Abstract
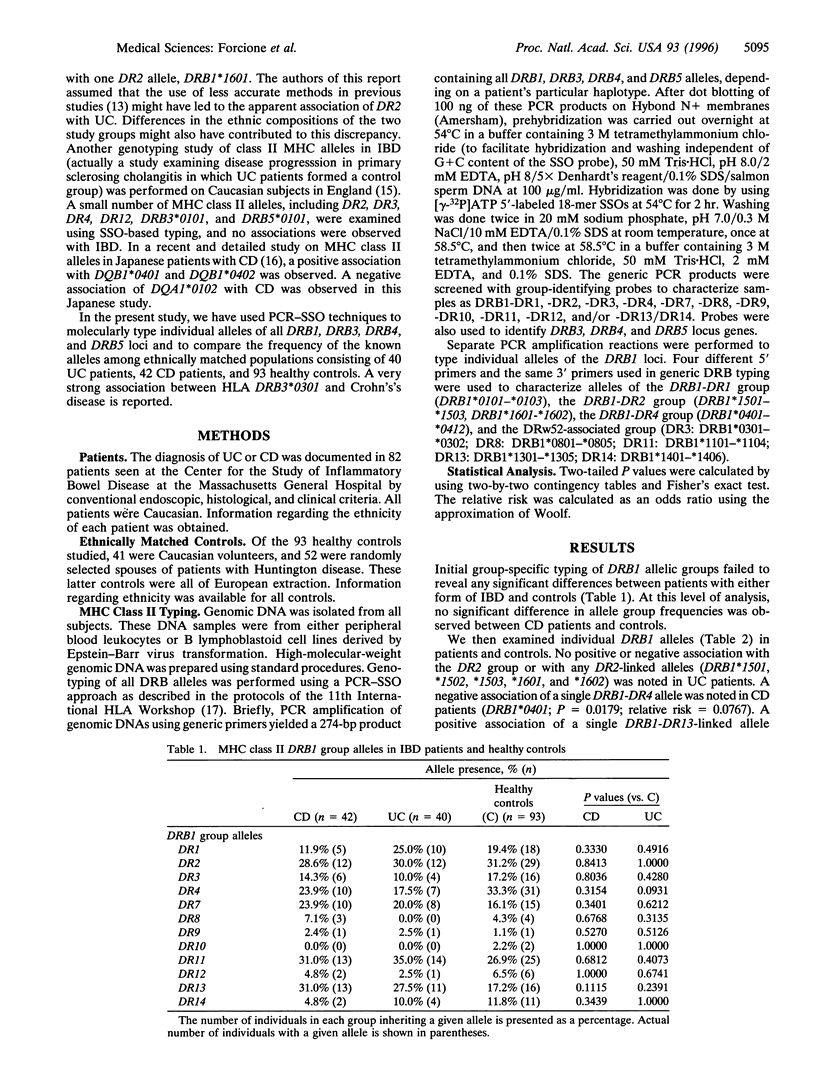
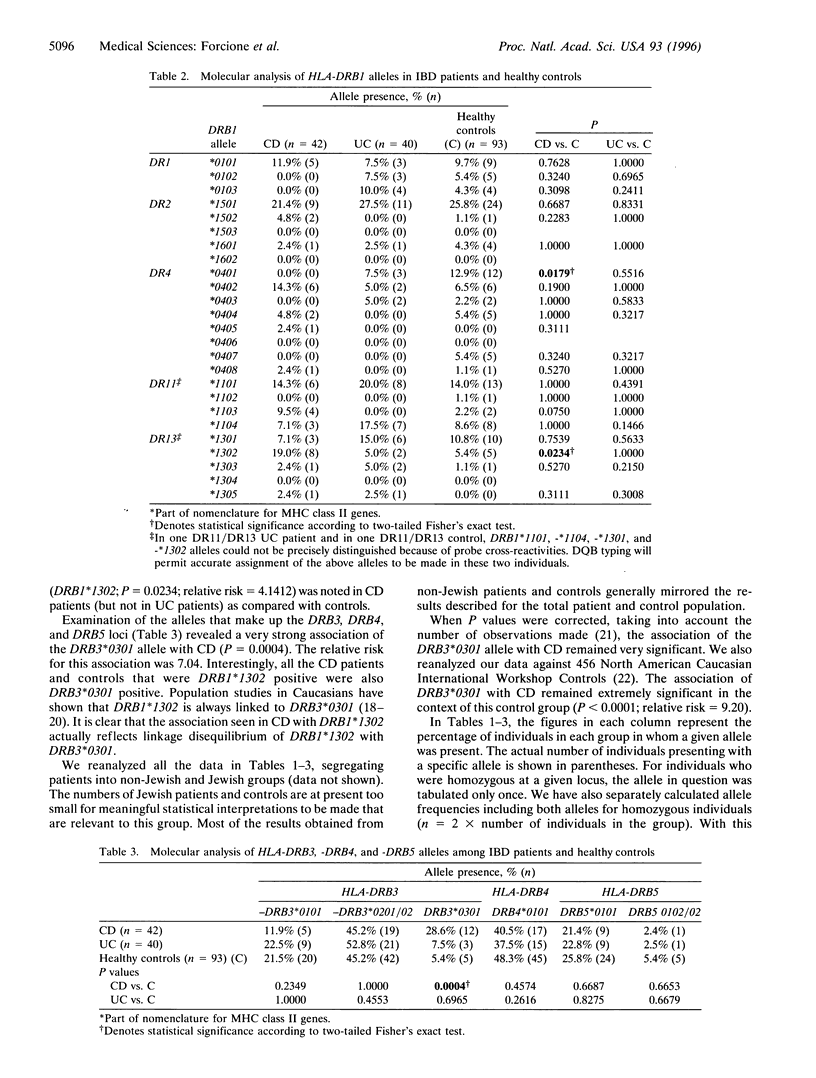
The role of inflammatory T cells in Crohn's disease suggests that inherited variations in major histocompatibility complex (MHC) class II genes may be of pathogenetic importance in inflammatory bowel disease. The absence of consistent and strong associations with MHC class II genes in Caucasian patients with inflammatory bowel disease probably reflects the use of less precise typing approaches and the failure to type certain loci by any means. A PCR-sequence-specific oligonucleotide-based approach was used to type individual alleles of the HLA class II DRB1, DRB3, DRB4, and DRB5 loci in 40 patients with ulcerative colitis, 42 Crohn's disease patients, and 93 ethnically matched healthy controls. Detailed molecular typing of the above alleles has previously not been reported in patients with inflammatory bowel disease. A highly significant positive association with the HLA-DRB3*0301 allele was observed in patients with Crohn's disease (P = 0.0004) but not in patients with ulcerative colitis. The relative risk for this association was 7.04. Other less significant HLA class II associations were also noted in patients with Crohn's disease. One of these associations involved the HLA-DRB1*1302 allele, which is known to be in linkage disequilibrium with HLA-DRB3*0301. These data suggest that a single allele of an infrequently typed HLA class II locus is strongly associated with Crohn's disease and that MHC class II molecules may be important in its pathogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura H., Sugimura K. HLA, antineutrophil cytoplasmic autoantibody, and heterogeneity in ulcerative colitis. Gastroenterology. 1995 Feb;108(2):597–599. doi: 10.1016/0016-5085(95)90091-8. [DOI] [PubMed] [Google Scholar]
