1/G1. Ldlr−/− mice were transplanted with Abca1−/−Abcg1−/− or wild-type bone marrow (BM) and fed a Western-type diet (WTD) for 6 weeks with or without T0 supplementation. Abca1/g1 BM deficiency increased atherosclerotic lesion complexity and inflammatory cell infiltration into the adventitia and myocardium. T0 markedly decreased lesion area, complexity and inflammatory cell infiltration in the Abca1−/−Abcg1−/− BM transplanted mice. To investigate whether this was due to macrophage Abca1/g1 deficiency, Ldlr−/− mice were transplanted with LysmCreAbca1fl/flAbcg1fl/fl or Abca1fl/flAbcg1fl/fl BM and fed WTD with or without the more specific LXR agonist GW3965 for 12 weeks. GW3965 decreased lesion size in both groups and the decrease was more prominent in the LysmCreAbca1fl/flAbcg1fl/fl group.
Conclusions
The results suggest that anti-inflammatory effects of LXR activators are of key importance to their anti-atherosclerotic effects in vivo independent of cholesterol efflux pathways mediated by macrophage ABCA1/G1. This has implications for the development of LXR activators that lack adverse effects on lipogenic genes while maintaining the ability to trans-repress inflammatory genes.
Keywords: Liver X Receptor, Atherosclerosis, ABC Transporters, Transrepression, Reverse Cholesterol Transport
INTRODUCTION
Liver X receptors (LXR) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily.1 LXR-α is expressed in the liver, adipose, intestine, kidney, and macrophages, while expression of LXR-β is nearly ubiquitous.2 LXRs are activated by oxysterols formed in response to increased intracellular cholesterol levels.3 Synthetic compounds such as T0901317 (T0) and GW3965 (GW) have also been established as active oral LXR agonists.4, 5 Further investigation into the role of LXR in cholesterol homeostasis, led to the discovery that synthetic LXR activators decrease atherosclerosis in multiple mouse models, including Apoe−/− and Ldlr−/− mice.6–8 Two principal properties of LXR activators that may contribute to their anti-atherogenic effects are (1) direct upregulation of genes involved in macrophage cholesterol efflux and reverse cholesterol transport (RCT) such as the ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1)9 and (2) anti-inflammatory effects mediated by a distinct molecular mechanism involving SUMOylation of LXR and transrepression of NFkB target genes.10 While (1) is known to be anti-atherogenic, the role of (2) in mediating anti-atherogenic effects of LXR activators has not been explored. We therefore investigated whether myeloid or macrophage deficiency of ABCA1 and ABCG1, two principal targets of LXR as well as key components of the cholesterol efflux pathway, would abolish the beneficial effects of LXR activation on atherosclerosis.
MATERIALS AND METHODS
GW3965 was kindly provided by Dr. Jon Collins, Glaxo Smith Kline. Materials and methods are available in the online-only Data Supplement.
RESULTS
LXR activation decreases inflammatory gene expression in LPS stimulated macrophages lacking ABCA1 and ABCG1 in vitro
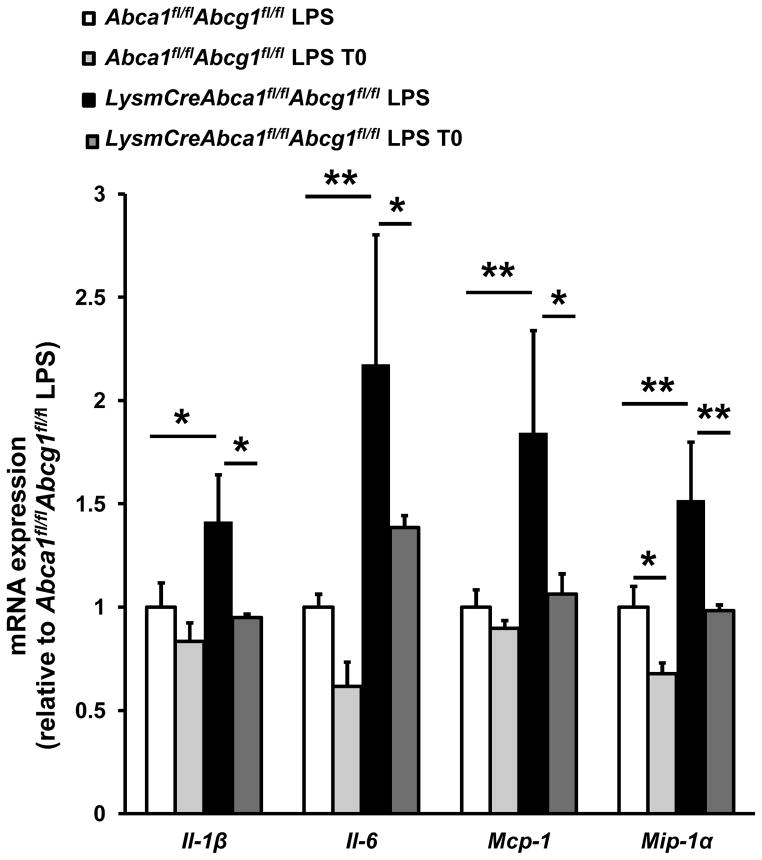
