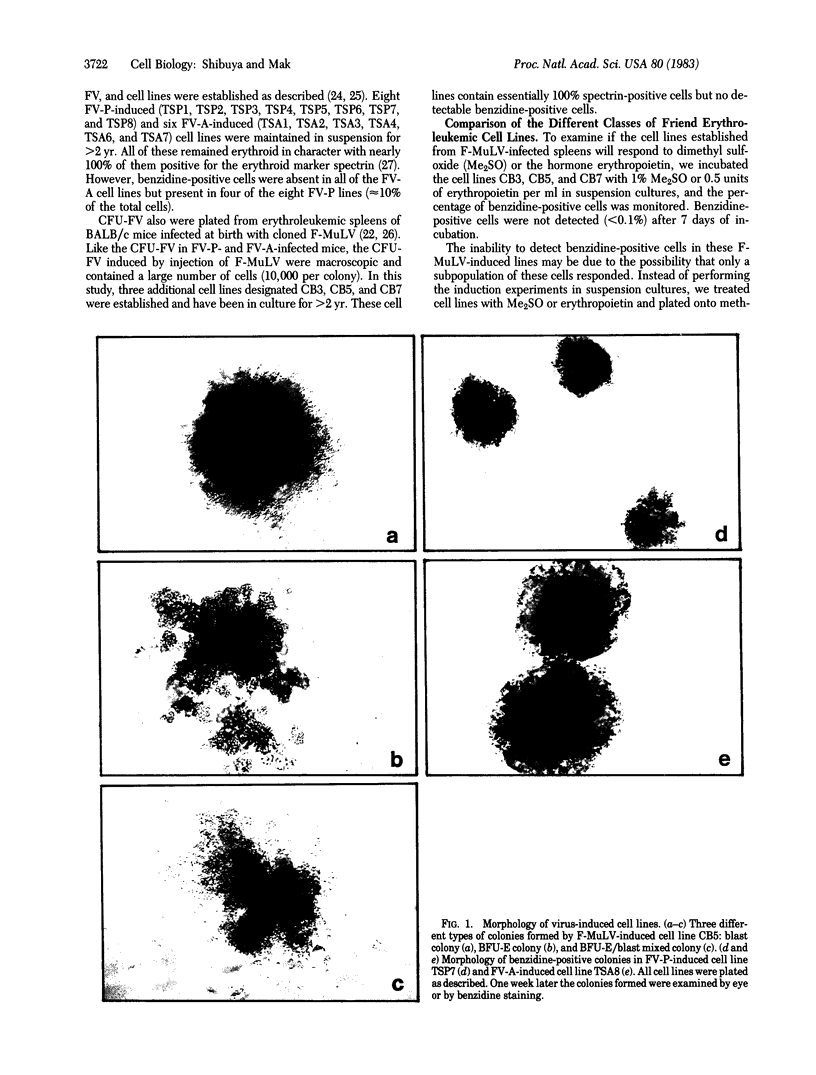
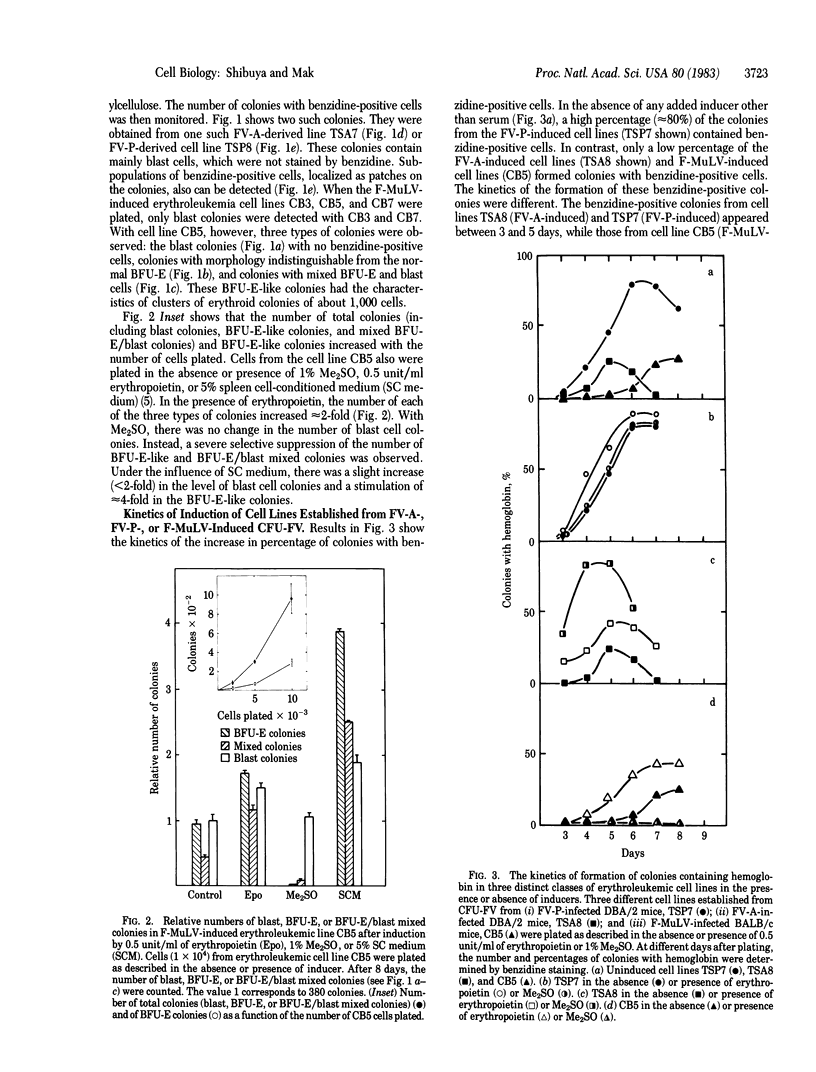
Abstract
We isolated erythroleukemic cell lines arrested at different levels of the erythroid differentiation pathway. One cell line (CB5), established from mice infected with the helper-independent Friend murine leukemia virus (F-MuLV), exhibited properties similar to those of the normal erythroid progenitor burst-forming cell (BFU-E). Six erythroleukemic cell lines, which were established from the anemia-inducing Friend virus complex (FV-A)-infected mice, formed erythroid colonies similar to the erythroid colony-forming precursor cell (CFU-E) after induction with dimethyl sulfoxide or erythropoietin. Three lines that were established from the polycythemia-inducing Friend virus complex (FV-P)-infected mice also formed low proportions (2-5%) of CFU-E-like colonies after induction by these same inducers. These data, together with the earlier findings that F-MuLV induces an increase in the levels of BFU-E and that FV-A or FV-P stimulates enhancement of CFU-E early after infection, indicate that the erythroleukemic cell lines isolated late in the diseases are at the same levels of differentiation as the leukemic cells in the corresponding initial stages. These cell lines with properties of BFU-E and CFU-E can be induced to differentiate in culture and should add to our understanding of the nature of erythroid progenitor cells and their early differentiation programs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Steeves R. How many types of erythroleukaemia are induced by retroviruses in mice? Nature. 1980 Aug 7;286(5773):615–617. doi: 10.1038/286615a0. [DOI] [PubMed] [Google Scholar]
- Aye M. T. Erythroid colony formation in cultures of human marrow: effect of leukocyte conditioned medium. J Cell Physiol. 1977 Apr;91(1):69–77. doi: 10.1002/jcp.1040910108. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Brickenden A. M., Ives R. A., Chui D. H. Effect of modulators of erythropoiesis on the hemoglobinization of human erythroid cell cultures. Blood. 1982 Aug;60(2):346–351. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Moore M. A., Sheridan A. P. Maintenance of hemopoietic stem cells and production of differentiated progeny in allogeneic and semiallogeneic bone marrow chimeras in vitro. J Exp Med. 1977 Jun 1;145(6):1612–1616. doi: 10.1084/jem.145.6.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- Eisen H., Bach R., Emery R. Induction of spectrin in erythroleukemic cells transformed by Friend virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3898–3902. doi: 10.1073/pnas.74.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Patterns of gene expression and the cellular origins of human leukaemias. Biochim Biophys Acta. 1978 Oct 27;516(2):193–230. doi: 10.1016/0304-419x(78)90008-2. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Kost T. A., Koury M. J., Krantz S. B. Erythroid bursts produced by Friend leukaemia virus in vitro. Nature. 1978 Nov 30;276(5687):506–508. doi: 10.1038/276506a0. [DOI] [PubMed] [Google Scholar]