- Choy M. Y., Walker-Smith J. A., Williams C. B., MacDonald T. T. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990 Dec;31(12):1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R. H., Neigut D. A. Molecularly defined HLA-DR2 alleles in ulcerative colitis and an antineutrophil cytoplasmic antibody-positive subgroup. Gastroenterology. 1995 Feb;108(2):423–427. doi: 10.1016/0016-5085(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Gleeson M. H., Walker J. S., Wentzel J., Chapman J. A., Harris R. Human leucocyte antigens in Crohn's disease and ulcerative colitis. Gut. 1972 Jun;13(6):438–440. doi: 10.1136/gut.13.6.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Tilanus M., Giphart M., Mach B. Oligonucleotide genotyping shows that alleles at the HLA-DR beta III locus of the DRw52 supertypic group segregate independently of known DR or Dw specificities. Immunogenetics. 1987;25(2):79–83. doi: 10.1007/BF00364271. [DOI] [PubMed] [Google Scholar]
- Mallas E. G., Mackintosh P., Asquith P., Cooke W. T. Histocompatibility antigens in inflammatory bowel disease. Their clinical significance and their association with arthropathy with special reference to HLA-B27 (W27). Gut. 1976 Nov;17(11):906–910. doi: 10.1136/gut.17.11.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehal W. Z., Lo Y. M., Wordsworth B. P., Neuberger J. M., Hubscher S. C., Fleming K. A., Chapman R. W. HLA DR4 is a marker for rapid disease progression in primary sclerosing cholangitis. Gastroenterology. 1994 Jan;106(1):160–167. doi: 10.1016/s0016-5085(94)95085-7. [DOI] [PubMed] [Google Scholar]
- Mullin G. E., Lazenby A. J., Harris M. L., Bayless T. M., James S. P. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not ulcerative colitis. Gastroenterology. 1992 May;102(5):1620–1627. doi: 10.1016/0016-5085(92)91722-g. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Matsuhashi N., Kodama T., Yazaki Y., Takazoe M., Kimura A. HLA-linked susceptibility and resistance genes in Crohn's disease. Gastroenterology. 1995 Nov;109(5):1462–1467. doi: 10.1016/0016-5085(95)90631-2. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Otten H. G., Tilanus M. G., Barnstijn M., van Heugten J. G., de Gast G. C. Serology versus PCR-SSP in typing for HLA-DR and HLA-DQ: a practical evaluation. Tissue Antigens. 1995 Jan;45(1):36–40. doi: 10.1111/j.1399-0039.1995.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Jewell D. P., Rosenberg W. M., Bell J. I. Genetics of inflammatory bowel disease. Gut. 1994 May;35(5):696–700. doi: 10.1136/gut.35.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Raedler A., Stenson W. F., MacDermott R. P. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992 Jun;21(2):451–502. [PubMed] [Google Scholar]
- Smolen J. S., Gangl A., Polterauer P., Menzel E. J., Mayr W. R. HLA antigens in inflammatory bowel disease. Gastroenterology. 1982 Jan;82(1):34–38. [PubMed] [Google Scholar]
- Svejgaard A., Ryder L. P. HLA and disease associations: detecting the strongest association. Tissue Antigens. 1994 Jan;43(1):18–27. doi: 10.1111/j.1399-0039.1994.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Tiercy J. M., Gorski J., Bétuel H., Freidel A. C., Gebuhrer L., Jeannet M., Mach B. DNA typing of DRw6 subtypes: correlation with DRB1 and DRB3 allelic sequences by hybridization with oligonucleotide probes. Hum Immunol. 1989 Jan;24(1):1–14. doi: 10.1016/0198-8859(89)90042-6. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Wang S. J., Yang H. Y., Redford A., Magalong D., Tyan D., McElree C. K., Pressman S. R., Shanahan F., Targan S. R. Distinct associations of HLA class II genes with inflammatory bowel disease. Gastroenterology. 1993 Mar;104(3):741–748. doi: 10.1016/0016-5085(93)91009-7. [DOI] [PubMed] [Google Scholar]
- Vaughan R. W., Lanchbury J. S., Marsh S. G., Hall M. A., Bodmer J. G., Welsh K. I. The application of oligonucleotide probes to HLA class II typing of the DRB sub-region. Tissue Antigens. 1990 Oct;36(4):149–155. doi: 10.1111/j.1399-0039.1990.tb01821.x. [DOI] [PubMed] [Google Scholar]