We have shown that the ability of LXR activator to increase cholesterol efflux to HDL or apoA-1 is abolished in macrophages lacking ABCA1 and ABCG1.11, 12 To determine if LXR activator treatment retained its anti-inflammatory effects, we treated LysmCreAbca1fl/flAbcg1fl/fl and Abca1fl/flAbcg1fl/fl control macrophages with T0, and then stimulated them with lipopolysaccharide (LPS). We subsequently examined expression of the inflammatory genes Il-1β, Il-6, Mcp-1, and Mip-1α, using qPCR. As reported, LPS-induced inflammatory gene expression was increased in LysmCreAbca1fl/flAbcg1fl/fl macrophages.11 T0 treatment led to significantly decreased inflammatory gene expression (Il-1β, 33%, Il-6, 36%, Mcp-1, 42%, and Mip-1α, 35%, (all P<0.05)) in LysmCreAbca1fl/flAbcg1fl/fl macrophages indicating that its anti-inflammatory effects occur independent of ABCA1/G1 expression (Figure 1). Interestingly, LXR activators showed similar effects in Abca1fl/flAbcg1fl/fl macrophages (Figure 1), however, the effect was less pronounced and statistical significance was only reached for Mip-1α (P<0.05). This is consistent with the known anti-inflammatory effects of LXR activators, which have been demonstrated in vitro and in vivo.10, 13, 14 The more pronounced anti-inflammatory effects in macrophages lacking ABCA1/G1 may reflect their higher baseline inflammatory gene expression.
Figure 1. LXR activation decreases inflammatory mRNA expression in macrophages deficient in Abca1 and Abcg1.
Bone marrow macrophages from LysmCreAbca1fl/flAbcg1fl/fl and Abca1fl/flAbcg1fl/fl mice were incubated with or without T0 for 18 h. Cells were then stimulated with or without LPS (50 ng/ml) for 4 h, lysed, and mRNA expression for the indicated genes was measured and corrected for the housekeeping gene m36B4. n=3–4 per group. *P< 0.05, **P< 0.01, ***P<0.001.
LXR activation decreases atherosclerosis in Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− bone marrow
Ldlr−/− mice were transplanted with either wild-type or Abca1−/−Abcg1−/− bone marrow (BM) and fed a WTD for 6 weeks supplemented with or without the LXR activator T0 (10mg/kg). Reconstitution of the BM was >90% (results not shown). Cholesterol and triglyceride (TG) levels were measured after 3 weeks of WTD feeding with or without T0 (Supplemental Figure I). BM Abca1/g1 deficiency decreased plasma cholesterol and TG levels by 64% and 31%, respectively (both P<0.001), which was mainly confined to the VLDL/LDL fraction for cholesterol (Supplemental Figure II). The decrease in plasma lipids has been previously observed in WTD-fed Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− BM15 and may partly reflect increased uptake of LDL by a non-Ldlr-dependent mechanism in the expanded myeloid cell compartment of these mice as has been shown in humans with myeloproliferative diseases.16 We also found an ~82% decrease in mRNA expression of sterol regulatory element binding protein 1c (Srebp-1c) in the liver of Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− bone marrow compared to their controls (P<0.001) (Supplemental Figure IIIA). This could perhaps reflect effects of decreased adipose tissue and insulin sensitization in myeloid ABCA1/G1 deficiency, 17 presumably decreasing plasma insulin levels and hepatic Akt signaling. In wild-type BM transplanted mice, chronic treatment with T0 caused a decrease in triglyceride and cholesterol levels in VLDL/LDL, as reported;7 however, in Abca1−/−Abcg1−/− BM transplanted mice, T0 treatment caused a slight increase in triglyceride and cholesterol levels in VLDL/LDL (Supplemental Figure I and II). In both groups of mice, T0 increased liver TG accumulation by 2.5-fold (P<0.001) (Supplemental Figure IIIB).