- Jacobs S. W., Miller R. G. Characterization of in vitro T-lymphocyte colonies from spleens of nude mice. J Immunol. 1979 Feb;122(2):582–584. [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A. Recombinant origins of leukemogenic murine viruses. Parke-Davis award lecture, 1978. Am J Pathol. 1978 Oct;93(1):10–26. [PMC free article] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- MacDonald M. E., Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R., Mak T. W., Bernstein A. Anemia- and polycythemia-inducing isolates of Friend spleen focus-forming virus. Biological and molecular evidence for two distinct viral genomes. J Exp Med. 1980 Jun 1;151(6):1477–1492. doi: 10.1084/jem.151.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L., Mak T. W., Bernstein A. Quantitative colony method for tumorigenic cells transformed by two distinct strains of Friend leukemia virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1703–1707. doi: 10.1073/pnas.78.3.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D., MacDonald M. E., Robson I. B., Mak T. W., Bernstein A. Clonal analysis of the late stages of erythroleukemia induced by two distinct strains of Friend leukemia virus. Mol Cell Biol. 1981 Aug;1(8):721–730. doi: 10.1128/mcb.1.8.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D., Mak T. W., Bernstein A. Friend leukaemia virus-transformed cells, unlike normal stem cells, form spleen colonies in Sl/sld mice. Nature. 1980 Dec 11;288(5791):592–594. doi: 10.1038/288592a0. [DOI] [PubMed] [Google Scholar]
- Mager D., Mak T. W., Bernstein A. Friend leukaemia virus-transformed cells, unlike normal stem cells, form spleen colonies in Sl/sld mice. Nature. 1980 Dec 11;288(5791):592–594. doi: 10.1038/288592a0. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Axelrad A. A., Bernstein A. Fv-2 locus controls expression of Friend spleen focus-forming virus-specific sequences in normal and infected mice. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5809–5812. doi: 10.1073/pnas.76.11.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., Gamble C. L., MacDonald M. E., Bernstein A. Host control of sequences specific to Friend erythroleukemia virus in normal and leukemic mice. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):893–899. doi: 10.1101/sqb.1980.044.01.096. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K., Mak T. W. Phorbol esters induce differentiation in human malignant T lymphoblasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2964–2968. doi: 10.1073/pnas.77.5.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niho Y., Shibuya T., Mak T. W. Modulation of erythropoiesis by the helper-independent Friend leukemia virus F-MuLV. J Exp Med. 1982 Jul 1;156(1):146–158. doi: 10.1084/jem.156.