Abstract
In this article, we discuss the significance of DNA replication proteins in human disease. There is a broad range of mutations in genes encoding replication proteins, which result in several distinct clinical disorders that share common themes. One group of replication proteins, the MCMs, has emerged as effective biomarkers for early detection of a range of common cancers. They offer practical and theoretical advantages over other replication proteins and have been developed for widespread clinical use.
Some inherited disorders (e.g., Meier–Gorlin syndrome) involve mutations in genes encoding replication proteins. One group of replication proteins, the MCMs, are emerging as effective markers for early cancer detection.
Semiconservative replication of DNA is essential for cellular proliferation. Therefore, mutation of genes encoding the replication machinery could be thought to be fundamentally harmful to an organism. However, inherited and acquired mutations in such genes do occur, resulting in a broad spectrum of disease. The first half of this article outlines the phenotypes associated with several classes of replication disorders, before focusing on the pre-replicative complex (pre-RC) and its involvement in both inherited and acquired human disease. In the second half, we discuss the utility of replication proteins as oncological disease markers, again with emphasis on pre-RC components. The function and mechanism of action of the replication proteins described here are covered in depth in other articles in this collection (Holt and Reyes 2012; Bell and Botchan 2013; Siddiqui et al. 2013).
INHERITED GENETIC DISORDERS OF REPLICATION
There are several clinically recognized genetic disorders resulting from mutations in replication genes. Although distinct from each other, there are common themes to their presentation. In particular, reduced growth and increased cancer risk are frequent features for genes involved in nuclear genome replication. In contrast, for mitochondrial replication genes, multisystem dysfunction of highly energy-dependent tissues is seen. Strikingly, particular developmental abnormalities are also observed in certain disorders. These are less easily attributable to core replication functions and include skeletal abnormalities involving the thumb and forearm (radial ray defects) and absence of the patella. In addition, skin rashes, premature aging, and immune and endocrine dysfunction can occur.
recQ HELICASES: PREMATURE AGING, SKIN SIGNS, CANCER PREDISPOSITION
The recQ helicase family has important roles in replication through their functions in ATP-dependent unwinding of double-stranded nucleic acids. They have roles in repairing damaged replication forks by homologous recombination and in stabilizing stalled forks (Bachrati and Hickson 2008).
Autosomal-recessive mutations in three of the recQ helicases—WRN, BLM, and RECQ4L—cause distinct but overlapping syndromes (Monnat 2010), with reduced growth and cancer predisposition being common to all these conditions.
Bloom’s Syndrome (BLM)
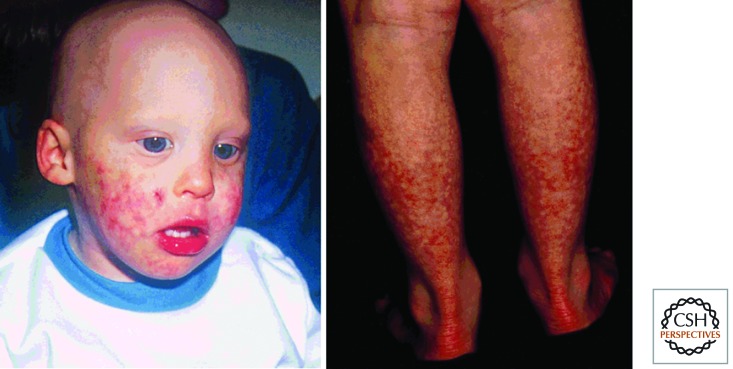
Alongside globally reduced growth, the defining clinical feature of Bloom’s syndrome is a red telangiectatic facial rash that develops on the face in early childhood (Fig. 1). This occurs in a butterfly distribution involving the cheeks and nose (Bloom 1954). Growth is impaired from before birth and is accompanied by significant reduction in head size (microcephaly). Adult height is markedly reduced, reaching only 149 cm in men and 138 cm in women (∼4 standard deviations [SD] below the population mean). There is a high risk of cancer, with both solid tumors and hematological malignancies being a major cause of early death (German 1997). Immunity is impaired with low immunoglobulin levels and an increased incidence of ear and chest infections in childhood, and with chronic chest disorders in adulthood. Diabetes and male infertility also occur. Cytogenetically, a high rate of sister-chromatid exchange (40–100 per metaphase) is pathognomonic for this condition. Mutations in the BLM gene are typically biallelic, truncating loss-of-function mutations (German et al. 2007).
Figure 1.
Bloom’s syndrome with a red facial rash in a butterfly distribution, affecting cheeks and nose. (Reprinted from German 1969.)
Werner’s Syndrome (WRN)
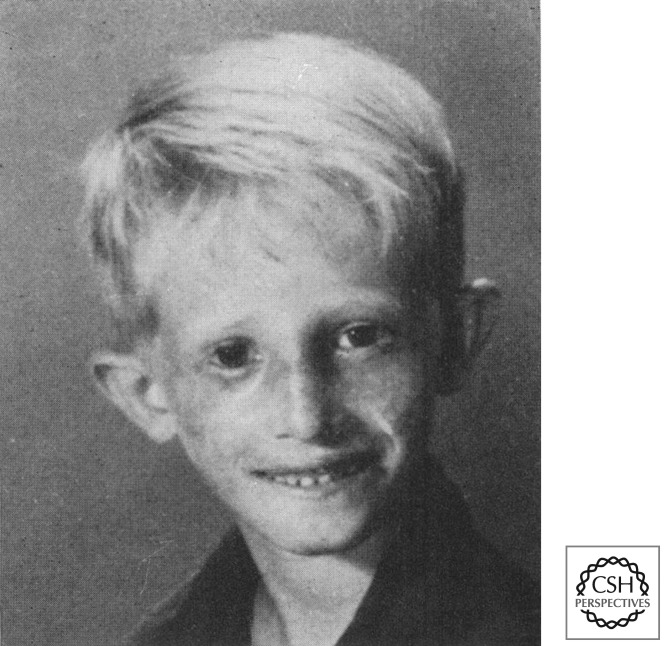
Werner’s syndrome (Oto Werner 1904) (Muftuoglu et al. 2008; Oshima et al. 2012) is the archetypal “progeric” syndrome, with premature aging from early adulthood. In adults, there is early graying of hair, hair loss, and skin atrophy, leading to a tight, wizened facial appearance (Fig. 2). Accelerated aging also results in premature heart disease and strokes (atherosclerosis), as well as osteoporosis and diabetes. There are some similarities with the other RecQ disorders, with increased cancer predisposition and reduced growth in adolescence. Mutations in Werner syndrome are generally truncating loss of function, although occasional missense mutations destabilizing protein stability have been reported (Huang et al. 2006).
Figure 2.
Premature aging in Werner’s syndrome. (Left) Patient aged 15; (right) aged 48. (From Muftuoglu et al. 2008; reprinted, with kind permission, from Springer Science and Business Media.)