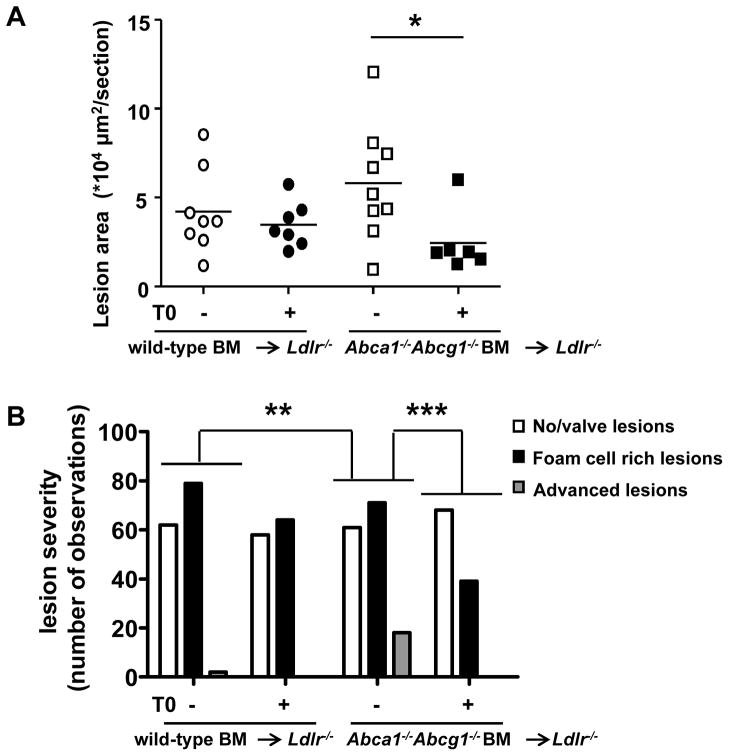
After 4 weeks of diet and after two consecutive IP injections of T0 or vehicle, blood samples were taken to analyze leukocyte subsets. Monocytes and neutrophils were assessed as a percentage of total leukocytes (Supplemental Figure IV). Abca1−/−Abcg1−/− BM transplanted mice showed a non-significant ~31% increase in monocytes and a ~147% increase in neutrophils (P<0.001) as compared to wild-type BM transplanted mice. While the LXR activator T0 had little effect in wild-type BM transplanted mice, it increased monocytes by ~38% in Abca1−/−Abcg1−/− BM transplanted mice (P<0.05), mainly due to an increase in the Ly6-Chi subpopulation (P<0.01) (Supplemental Figure IV). The increase in blood lipids and monocytes resulting from T0 treatment would not be expected to have a beneficial effect on atherosclerosis. After 6 weeks of diet, all mice were sacrificed and the aortic root was collected to analyze atherosclerotic lesions. Similar to a previous report in this model15 atherosclerotic lesion area was slightly but not significantly increased in Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− BM compared to wild-type (Figure 2A and Supplemental Figure V). The nonsignificant increase in lesion area was likely due to the marked lowering of plasma lipids in the Abca1−/−Abcg1−/− group; the lowering of plasma lipids does not occur in the Ldlr+/− background.12, 18 In the control group, the LXR activator did not affect lesion area (Figure 2A and Supplemental Figure V). This may be attributed to the short time course of WTD feeding and to the fact that these were early foam cell lesions. The impact of T0 treatment was much greater in the Abca1−/−Abcg1−/− bone marrow transplanted group, which showed a 58% decrease in atherosclerotic lesion area in response to the LXR activator T0 (P<0.05) (Figure 2A).
Figure 2. LXR activation decreases atherosclerosis in Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− BM.
Ldlr−/− mice were transplanted with wild-type or Abca1−/−Abcg1−/− BM and fed WTD with or without T0 supplementation for 6 weeks. A. Atherosclerotic lesion area in the aortic root. Each datapoint represents a single mouse. B. Lesion severity expressed as number of observations. Differences of distribution of lesions between groups are indicated. n=6–9 mice per group. *P< 0.05, **P<0.01, ***P<0.001.
We then analyzed the sections for lesion severity and distinguished 3 lesion types: sections containing no lesions or valve lesions, sections containing foam cell rich lesions, and sections containing advanced, complex lesions characterized by compromised integrity of the media, necrotic core formation, and cholesterol clefts (Figure 2B). A comparison of lesion severity between Abca1−/−Abcg1−/− and wild-type BM transplanted Ldlr−/− mice revealed more advanced lesions in Abca1−/−Abcg1−/− BM transplanted Ldlr−/− mice (P<0.01 for the distribution of lesion types, expressed as number of observations) (Figure 2B). T0 treatment did not affect the lesion severity in wild-type bone marrow transplanted Ldlr−/− mice (Figure 2B). However, T0 treatment in Abca1−/−Abcg1−/− BM transplanted Ldlr−/− mice caused a shift from advanced lesions (in mice treated with vehicle) to sections containing no lesions or valve lesions (in mice treated with T0), and a decrease in foam cell rich lesions (P<0.001 for the distribution of lesion types, expressed as number of observations) (Figure 2B). Overall, Abca1−/−Abcg1−/− BM transplanted Ldlr−/− mice showed the most advanced lesions, and T0 treatment prevented the development of advanced lesions in Abca1−/−Abcg1−/− BM transplanted Ldlr−/− mice (Figure 2B).