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Ruscetti S., Douglass E. C., Scolnick E. Isolation of transplantable erythroleukemia cells from mice infected with helper-independent Friend murine leukemia virus. Blood. 1981 Aug;58(2):244–254. [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Feild J., Davis L., Oliff A. Factors determining the susceptibility of NIH swiss mice to erythroleukemia induced by Friend murine leukemia virus. Virology. 1982 Mar;117(2):357–365. doi: 10.1016/0042-6822(82)90475-5. [DOI] [PubMed] [Google Scholar]
- Sachs L. Regulation of membrane changes, differentiation, and malignancy in carcinogenesis. Harvey Lect. 1974;68:1–35. [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. A host gene controlling early anaemia or polycythaemia induced by Friend erythroleukaemia virus. Nature. 1982 Apr 8;296(5857):577–579. doi: 10.1038/296577a0. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. Host control of susceptibility to erythroleukemia and to the types of leukemia induced by Friend murine leukemia virus: initial and late stages. Cell. 1982 Dec;31(2 Pt 1):483–493. doi: 10.1016/0092-8674(82)90141-6. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. Induction of erythroid tumorigenic colonies by Friend helper virus F-MuLV alone and isolation of a new class of friend erythroleukemic cells. J Cell Physiol Suppl. 1982;1:185–194. doi: 10.1002/jcp.1041130426. [DOI] [PubMed] [Google Scholar]
- Shibuya T., Niho Y., Mak T. W. Erythroleukemia induction by Friend leukemia virus. A host gene locus controlling early anemia or polycythemia and the rate of proliferation of late erythroid cells. J Exp Med. 1982 Aug 1;156(2):398–414. doi: 10.1084/jem.156.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves R., Lilly F. Interactions between host and viral genomes in mouse leukemia. Annu Rev Genet. 1977;11:277–296. doi: 10.1146/annurev.ge.11.120177.001425. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Coleman M. B., Garver F. A., Grenett H. E., Pressley A., Adams J. G., 3rd Effects of dexamethasone on fetal hemoglobin synthesis in peripheral blood erythroid burst-forming units. Am J Hematol. 1981;10(1):37–45. doi: 10.1002/ajh.2830100107. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Axelrad A. A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980 Jan;19(1):225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Urabe A., Hamilton J., Sassa S. Dexamethasone and erythroid colony formation: contrasting effects in mouse and human bone marrow cells in culture. Br J Haematol. 1979 Nov;43(3):479–480. doi: 10.1111/j.1365-2141.1979.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Gamble C. L., Clark S. P., Joyner A., Shibuya T., MacDonald M. E., Mager D., Bernstein A., Mak T. W. Clonal analysis of early and late stages of erythroleukemia induced by molecular clones of integrated spleen focus-forming virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6893–6897. doi: 10.1073/pnas.78.11.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]