RECQ4L: Rothmund–Thompson, RAPADILLINO, and Ballier–Gerold Syndromes
Like Bloom’s syndrome, dermatological signs are strong diagnostic features of Rothmund–Thompson syndrome (RTS) (Larizza et al. 2010). At a few months of age, reddening and blistering of the skin occur on the face (Fig. 3A), and then this spreads to the limbs and buttocks. With time, a reticulate pattern of over- and underpigmentation occurs, known as “poikiloderma” (Fig. 3B). Hair is sparse in this disorder and there are skeletal abnormalities, which can include radial ray defects (thumb abnormalities and/or absence of the radius in the forearm), as well as reduced size of knee caps (patella hypoplasia). Cataracts and chronic diarrhea are often also features. Growth is reduced prenatally and in childhood, but not as severely as in Bloom’s syndrome (Vennos et al. 1992), and there is an increased rate of cancers, particularly osteosarcomas (Wang et al. 2001).
Figure 3.
Rothmund–Thompson syndrome. (A) Facial rash. (B) Poikiloderma, a reticulate hypo/hyperpigmented rash with areas of skin atrophy and telangiectasia (small dilated blood vessels). (From Wang et al. 2001; reprinted, with permission.)
Two other conditions have also been found to have mutations in RecQ4L, and both have clinical features that substantially overlap with RTS (Sznajer et al. 2008). In RAPADILLINO syndrome (Jam et al. 1999), a cleft palate may be present and poikiloderma is said to be absent, whereas Ballier–Gerold syndrome is defined by premature fusion of skull sutures (coronal craniosynostosis), leading to abnormal skull shape and ocular proptosis (protrusion of the eyes).
Although most cases have biallelic truncating mutations, patient cells appear to have some residual protein activity (Siitonen et al. 2009).
MUTATIONS IN THE REPLICATIVE POLYMERASE γ AND TWINKLE HELICASE CAUSE MITOCHONDRIAL DNA DELETION AND DEPLETION SYNDROMES
Mitochondrial diseases are a heterogeneous group of disorders that all result from respiratory chain dysfunction. One or multiple organ systems may be involved, with the tissues involved being those with highest demands for oxidative metabolism. These include the brain, heart, muscle, retina, liver, and endocrine systems. In addition to mutations of genes encoded by the mitochondrial DNA (mtDNA), nuclear-encoded genes also cause mitochondrial diseases. The mitochondrial replicative polymerase POLG1 (Van Goethem et al. 2001; Hudson and Chinnery 2006; Copeland 2010), POLG2 genes (Longley et al. 2006; Young et al. 2011), and replicative helicase TWINKLE (Spelbrink et al. 2001) are three such genes. Mutations in these genes perturb replication and result in multiple large-scale deletions and point mutations in the mtDNA. In addition, they can also lead to an overall depletion of mtDNA in cells. Consequently the production of respiratory chain enzymes (encoded by the mtDNA) is impaired and leads to reduced mitochondrial oxidative function (Suomalainen and Isohanni 2010). As is often the case with mitochondrial disorders, different mutations within the same gene cause a spectrum of clinical disease that varies widely in severity, age of onset, and systems involved. Although distinct clinical syndromes exist, individual patients may not fit neatly into one category.
At the most severe, mitochondrial depletion syndromes (in which total cellular levels of mtDNA can be a few percent of normal) result in severe metabolic disturbance, with lactic acidosis and liver dysfunction. Alongside metabolic issues, muscle and neurological involvement occurs. Epilepsy, ataxia (incoordination), neuropathy, and deafness can all occur, and death generally ensues in early childhood (Suomalainen and Isohanni 2010). As well as autosomal-recessive mutations in POLG1 and TWINKLE, genes encoding many proteins regulating mitochondrial dNTP pools have also been implicated. These include thymidine kinase 2, deoxyguanosine kinase, and the P53-R2 subunit of ribonucleotide reductase (RRM2B) (Mandel et al. 2001; Saada et al. 2001; Bourdon et al. 2007).
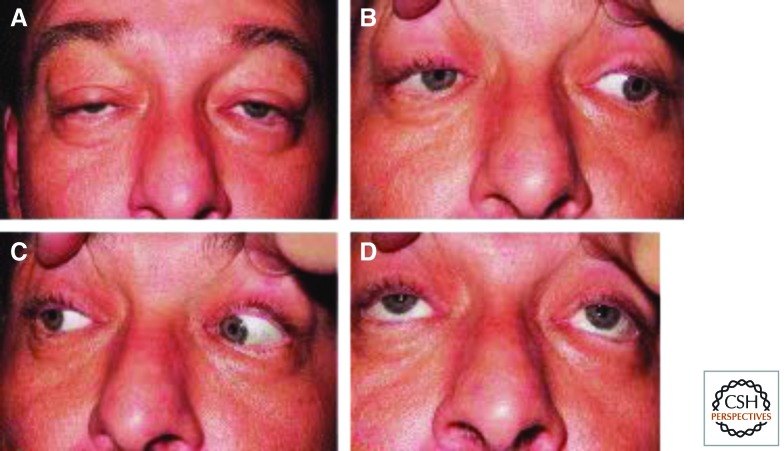
At the other end of the spectrum are adult-onset disorders with neurological or muscular involvement. In such patients, Parkinsonism, skeletal muscle weakness, sensory neuropathy, cognitive decline, and/or ataxia occur. At its mildest, involvement may even simply be limited to the muscles surrounding the eye. In progressive external opthalmoplegia (PEO), there is impaired movement of the eyes, due to weak ocular muscles along with drooping eyelids (ptosis) (Fig. 4). Dominant or recessive mutations in POLG1, TWINKLE, and occasionally POLG2 cause PEO as the result of multiple mitochondrial deletions in muscle tissue.
Figure 4.
Chronic progressive external opthalmoplegia. (A) Drooping eyelids (ptosis) result from weakness in levator palpebrae muscles. (B–D) Weakness of external eye muscles leads to restricted eye movements. (Reprinted from Amato et al. 2006.)
THE PRE-REPLICATIVE COMPLEX, GROWTH, EARS, AND KNEE CAPS
Recently mutations have been identified in multiple components of the pre-replicative complex (pre-RC). The pre-RC forms at origins of replication. Firstly, the heterohexameric origin recognition complex (ORC1-6) becomes bound to DNA during early G1 phase of the cell cycle. Then, in conjunction with the accessory proteins CDT1 and CDC6, it iteratively loads the MCM helicase to license the origin for replication. Recessive biallelic partial of loss-of-function mutations in ORC1, 4, 6, CDT1, and CDC6 have been identified in patients with Meier–Gorlin syndrome (Bicknell et al. 2011a,b; Guernsey et al. 2011). A mutation in MCM4 has also been reported in individuals with immune deficiency, genome instability, and adrenal failure (Gineau et al. 2012; Hughes et al. 2012).