LXR activation decreases inflammatory cell infiltration in Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− BM
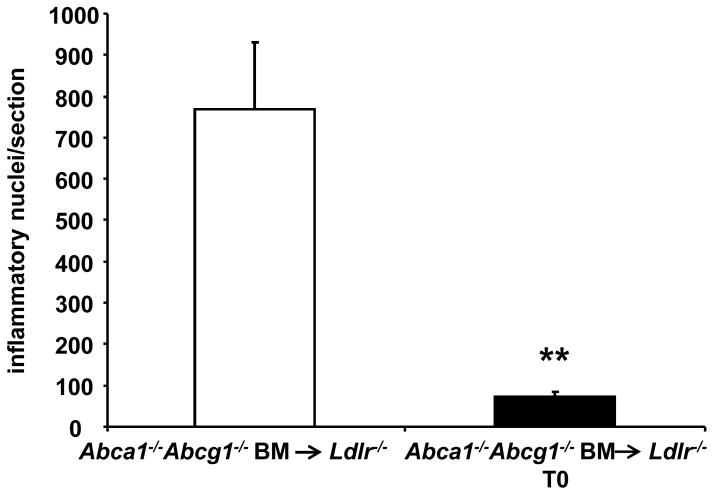
As previously reported,12 H&E staining of the aortic sinus revealed that Abca1−/−Abcg1−/− BM transplanted mice had increased inflammatory cell infiltration in the adventitia and surrounding myocardium, and this was not found in wild-type bone marrow transplanted mice. Staining of aortic root sections with Mac3 confirmed that these cells were indeed inflammatory cells (macrophages). LXR activator treatment virtually abolished the appearance of inflammatory cells in the adventitia and surrounding myocardium (90% decrease; P<0.01) (Figure 3 and Supplemental Figure VI). These results suggest that LXR activation decreases inflammation in vivo in the setting of hematopoietic Abca1/g1 deficiency.
Figure 3. LXR activation decreases inflammatory cell infiltration in the myocardium and adventitia of Ldlr−/− mice transplanted with Abca1−/−Abcg1−/− BM.
Inflammatory nuclei in the adventitia of H&E stained sections were quantified. n=6–9 mice per group; **P< 0.01.
LXR activation decreases atherosclerosis in Ldlr−/− mice transplanted with LysmCreAbca1fl/flAbcg1fl/fl BM
We next investigated whether LXR activation would decrease atherosclerosis in a longer term study in Ldlr−/− mice with macrophage Abca1/g1 deficiency. We used the GW3965 (GW) compound to activate LXR, which has been reported to induce less hepatic steatosis that the T0 compound,19 and does not exhibit the off target effects of T0 such as activation of the farnesoid X receptor,20 the pregnane X receptor,19 and the retinoic acid receptor-related orphan receptor.21 Eight weeks old Ldlr−/− mice were transplanted with LysmCreAbca1fl/flAbcg1fl/fl or Abca1fl/flAbcg1fl/fl BM. At 5 weeks after transplantation, BM reconstitution was >90% (results not shown), and the mice were fed WTD with or without GW supplementation (10 mg/kg) for a period of 12 weeks. Similar to our previous observations,11 macrophage Abca1/g1 deficiency decreased plasma cholesterol levels by ~48% in WTD-fed Ldlr−/− mice (P<0.001) and there was a tendency to decreased TG levels (Supplemental Figure VII). There was ~47% decreased Srebp-1c mRNA in the liver from LysmCreAbca1fl/flAbcg1fl/fl bone marrow transplanted WTD-fed Ldlr−/− mice (P<0.01), concomitant with decreased liver TG levels (57%; P<0.01) (Supplemental Figure VIII). GW increased liver TG levels in both groups of mice (P<0.001) (Supplemental Figure VIIIB), albeit to a lower extent than T0 (Supplemental Figure III). As we reported previously,11 LysmCreAbca1fl/flAbcg1fl/fl BM transplanted WTD-fed Ldlr−/− mice showed increased monocyte and neutrophil levels. These increases were not affected by GW treatment (Supplemental Figure IX).
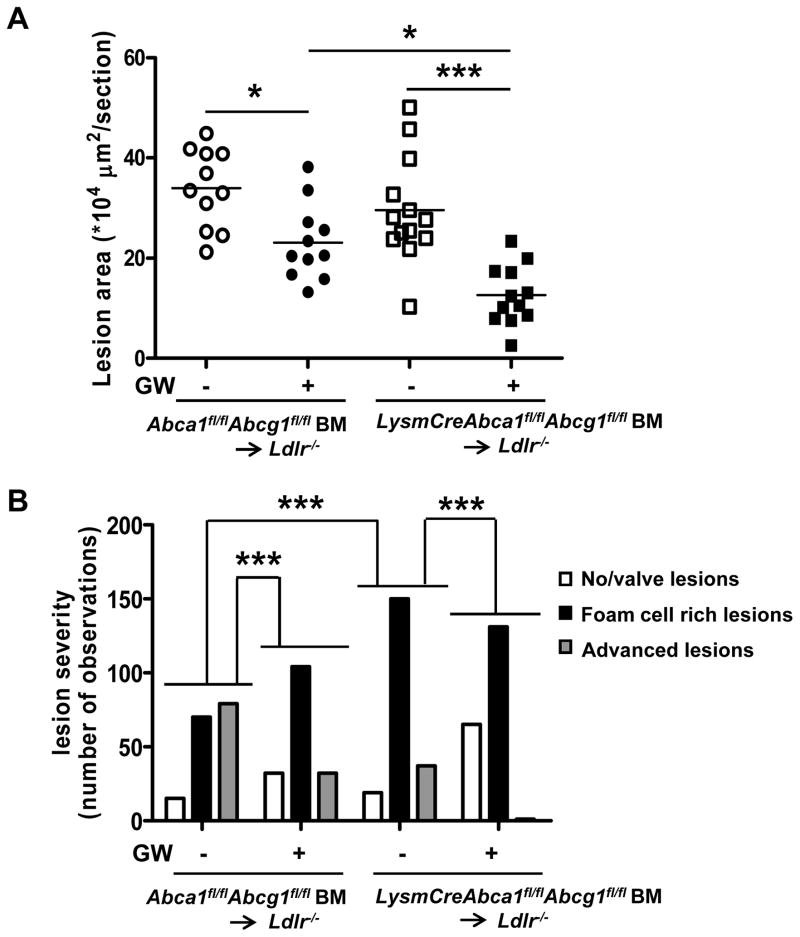
After 12 weeks of WTD, mice were sacrificed and atherosclerosis was assessed in the aortic root. Macrophage Abca1/g1 deficiency did not affect atherosclerotic lesion size (Figure 4A and Supplemental Figure X), likely because of the ~50% decreased cholesterol levels, as we observed previously in this model.11 Under these conditions, the control group developed more advanced lesions than the LysmCreAbca1fl/flAbcg1fl/fl group (Figure 4B). GW treatment significantly decreased lesion size and lesion severity in both groups of mice (Figure 4B). Again, the reduction in lesion size appeared to be greater in the LysmCreAbca1fl/flAbcg1fl/fl bone marrow transplanted Ldlr−/− mice (Figure 4A). Macrophage Abca1/g1 deficiency increased plasma MCP-1 levels (3.7-fold; P<0.001) (Figure 4C). GW treatment decreased plasma MCP-1 in mice lacking Abca1/g1 in macrophages (Figure 4C), further substantiating the observation that the anti-atherogenic effect of LXR activation may reflect its anti-inflammatory effects.
Figure 4. LXR activation decreases atherosclerosis in Ldlr−/− mice transplanted with BM deficient in macrophage Abca1 and Abcg1.
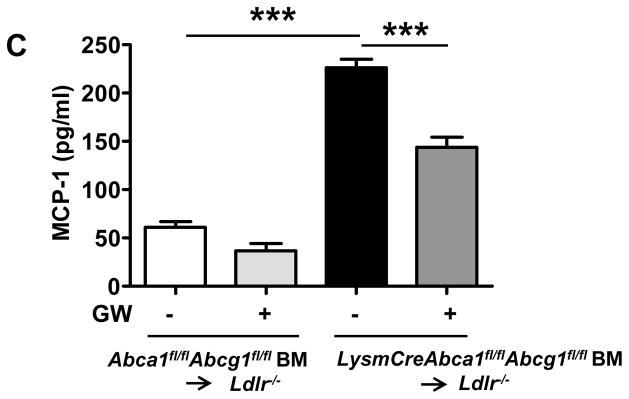
Ldlr−/− mice were transplanted with Abca1fl/flAbcg1fl/fl or LysmCreAbca1fl/flAbcg1fl/fl BM and fed WTD with or without GW supplementation for 12 weeks. A. Atherosclerotic lesion area in the aortic root. Each datapoint represents a single mouse. B. Lesion severity expressed as number of observations. Differences of distribution of lesions between groups are indicated. C. MCP-1 plasma levels. n=11–13 mice per group. *P< 0.05, **P<0.01, ***P<0.001.