Meier–Gorlin Syndrome, ORC Complex, CDT1, and CDC6
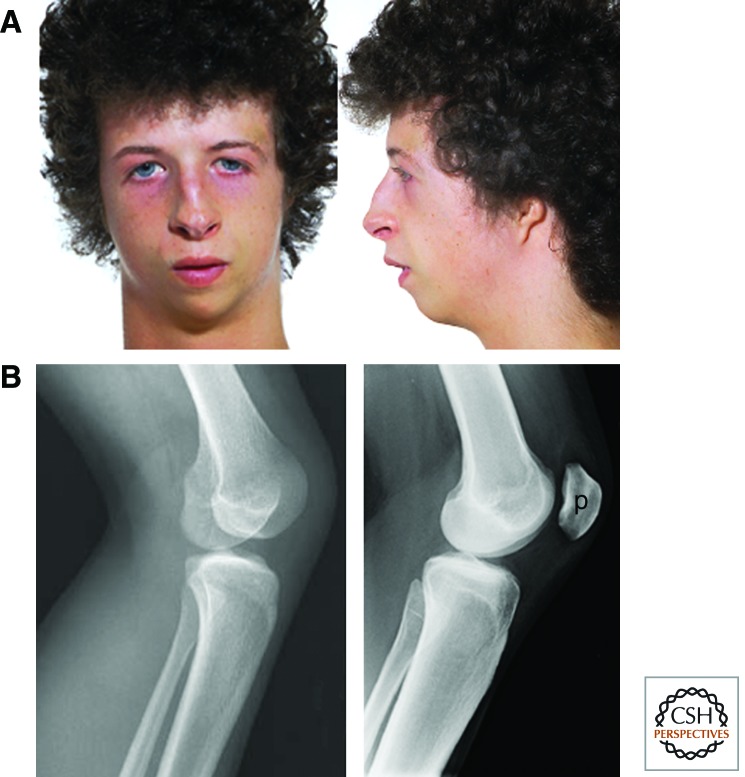
Meier–Gorlin syndrome is an autosomal-recessive single-gene disorder defined by the triad of short stature, microtia (reduced external ear size), and hypoplasia/absence of the patella (Fig. 5) (Gorlin et al. 1975; Bongers et al. 2001). Growth in Meier–Gorlin syndrome is reduced before birth and postnatally, and accompanied by proportionate microcephaly. This growth pattern is similar to that seen in Bloom’s syndrome. Height reduction of those with ORC1 mutations can be extreme (≤6 SD), whereas mutations in the other genes can result in final height being at the lower end of the normal range (de Munnik et al. 2012). Intellect is generally normal.
Figure 5.
Meier–Gorlin syndrome. (A) Marked reduction in size of the external ear (pinna). (Adapted from Bicknell et al. 2011a; images originally kindly provided by Dr. S. Aftimos.) (B) Absence of the patella. Lateral X ray of the knee of a patient with Meier–Gorlin syndrome (left), control subject (right). p, Patella. ([Left] From Guernsey et al. 2011; reprinted, with permission, © Nature Publishing Group. [Right] Courtesy of N. Morley.)
Consistent with the pre-RC cell-essential function, mutations in the ORC1, 4, 6, CDC6, and CDT1 genes are thought to impair rather than abrogate pre-RC function (Bicknell et al. 2011a,b; Guernsey et al. 2011). Recurrent missense mutations are seen in evolutionarily conserved amino acids within domains important for pre-RC function. Additionally, localized (lobar) lung emphysema and retroflexion of the knees may be evident at birth, and in adult women, breast hypoplasia is evident. Although general failure in growth seems to be the result of impaired replication licensing, the reason for the microtia, absent patella, and additional clinical features is not readily evident. In this regard, it is interesting that Recq4L mutations can also specifically affect patella size.
MCM Helicase Mutations: Mice and Humans
The ENU-induced Chaos3 mutation in the Mcm4 gene has been associated with genome instability and breast adenocarcinomas in mice (Shima et al. 2007). It is therefore surprising that there is no increased incidence of cancer in Meier–Gorlin syndrome. This and the lack of any reported mutations in the MCM genes in Meier–Gorlin syndrome suggest that these may result in a different phenotype. Recently, this has been shown to be the case, with two research groups reporting a new syndrome of adrenal insufficiency, natural killer (NK) cell deficiency, a chromosome instability that is associated with a homozygous splice-site mutation in the MCM4 gene (Gineau et al. 2012; Hughes et al. 2012). This mutation has been found in multiple individuals in an extended consanguineous Irish Traveller family and results in a small amino-terminal truncation of the MCM4 protein. As with MGS, the human phenotype suggests that additional functions for this protein need to be established, because these features seem to beyond those expected from its core function as a component of the helicase that establishes the replication fork.
DNA REPLICATION AND CANCER DIAGNOSIS
Many of the commonest cancers can be treated more successfully when they are detected early, either as precursor lesions or early-stage malignancies. The choice of markers for early detection poses a dilemma. The aims are to distinguish normal cells from cancer cells, irrespective of the oncogenes or tumor-suppressor genes that the latter misexpress. Abundant evidence indicates that DNA replication proteins are exceptionally good markers for early detection of many of the common carcinomas and that they can also provide clinically useful prognostic information. This may seem surprising in view of the fact that these proteins are required for the replication of normal as well as cancer cells. However, if cells are recovered from body fluids after they have been exfoliated from the tissue surface, then replication proteins, such as the MCM proteins, can provide decisive clinical information. The reason for this is because MCM proteins are broken down before cells are shed from normal tissues into the body fluids. Therefore, cells shed from normal tissues should not contain MCM proteins, and this prediction is confirmed in practice. In contrast, malignant and premalignant (dysplastic) cells are not programmed to break down MCM proteins before they are lost from the tumor surface.
We have investigated the clinical value of antibodies against DNA replication proteins for detection of malignant and premalignant cells shed into body fluids. We have focused on those epithelia that are the sites of common cancers. MCM proteins are particularly valuable in this role, outperforming both PCNA and Ki67, both of which have also been used for this purpose. We discuss the properties of an ideal marker for early cancer screening and argue that MCM 2-7 proteins provide an exceptionally good match to those criteria.
IMPROVED CANCER SCREENING BY DETECTING MCMs
MCMs Are Sensitive Markers of Malignant and Dysplastic Cells
The essential role of MCMs 2–7 in proliferation led us to test the hypothesis that these proteins would be useful as biomarkers for early detection of cancer by screening. All six subunits are expressed through all phases of the cell cycle but are lost when cells exit the cycle into quiescence, senescence, or differentiation (Musahl et al. 1978; Madine et al. 2000; Stoeber et al. 2001). Although they bind to and detach from DNA according to cell cycle phase, they remain in the nucleus of higher eukaryotes throughout the cell cycle. Of greatest relevance to clinical applications, MCMs are rapidly lost from differentiating epithelial cells, for example, in the squamous epithelium of the cervix, glandular epithelium of the large bowel, and transitional epithelium of the urinary tract (Freeman et al. 1999; Gonzalez et al. 2005). The mechanisms involved are uncertain, although there is evidence of differentiation-associated posttranslational modification of MCM2, resulting in a cleaved fragment that may regulate pre-RC activity (Harada et al. 2008). In contrast, in malignant and premalignant lesions of differentiating epithelia, there is a substantial increase in the number of cells expressing MCMs (Hiraiwa et al. 1998; Todorov et al. 1998; Freeman et al. 1999; Gonzalez et al. 2005; Tachibana et al. 2005).