DISCUSSION
It is well known that LXR activation causes increased expression of genes involved in cholesterol efflux and RCT such as ABCA1 and ABCG1 in macrophages.9 A therapeutic agent with the ability to increase cholesterol efflux would have obvious positive implications for the treatment of atherosclerosis because it would decrease the number of lipid-laden macrophages or foam cells embedding themselves into the artery wall. Indeed LXR activators have been shown to decrease atherosclerosis in multiple mouse models and this has been assumed to be mainly due to its effects on cholesterol efflux and reverse cholesterol transport.6–8 LXR also mediates a distinct mechanism of transrepression of the expression of inflammatory genes.10 When LXR becomes bound by a ligand, it can then be conjugated to a SUMO protein. This process targets the SUMOylated LXR to the promoters of NFkB target genes where they prevent the signal-dependent removal of co-repressor complexes required for transcriptional activation.10 Thus inflammatory genes downstream of NFkB are maintained in a repressed state.10 Previous studies have shown beneficial effects of LXR activator treatment in skin inflammation models,22 rheumatoid arthritis models,23 and hepatic injury caused by endotoxemia.24 However the role of inflammatory gene suppression in atherosclerosis has not been addressed.
ABCA1 and ABCG1 are important targets of LXR activation as well as key players in macrophage cholesterol efflux and initiation of RCT.9 We showed that the ability of LXR activator to increase cholesterol efflux to apoA1 and HDL was abolished in Abca1/g1 deficient macrophages,11 but that anti-inflammatory effects were actually increased in these cells (Figure 1). In the absence of ABCA1 and ABCG1, LXR activation by T0 exerted potent anti-atherosclerotic effects, reducing early lesion area, complexity and inflammation (Figure 2). In a longer term study, LXR activation by GW decreased lesion size and pro-atherogenic plasma MCP-1 levels in Ldlr−/− mice with macrophage Abca1/g1 deficiency (Figure 4). Our findings thus suggest that the anti-atherosclerotic effects of LXR activation in macrophage and bone marrow Abca1/g1 deficiency were due to anti-inflammatory effects of LXR presumably mediated by the well estasblished transrepression mechanism.10,14 This interpretation was supported by the marked decrease in inflammatory nuclei in the adventitia, and myocardium of Abca1−/−Abcg1−/− BM transplanted mice treated with T0 for 6 weeks (Figure 3), and by the decreased MCP-1 levels in macrophage Abca1/g1 deficient mice treated with GW for 12 weeks (Figure 4C). It is notable that effects of LXR activator treatment on atherosclerosis were more pronounced in Abca1−/−Abcg1−/− BM transplanted and macrophage Abca1/g1 deficient mice than in controls. This likely reflects the fact that macrophage inflammatory gene expression, lesional inflammatory features and lesion complexity were increased in mice transplanted with Abca1−/−Abcg1−/− BM, and suggests that the effects of LXR activators on lesion inflammation and complexity may be at least as important as their effects on macrophage foam cell formation. Whereas we also observed increased inflammation in macrophage Abca1/g1 deficiency compared to controls (Figure 4C), the atherosclerotic lesion complexity was not more advanced than in controls (Figure 4B), likely reflecting the difference in plasma cholesterol levels between these two groups.
A number of other potential explanations for our findings need to be considered. LXR activation has been shown to increase expression of AIM (apoptosis inhibitor expressed by macrophages; also called Spα or Api6),25, 26 which supports survival of macrophages in response to apoptosis-inducing stimuli,27 such as oxidized lipids.28 As a consequence AIM−/−Ldlr−/− mice show decreased atherogenesis.28 Since LXR treatment reduced atherogenesis in bone marrow and macrophage Abca1/g1 deficiency in our study, it is unlikely that this effect was mediated by AIM. LXR has multiple gene targets in addition to ABCA1 and ABCG1 that may affect cholesterol homeostasis, including IDOL (inducible degrader of the Ldlr), a molecule that acts to suppress LDL uptake.29 In our experiments bone marrow donor cells were Ldlr+/+ and thus induction of IDOL could act to suppress Ldlr in BM cells. However, Herijgers et al., have demonstrated that there was no difference in mean atherosclerotic lesion area in Ldlr−/− or Ldlr+/+ bone marrow transplanted Ldlr−/− mice when fed a high cholesterol diet,30 likely due to down-regulation of Ldlr by the diet, and thus this scenario is also unlikely. LXR activators have been shown to exert modest anti-atherogenic effects independent of LXR expression in bone marrow-derived cells.31 This could reflect beneficial effects on expression of ABCA1 and ABCG1 in vascular endothelial cells.32 LXR activators also induce hepatic ABCG5/833 and appear to stimulate RCT at the level of the small intestine34 and such effects cannot be excluded in our study. However, studies in isolated macrophages11, 12 and of macrophage RCT in vivo35 indicate a major role of ABCA1 and ABCG1 in cholesterol efflux and initiation of RCT from macrophages. Thus, it seems improbable that stimulating cholesterol flux across the liver or intestine would lead to increased cholesterol removal from macrophage foam cells, in the absence of up-regulating the initial step of macrophage reverse cholesterol transport.
In addition to its beneficial effects, LXR activators have also been shown to induce expression of lipogenic genes and cause hepatic steatosis.36 An additional potential concern is that LXR activators may induce hepatic IDOL expression leading to reduced levels of LDLrs and increased plasma LDL levels.29, 37 Since we have shown the anti-inflammatory effects of LXR are of key importance to its anti-atherosclerotic effects in vivo, independent of the ABCA1/G1-mediated cholesterol efflux pathway, it should be possible to develop selective LXR activators that mediate inflammatory transrepression without direct targeting of cholesterol efflux, IDOL and lipogenic genes. These could act as anti-atherogenic agents without inducing the adverse effect of fatty liver. Indeed compounds that have selective anti-inflammatory effects with minimal induction of lipogenic gene expression in cultured hepatocytes have been developed,38 but their role in treating atherosclerosis has not yet been assessed. Our studies suggest that LXR activators selective for their trans-repressive effects should be further developed and tested for effects on atherosclerosis, especially in models of advanced, complex disease.
Supplementary Material
SIGNIFICANCE.
Liver X receptor (LXR) agonists are athero-protective in mice. The athero-protective properties of LXR have been attributed to the upregulation of genes involved in reverse cholesterol transport (RCT) and to anti-inflammatory effects mediated by transrepression of NFκB target genes.
Nonetheless, the promise of LXR as a therapeutic target has been diminished by the findings that LXR also induces lipogenic gene expression in the liver, leading to hepatic steatosis. Our data show that in the absence of cholesterol transporters that initiate RCT in macrophages, LXR agonists reduce inflammation in vitro and in vivo and are still athero-protective in mice. These findings suggest that the anti-inflammatory effects of LXR are of key importance to their anti-atherogenic properties and have implications for the development of selective LXR activators that mediate inflammatory trans-repression without direct targeting of cholesterol efflux and lipogenic genes.
Acknowledgments
SOURCES OF FUNDING
Mojdeh S. Kappus was supported by the Sarnoff Cardiovascular Research Foundation. Marit Westerterp was supported by Postdoctoral Fellowship 09POST2110109 from the American Heart Association and by NWO – VENI grant 916.11.072 from The Netherlands Organization of Scientific Research. This project was supported by NIH grant HL107653 (to A.T.).
ABBREVIATIONS
- ABCA1/G1
ATP Binding Cassette Transporter A1/G1
- BM
Bone marrow
- GW
GW3965
- HDL
High density lipoprotein
- H&E
Haematoxylin and Eosin
- LDLr
Low density lipoprotein receptor
- LPS
Lipopolysaccharide
- LXR
Liver X Receptor
- Mcp-1
Monocyte chemoattractant protein 1
- Mip-1α
Macrophage inflammatory protein 1α
- RCT
Reverse cholesterol transport
- Srebp-1c
Sterol Regulatory Element Binding Protein-1c
- T0
T0901317
- TG
Triglyceride
- VLDL
Very low density lipoprotein
- WTD
Western-type diet
Footnotes
DISCLOSURES
A.R. Tall is a consultant to Merck, Roche, Amgen, Arisaph, and CSL. The other authors report no conflicts.