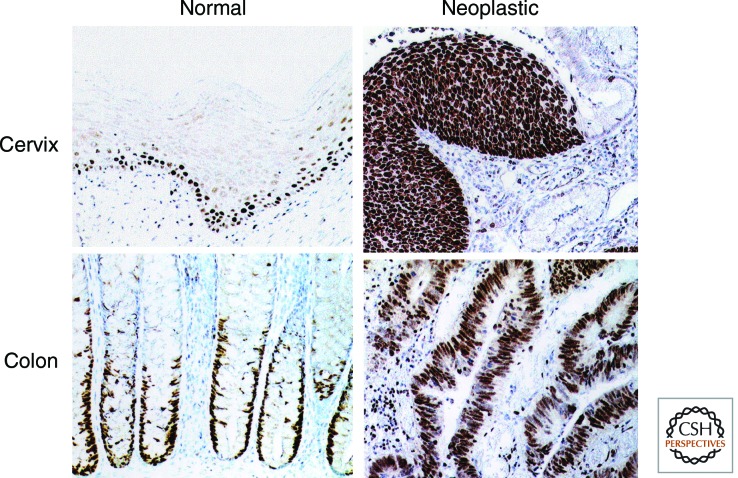
Approximately 80%–100% of cells are MCM immunopositive in malignant and high-grade premalignant lesions, for example, high-grade squamous intraepithelial lesions (SILs) of the cervix (Williams et al. 1998) and dysplastic adenomas of the large bowel (Fig. 6) (Freeman et al. 1999). We observed that there is essentially no difference between the expression patterns of each of the six pre-replication complex MCMs in a large number of tissue samples (Freeman et al. 1999). Based on these findings, we have argued that one of the characteristic features of dysplastic and malignant cells may be that they are continuously licensed for DNA replication.
Figure 6.
Expression of Mcm5 in normal and neoplastic tissue from cervix and colon. The protein is restricted to basal proliferative compartments in normal cervical squamous epithelium and normal large bowel crypts (brown immunoperoxidase staining) and is lost as cells differentiate as they move toward the epithelial surface. In contrast, neoplastic epithelia at the same sites (both malignant and premalignant) show full thickness expression of Mcm5. Numerous immunopositive cells are present at the epithelial surface, from which they can be sampled either actively or passively. Expression of MCMs 2–7 is essentially the same in all tissues examined. (From Freeman et al. 1999; reprinted, with permission, © AACR.)
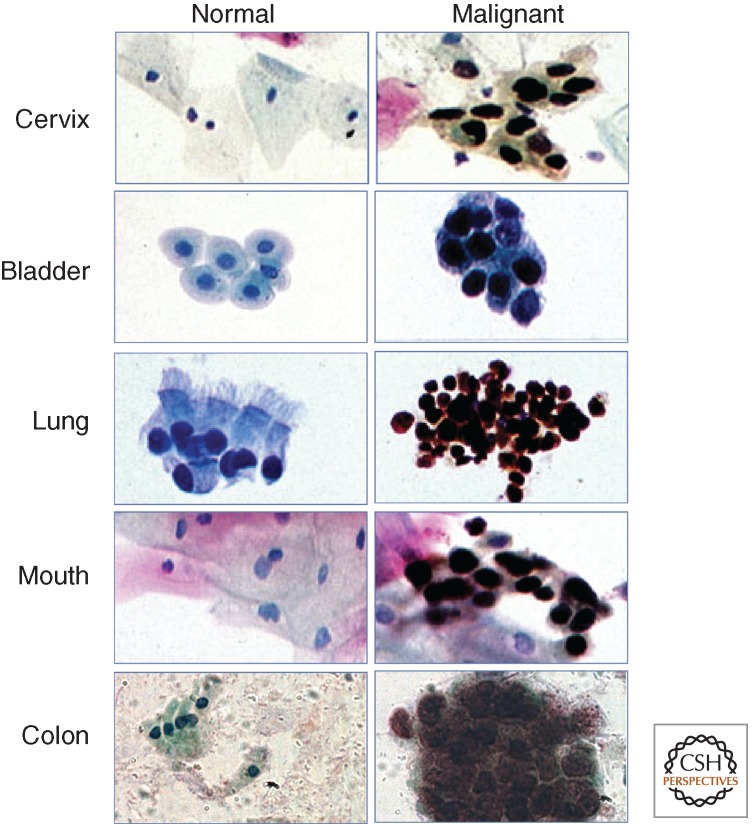
An important consequence of the expansion of the proliferative compartment in neoplasia is that MCM-positive cells appear at the surface of epithelia, and will therefore be included in cytological samples. Such samples can be obtained actively, for example, by scraping, brushing, or washing, or passively, following spontaneous exfoliation into body fluids or solids, such as urine or stool (Fig. 7) (Stoeber et al. 1999, 2002; Davies et al. 2002; Gonzalez et al. 2005). MCMs are therefore very promising biomarkers for early detection of malignancy and premalignancy by population screening. Abnormal cells in cytological preparations generally stain with crisp nuclear signals, making them easy to identify, even at low magnification (Williams et al. 1998; Davies et al. 2002; Chatrath et al. 2003).
Figure 7.
In cytological preparations, immunocytochemistry for Mcm5 enables discrimination between normal cells (left), which are immunonegative, and malignant (and premalignant) cells (right), which are immunopositive (brown nuclei). Equally good discrimination is obtained by staining for other pre-replication complex MCMs, particularly Mcm2. We have used antibodies against Mcm2 extensively in clinical studies, either singly or in combination with anti-Mcm5 antibodies. (From Laskey 2004; reprinted, with permission, © Cambridge University Press.)
These properties make MCMs highly reliable for detecting abnormal cells in cytological samples. This is particularly important where abnormal cells are rarely represented, for example, following sampling of a small lesion or only a small part of a larger lesion. MCM-based tests consequently show high sensitivity4 for detecting malignancy and premalignancy and can reduce the rate of false-negative results associated with conventional cytological screening.
MCMs Have Advantages Over Other Markers
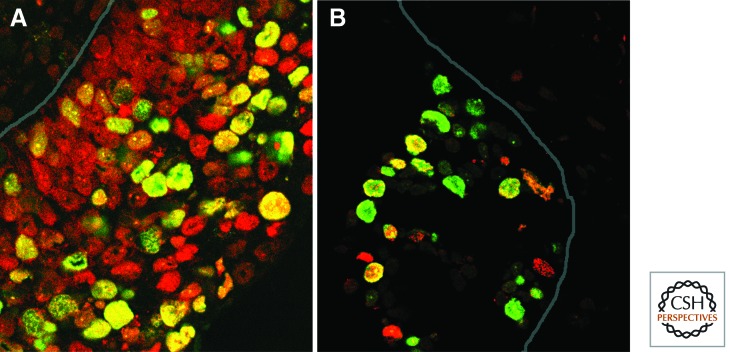
The sensitivity of MCM-based tests would be predicted to be superior to those using other currently used markers of cell cycle entry, such as Ki67 and PCNA. Ki67 was expressed by fewer cells than MCMs in most series of malignant and dysplastic lesions examined (Wharton et al. 2001; Chatrath et al. 2003; Scott et al. 2003; Davies et al. 2004; Dudderidge et al. 2005). This is in keeping with observations that Ki67 is not expressed by all cycling cells, including cells in S phase (Fig. 8) (Scholzen and Gerdes 2000). Indeed, the function of Ki67 in the cell cycle remains poorly understood. This is reflected in the threshold values used to define Ki67 overexpression in tissue samples, which are determined empirically and vary substantially between different centers (Yerushalmi et al. 2010).
Figure 8.
Dual immunofluorescence staining for Ki67 and other cell cycle markers in sections of premalignant cervical high-grade squamous intraepithelial lesions. In each image, the site of the basement membrane is indicated by a continuous line. (A) Staining for Ki67 (green) and Mcm5 (red). Although many nuclei express both proteins (appearing yellow), many others are red, indicating that Ki67 is not present in many cells expressing Mcm5. (B) Staining for Ki67 (green), together with our in situ DNA replication assay (Mills et al. 2000), in which S-phase nuclei are stained (here in red) as a result of their ability to incorporate labeled nucleotides. In situ replication is a sensitive and specific indicator of S-phase cells, not all of which are Ki67 positive. In the image shown, 13% of S-phase cells are Ki67 negative. (Image kindly provided by Tony Mills).
PCNA shows wide variation in staining intensity in vivo, which is much greater than that seen for MCMs. This is consistent with fluctuations in expression levels of PCNA during the cell cycle, with greater expression in S than in G1, G2, and M (Celis and Celis 1985). Moreover, important roles for PCNA in DNA repair mean that it is still present in non-proliferating cells and therefore less useful as a specific marker of the deregulation of proliferation characteristic of malignancy and dysplasia (Toschi and Bravo 1988). Instead, semiarbitrary thresholds must be set for its use as a proliferation marker.
MCMs also offer theoretical advantages over other molecules suggested as being useful biomarkers for cancer detection. Many of the latter are involved in growth signaling pathways, which are frequently disrupted in neoplastic cells. However, there is an enormous degree of redundancy in such pathways, and detection of particular disruptions would be unlikely to provide adequate sensitivity for detection of neoplastic cells in clinical samples. Testing for MCMs represents a “reductionist” alternative, by detecting expression of proteins at the point where growth signaling pathways converge in the initiation of DNA replication (Fig. 9) (Gonzalez et al. 2005).
Figure 9.
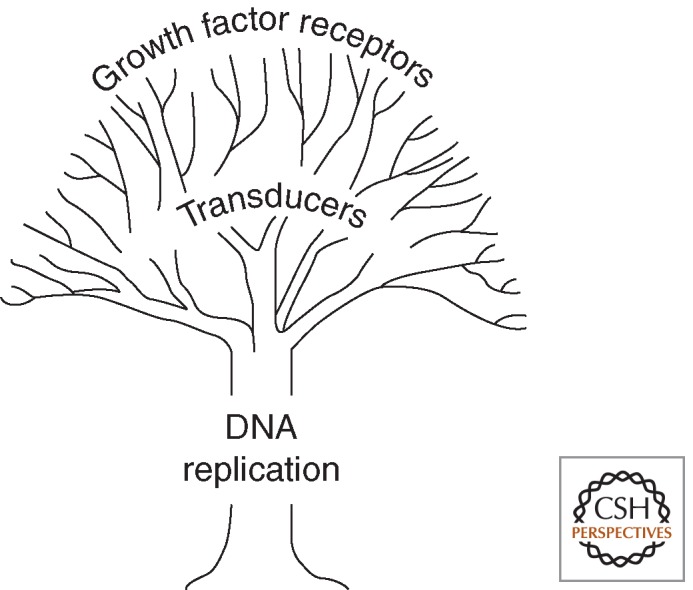
Cartoon illustrating the reductionist approach of using MCM proteins for early detection of malignancy and premalignancy. Other candidate tumor markers include molecules involved in signaling cell growth, many of which are mutated or otherwise disrupted in cancer. However, each growth signaling pathway is inherently redundant, and individual signaling molecules would not be expected to offer adequate sensitivity for detection of neoplastic cells. In contrast, DNA replication proteins, such as MCMs, represent the point of convergence of all signals leading to DNA replication, making them particularly sensitive markers for detecting neoplastic cells showing ectopic cell cycle entry at epithelial surfaces.
Far less literature is available regarding the potential translational value of other replication proteins. In our initial work (Williams et al. 1998), we attempted to use Cdc6 as a biomarker for early cancer detection. However, the relatively low nuclear abundance of Cdc6, compared with that of the MCMs, led to an inferior sensitivity/specificity balance that precluded further development. In contrast, antibodies against ORC proteins stained a wide range of normal cells, consistent with the chromatin-bound state of ORC proteins in quiescent mammalian nuclei (Madine et al. 2000). Cdt1 and geminin would not be expected to provide as much sensitivity for detecting abnormally proliferating cells as MCMs, because expression of each protein is restricted to particular cell cycle phases, rather than being abundant throughout the cell cycle (Gonzalez et al. 2004). On the other hand, these very features make geminin and Cdt1 potentially important markers for indicating tumor prognosis, based on immunohistochemical analysis of tissue sections (Gonzalez et al. 2004). Whether DNA replication initiation factors such as Mcm10, Cdc45, GINS complex members, and so on will offer any practical benefits over MCMs for cancer detection remains to be determined, although there is no strong theoretical basis for believing they would.
Detection of MCM-Positive Cells
A major advantage of using biomarkers in cancer screening is the potential for automated assessment of test results. The algorithm required to detect immunopositive cells by automated microscopy is straightforward (i.e., whether nuclei of a particular color are present or not) and much simpler than those used to assess morphological features (nuclear size, nuclear outline, staining intensity, etc.) in conventionally prepared slides. A simple algorithm should substantially increase throughput and reduce costs. The latter would potentially make automated screening affordable not only in industrialized countries but also in the developing world, where for many cancers the clinical need for a screening test is greatest.