References
- 1.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. Lxr, a nuclear receptor that defines a distinct retinoid response pathway. Gene Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 2.Peet DJ, Janowski BA, Mangelsdorf DJ. The lxrs: A new class of oxysterol receptors. Curr Opin Gen Dev. 1998;8:571–575. doi: 10.1016/s0959-437x(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 3.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor lxr alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 4.Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, Plunket KD, Morgan DG, Beaudet EJ, Whitney KD, Kliewer SA, Willson TM. Identification of a nonsteroidal liver x receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 5.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of lxrs in control of lipogenesis. Gene Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph SB, Tontonoz P. Lxrs: New therapeutic targets in atherosclerosis? Curr Opin Pharmacol. 2003;3:192–197. doi: 10.1016/s1471-4892(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 7.Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver x receptor is required for antiatherogenic activity of lxr agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 8.Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T. T-0901317, a synthetic liver x receptor ligand, inhibits development of atherosclerosis in ldl receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 9.Zanotti I, Poti F, Pedrelli M, Favari E, Moleri E, Franceschini G, Calabresi L, Bernini F. The lxr agonist t0901317 promotes the reverse cholesterol transport from macrophages by increasing plasma efflux potential. J Lipid Res. 2008;49:954–960. doi: 10.1194/jlr.M700254-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel sumoylation-dependent pathways mediate gene- and signal-specific transrepression by lxrs and ppargamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of atp-binding cassette transporters a1 and g1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of abca1 and abcg1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver x receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 14.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage abca1 and abcg1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg H, Gilbert HS, Gibson JC, Le NA, Brown WV. Increased low-density-lipoprotein catabolism in myeloproliferative disorders. Ann Int Med. 1982;96:311–316. doi: 10.7326/0003-4819-96-3-311. [DOI] [PubMed] [Google Scholar]
- 17.Gautier EL, Westerterp M, Bhagwat N, Cremers S, Shih A, Abdel-Wahab O, Lutjohann D, Randolph GJ, Levine RL, Tall AR, Yvan-Charvet L. Hdl and glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J Exp Med. 2013;210:339–353. doi: 10.1084/jem.20121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitro N, Vargas L, Romeo R, Koder A, Saez E. T0901317 is a potent pxr ligand: Implications for the biology ascribed to lxr. FEBS Lett. 2007;581:1721–1726. doi: 10.1016/j.febslet.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Houck KA, Borchert KM, Hepler CD, Thomas JS, Bramlett KS, Michael LF, Burris TP. T0901317 is a dual lxr/fxr agonist. Mol Gen Metab. 2004;83:184–187. doi: 10.1016/j.ymgme.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. The benzenesulfoamide t0901317 [n-(2,2,2-trifluoroethyl)-n-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethy l]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver x receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: Liver-x-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 23.Asquith DL, Miller AM, Hueber AJ, McKinnon HJ, Sattar N, Graham GJ, McInnes IB. Liver x receptor agonism promotes articular inflammation in murine collagen-induced arthritis. Arthr Rheum. 2009;60:2655–2665. doi: 10.1002/art.24717. [DOI] [PubMed] [Google Scholar]
- 24.Wang YY, Dahle MK, Agren J, Myhre AE, Reinholt FP, Foster SJ, Collins JL, Thiemermann C, Aasen AO, Wang JE. Activation of the liver x receptor protects against hepatic injury in endotoxemia by suppressing kupffer cell activation. Shock. 2006;25:141–146. doi: 10.1097/01.shk.0000191377.78144.d9. [DOI] [PubMed] [Google Scholar]
- 25.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. Lxr-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver x receptors and retinoid x receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA. 2004;101:17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking aim, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999;189:413–422. doi: 10.1084/jem.189.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor aim/spalpha/api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Zelcer N, Hong C, Boyadjian R, Tontonoz P. Lxr regulates cholesterol uptake through idol-dependent ubiquitination of the ldl receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herijgers N, Van Eck M, Groot PH, Hoogerbrugge PM, Van Berkel TJ. Effect of bone marrow transplantation on lipoprotein metabolism and atherosclerosis in ldl receptor-knockout mice. Arterioscler Thromb Vasc Biol. 1997;17:1995–2003. doi: 10.1161/01.atv.17.10.1995. [DOI] [PubMed] [Google Scholar]
- 31.Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for lxralpha and lxrbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao H, Langmann T, Schmitz G, Zhu Y. Native ldl upregulation of atp-binding cassette transporter-1 in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:127–132. doi: 10.1161/hq1201.101772. [DOI] [PubMed] [Google Scholar]
- 33.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of atp-binding cassette sterol transporters abcg5 and abcg8 by the liver x receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 34.Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific lxr activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage abca1 and abcg1, but not sr-bi, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver x receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Reue K, Fong LG, Young SG, Tontonoz P. Feedback regulation of cholesterol uptake by the lxr-idol-ldlr axis. Arterioscler Thromb Vasc Biol. 2012;32:2541–2546. doi: 10.1161/ATVBAHA.112.250571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao EY, Caravella JA, Watson MA, et al. Structure-guided design of n-phenyl tertiary amines as transrepression-selective liver x receptor modulators with anti-inflammatory activity. J Med Chem. 2008;51:5758–5765. doi: 10.1021/jm800612u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.