An alternative approach to high-throughput test analysis is to lyse samples and detect the marker or markers of interest using a liquid phase assay such as DELFIA or ELISA. This approach has shown promise in samples in which neoplastic cells are not accompanied by unduly large numbers of normal cells, most notably screening for neoplasia in the urinary tract using urine sediments (Stoeber et al. 1999, 2002). In settings in which abnormal cells may be rare and/or accompanied by numerous normal cells (e.g., cervical smears, sputum, and fecal washings), the sensitivity of liquid phase assays will be substantially less than that of microscopic approaches in which signal is retained in abnormal cells rather than being diluted by lysis.
SPECIFIC APPLICATIONS OF MCM TESTING IN CANCER SCREENING
Cervical Cancer and Precancer
In a cervical smear test, cells are scraped from the cervical epithelial surface. An important premise underlying this approach is that changes at the surface of the cervical squamous epithelium (if detected correctly) can predict abnormalities in the entire epithelium (Baldwin et al. 2003). The current Papanicolaou (Pap) smear test involves subjective assessment of cell morphological features and requires highly trained observers. A liquid-based cytology cervical smear samples about 70,000 cells and in the United Kingdom is examined in 10–15 min, meaning that individual high-magnification microscopic fields are typically assessed for ∼1–2 sec. Because the morphological abnormalities in neoplastic cells are often subtle, it is easy to miss cases of cervical cancer or precancer (SILs). Accordingly, despite expert cytoscreeners, the sensitivity of an individual cervical smear test for neoplastic disease is ∼60% (Baldwin et al. 2003).
An important factor that contributes to this limited sensitivity is that most cervical smears are normal. In consequence, cases containing abnormal cells may be missed if the cytoscreener loses concentration and/or the abnormal cells are rare. This problem will worsen in years to come as the impact of human papillomavirus vaccines reduces the overall burden of cervical neoplastic disease. On the other hand, current vaccines are only predicted to prevent ∼70% of cervical cancers (assuming 100% vaccine uptake) (Stanley 2010), so the requirement for regular cervical screening will remain for the foreseeable future. In view of these issues, there is an important clinical need for new biomarker-based approaches to cervical screening that permit objective disease detection and can be automated for high throughput.
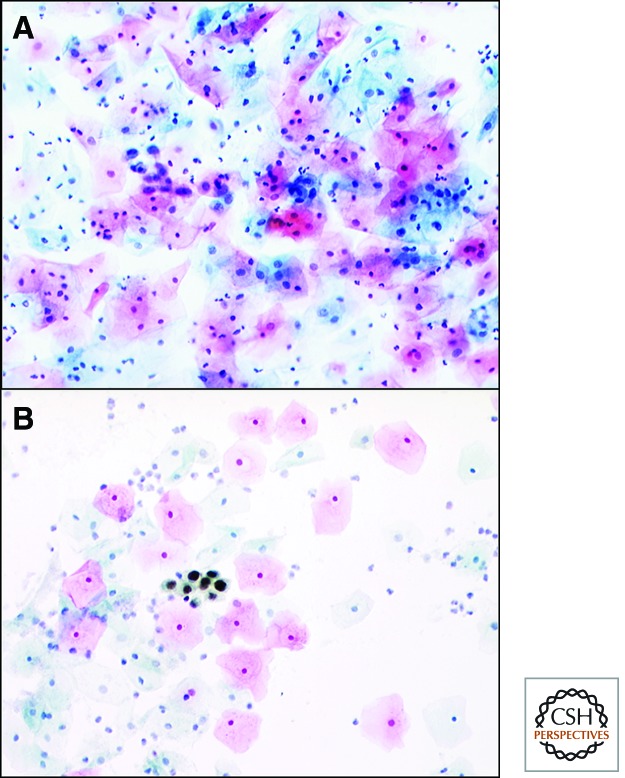
Identifying the presence of MCM-containing cells in a smear is an exciting approach to cervical screening (Fig. 10). In our initial studies, MCM testing showed high sensitivity and specificity for cervical cancer/SILs in smears obtained in the United Kingdom and India (Williams et al. 1998; Baldwin et al. 2003; Mukherjee et al. 2007), providing greater accuracy and faster throughput than conventional Pap testing. The MCM detection technique was subsequently incorporated into diagnostic test kits for widespread clinical use. The first-generation product, ProExC (Becton Dickinson), combined antibodies versus MCM2 and topoisomerase-2A and has been shown in more than 30 studies in Europe and the United States to accurately identify neoplastic cells in cervical smears, with reported sensitivity and specificity values of up to 98% (Tambouret et al. 2008; Brown et al. 2012). A second product, SurePath Plus (Becton Dickinson), is based on combined antibodies against several MCM subunits and is currently undergoing large-scale clinical trials pending detailed evaluation by the U.S. Food and Drug Administration.
Figure 10.
The high sensitivity of MCM immunocytochemistry is exemplified in the assessment of cervical smears. (A) A microscopic field from the cervical smear of a patient with a premalignant cervical high-grade squamous intraepithelial lesion, stained by the routine Papanicolaou (Pap) method. A field of this size has to be examined in routine cytological practice in a second or so. The premalignant cells near the center of the field are easy to miss because they do not stand out from the normal cells around them and can be identified only by highly trained cytoscreeeners and cytopathologists. (B) In contrast, cells from the same patient stained by immunocytochemistry for MCMs. By this approach, abnormal cells are stained a particular color (in this case, brown), enabling them to be recognized much more easily and quickly. Errors are considerably less likely to be made (particularly when the abnormal cells are rare), providing MCM testing with very high sensitivity for detecting disease in routine clinical samples. (from Baldwin et al. 2003; reprinted, with permission, © Nature Publishing Group.)
An important question is how to combine MCM-based tests with other clinical screening methods, for maximal clinical benefit. In Europe, MCM testing may best be used as a reflex investigation in HPV-positive smears, either in isolation or with other tests, such as additional biomarkers or Pap-based cytology. Indeed, an interesting recent study compared eight cervical screening strategies in more than 3000 smear samples and showed that the optimal screening approach was primary HPV testing, followed by ProExC triage of HPV-positive samples (Depuydt 2011). Elsewhere, including the United States, the order of using multiple biomarker-based tests may be different, with MCM and/or HPV tests being used as a reflex to triage smears with mild cytological abnormalities.
A further benefit of ProExC is in improving histological grading of cervical biopsies or resections. Histological examination of tissue sections stained with hematoxylin and eosin is complicated by other pathological or physiological processes that can affect cell morphology, such as inflammation or hormonal changes (including pregnancy). Accordingly, the interobserver and intraobserver reproducibilities of routine histological assessment are limited. Areas of particular difficulty include distinguishing some cases of non-neoplastic squamous metaplasia from squamous intraepithelial lesions (SILs) and some cases of low-grade SILs from high-grade SILs. It is important to resolve these difficulties, because clinical management differs substantially between the diagnostic categories. Immunohistochemical staining with ProExC gives characteristic staining patterns in cervical SILs, including increasing expression with increasing grade (Ozaki et al. 2011). The use of ProExC, alone or in combination, therefore provides a more objective and accurate method of distinguishing high-grade SILs from non-neoplastic mimics in tissue sections.
Colorectal Cancer
It is thought to take two to three years for an asymptomatic early colorectal cancer to develop into a symptomatic advanced lesion (Rhodes 2000). An effective strategy for early detection of colorectal cancer would produce very substantial benefits in overall survival. Current screening tests either detect the presence of occult blood in stool or identify gross abnormalities by endoscopy (Davies et al. 2005). All current tests are limited in their effectiveness and/or patient acceptability. There is a pressing need for new screening tests based on our increasing understanding of the biology and natural history of colorectal cancer. Stool testing is likely to be particularly valuable, because it represents a non-invasive method for screening all of the colon and rectum, without the need for bowel preparation. Methods for retrieving colonocytes from stool washings are now available (although stool samples should be processed within eight hours) (Davies et al. 2002), as are several assays for detecting neoplastic colonocytes. DNA testing is considered to be a promising approach, but clonal heterogeneity within tumors means that multitarget assays are required. These are very expensive and showed disappointing sensitivity for colorectal cancer in a recent large study of average-risk asymptomatic people (Imperiale et al. 2004). The “reductionist” approach offered by MCMs is an inexpensive alternative. In an initial clinical evaluation study, MCM-positive cells were retrieved from the stool of 37 of 40 patients with colorectal cancer, including all nine early-stage cancers, but from none of 25 control participants (Davies et al. 2002). Because colonocytes were often retrieved from stool in small numbers and obscured by fecal debris, we developed a new method of colonocyte retrieval, using stool-derived mucus retained by a 125-μm filter and subsequently processed in a fibrin clot (White et al. 2009). The method provided a >30-fold increase in yield of colonocytes, which are suitable for nucleic acid– and protein-based screening assays. Future work will aim to combine this cell retrieval technique with MCM staining in order to develop a test for high-throughput bowel cancer screening.
Other Cancers
MCM detection is also being developed for early diagnosis of other common cancers. The most advanced of these further applications is screening for bladder and prostate cancer using urinary sediments, based on immunocytochemistry of cytological preparations or liquid phase assays (Stoeber et al. 1999, 2002). Other promising cytological applications include screening for cancers and precancers of squamous epithelium at other sites (analogous to the cervical smear), particularly the oral cavity (Scott et al. 2006) and the anal canal (Scarpini et al. 2008).
IMPROVED PREDICTION OF CLINICAL OUTCOME BY DETECTING CELL CYCLE MARKERS
Immunohistochemistry for cell cycle proteins can address a further important challenge in diagnostic pathology, predicting the outcome of tumors using tissue sections of clinical samples. Markers that can be detected in routinely processed sections of formalin-fixed, paraffin-embedded tissue are particularly valuable. For many tumors, powerful prognostic information is provided by quantitative assessment of cell cycle entry and/or particular cell cycle phases.
As accurate indicators of cell cycle state, MCMs have been shown to predict survival in patients with a range of tumors, including malignancies of breast (Gonzalez et al. 2003), prostate (Meng et al. 2001), kidney (Rodins et al. 2002), bladder (Kruger et al. 2003), esophagus (Kato et al. 2003), mouth (Kodani et al. 2003), lung (Ramnath et al. 2001; Hashimoto et al. 2004), and brain (Wharton et al. 2001; Hunt et al. 2002; Scott et al. 2005). Because MCMs provide a strong nuclear signal in immunohistochemistry, assessment of MCM labeling is straightforward and in our hands highly reproducible. We showed by multivariate analysis that the MCM2 labeling index is superior to the Ki67 labeling index in predicting overall survival in breast cancer (Gonzalez et al. 2003). Moreover, for the data set analyzed, the MCM2 labeling index was also superior to clinico-pathological parameters that are currently widely used to predict outcome, namely, histological grade and lymph node stage.
In addition to information on cell cycle state in tumor cells provided by MCMs, markers of the advanced phases of the cell cycle (S, G2, and M) indicate cell cycle progression and thereby provide a useful representation of cell cycle rate. A promising candidate marker in this regard is geminin (Wohlschlegel et al. 2000; Tada et al. 2001; Gonzalez et al. 2004), which is expressed in S, G2, and M and down-regulated following cell cycle exit, making it a useful marker of cell cycle progression (McGarry and Kirschner 1998; Wohlschlegel et al. 2000). In breast cancer, geminin was a strong independent predictor of poor overall survival and the development of distant metastases. The geminin labeling index was superior to the Ki67 labeling index as to tumor grade, stage, and size (Gonzalez et al. 2004). The MCM labeling indices for the data set examined could not be included in multivariate analysis, because none of the patients with a low MCM index had died or developed metastatic disease at the time of their last follow-up. It was therefore not possible to compare the relative prognostic values of MCMs versus geminin, as indicators of cell cycle state and rate, respectively. It may prove to be the case that the greatest prognostic value is provided by the geminin/MCM fraction, which would represent the rate of proliferation of those tumor cells that are in cycle and therefore responsible for tumor growth.
CONCLUSIONS
Despite the replication machinery’s central role in cellular proliferation, several inherited disorders of replication have been identified. Although these conditions often manifest phenotypes such as growth failure and cancer predisposition, distinct clinical disease entities are recognizable and associated with specific genes. It is therefore likely that in the future, additional replication proteins will be identified for further human genetic syndromes.
Initiation of DNA replication is a critical point of convergence of growth signaling pathways. MCMs are exceptionally promising candidate markers for early detection of malignancy and premalignancy at a wide range of anatomical sites, and clinical evaluation studies from numerous independent laboratories worldwide have provided strong evidence in support of this. The abundance of MCM proteins in the surface layers of dysplastic and malignant lesions and the strong nuclear signal produced in immunocytochemistry make MCMs suitable for high-throughput population screening or pre-screening for a range of common cancers. Automated microscopy is likely to be the most useful detection method across the broad range of potential clinical applications. Cell cycle markers that can be detected in routine formalin-fixed, paraffin-embedded tissue sections may refine existing clinico-pathological approaches for predicting outcome in the common cancers. MCMs and geminin are particularly promising as surrogate markers of cell cycle state and rate, respectively.
ACKNOWLEDGMENTS
We thank Dr. Tony Mills and Lesley Morris for some of the images used in the figures. We also thank Sally Hames for help with preparation of the manuscript. We thank Cancer Research UK for supporting the development of MCMs as cancer screening biomarkers and the Medical Research Council, Lister Institute for Preventative Medicine and European Research Council for funding.
Sensitivity, the proportion of disease positives that are test positive; specificity, the proportion of disease negatives that are test negative; positive predictive value, the proportion of test positives that are disease positive; negative predictive value, the proportion of test negatives that are disease negative.
Editors: Stephen D. Bell, Marcel Méchali, and Melvin L. DePamphilis
Additional Perspectives on DNA Replication available at www.cshperspectives.org
Competing Interest Statement
Nicholas Coleman and Ronald A. Laskey are entitled to a share of royalties received by Cancer Research Technology Ltd. on sales of products related to the use of MCM detection in cancer diagnosis.
REFERENCES
- Amato MM, Monheit B, Shore JW 2006. Ptosis surgery. In Duane’s opthalmology (ed. Tasman W, Jaeger EA), Chap. 78, Fig. 6 Lippincott Williams and Wilkins, London [Google Scholar]
- Bachrati CZ, Hickson ID 2008. RecQ helicases: Guardian angels of the DNA replication fork. Chromosoma 117: 219–233 [DOI] [PubMed] [Google Scholar]
- Baldwin P, Laskey R, Coleman N 2003. Translational approaches to improving cervical screening. Nat Rev Cancer 3: 217–226 [DOI] [PubMed] [Google Scholar]
- Bicknell LS, Bongers EM, Leitch A, Brown S, Schoots J, Harley ME, Aftimos S, Al-Aama JY, Bober M, Brown PA, et al. 2011a. Mutations in the pre-replication complex cause Meier–Gorlin syndrome. Nat Genet 43: 356–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell LS, Walker S, Klingseisen A, Stiff T, Leitch A, Kerzendorfer C, Martin CA, Yeyati P, Al Sanna N, Bober M, et al. 2011b. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier–Gorlin syndrome. Nat Genet 43: 350–355 [DOI] [PubMed] [Google Scholar]
- Bloom D 1954. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am J Dis Child 88: 754–758 [PubMed] [Google Scholar]
- Bongers EM, Opitz JM, Fryer A, Sarda P, Hennekam RC, Hall BD, Superneau DW, Harbison M, Poss A, van Bokhoven H, et al. 2001. Meier–Gorlin syndrome: Report of eight additional cases and review. Am J Med Genet 102: 115–124 [DOI] [PubMed] [Google Scholar]
- Bourdon A, Minai L, Serre V, Jais JP, Sarzi E, Aubert S, Chretien D, de Lonlay P, Paquis-Flucklinger V, Arakawa H, et al. 2007. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 39: 776–780 [DOI] [PubMed] [Google Scholar]
- Brown CA, Bogers J, Sahebali S, Depuydt CE, De Prins F, Malinowski DP 2012. Role of protein biomarkers in the detection of high-grade disease in cervical cancer screening programs. J Oncol 2012: 289315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis JE, Celis A 1985. Cell cycle–dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: Subdivision of S phase. Proc Natl Acad Sci 82: 3262–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrath P, Scott IS, Morris LS, Davies RJ, Bird K, Vowler SL, Grant JW, Saeed IT, Howard D, Laskey RA, et al. 2003. Aberrant expression of minichromosome maintenance protein-2 and Ki67 in laryngeal squamous epithelial lesions. Br J Cancer 89: 1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WC 2010. The mitochondrial DNA polymerase in health and disease. Subcell Biochem 50: 211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RJ, Freeman A, Morris LS, Bingham S, Dilworth S, Scott I, Laskey RA, Miller R, Coleman N 2002. Analysis of minichromosome maintenance proteins as a novel method for detection of colorectal cancer in stool. Lancet 359: 1917–1919 [DOI] [PubMed] [Google Scholar]
- Davies RJ, Scott IS, Morris LS, Rushbrook SM, Bird K, Vowler SL, Arends MJ, Miller R, Coleman N 2004. Increased expression of minichromosome maintenance protein 2 in active inflammatory bowel disease. Colorectal Dis 6: 103–110 [DOI] [PubMed] [Google Scholar]
- Davies RJ, Miller R, Coleman N 2005. Colorectal cancer screening: Prospects for molecular stool analysis. Nat Rev Cancer 5: 199–209 [DOI] [PubMed] [Google Scholar]
- de Munnik SA, Bicknell LS, Aftimos S, Al-Aama JY, van Bever Y, Bober MB, Clayton-Smith J, Edrees AY, Feingold M, Fryer A, et al. 2012. Meier–Gorlin syndrome genotype–phenotype studies: 35 individuals with pre-replication complex gene mutations and 10 without molecular diagnosis. Eur J Hum Genet 20: 598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt CE, Makar AP, Ruymbeke MJ, Benoy IH, Vereecken AJ, Bogers JJ 2011. BD-ProExC as adjunct molecular marker for improved detection of CIN2+ after HPV primary screening. Cancer Epidemiol Biomarkers Prev 20: 628–637 [DOI] [PubMed] [Google Scholar]
- Dudderidge TJ, Stoeber K, Loddo M, Atkinson G, Fanshawe T, Griffiths DF, Williams GH 2005. Mcm2, Geminin, and KI67 define proliferative state and are prognostic markers in renal cell carcinoma. Clin Cancer Res 11: 2510–2517 [DOI] [PubMed] [Google Scholar]
- Freeman A, Morris LS, Mills AD, Stoeber K, Laskey RA, Williams GH, Coleman N 1999. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res 5: 2121–2132 [PubMed] [Google Scholar]
- German J 1969. Bloom’s syndrome. I. Genetical and clinical observations in the first twenty-seven patients. Am J Hum Genet 21: 196–227 [PMC free article] [PubMed] [Google Scholar]
- German J 1997. Bloom’s syndrome. XX. The first 100 cancers. Cancer Genet Cytogenet 93: 100–106 [DOI] [PubMed] [Google Scholar]
- German J, Sanz MM, Ciocci S, Ye TZ, Ellis NA 2007. Syndrome-causing mutations of the BLM gene in persons in the Bloom’s Syndrome Registry. Hum Mutat 28: 743–753 [DOI] [PubMed] [Google Scholar]
- Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, Picard C, Trouillet C, Eidenschenk C, Aoufouchi S, et al. 2012. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest 122: 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MA, Pinder SE, Callagy G, Vowler SL, Morris LS, Bird K, Bell JA, Laskey RA, Coleman N 2003. Minichromosome maintenance protein 2 is a strong independent prognostic marker in breast cancer. J Clin Oncol 21: 4306–4313 [DOI] [PubMed] [Google Scholar]
- Gonzalez MA, Tachibana KK, Chin S-F, Callagy G, Madine MA, Vowler SL, Pinder SE, Laskey RA, Coleman N 2004. Geminin predicts adverse clinical outcome in breast cancer by reflecting cell-cycle progression. J Pathol 204: 121–130 [DOI] [PubMed] [Google Scholar]
- Gonzalez MA, Tachibana KK, Laskey RA, Coleman N 2005. Control of DNA replication and its potential clinical exploitation. Nat Rev Cancer 5: 135–141 [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cervenka J, Moller K, Horrobin M, Witkop CJ Jr. 1975. Malformation syndromes. A selected miscellany. Birth Defects Orig Artic Ser 11: 39–50 [PubMed] [Google Scholar]
- Guernsey DL, Matsuoka M, Jiang H, Evans S, Mac-gillivray C, Nightingale M, Perry S, Ferguson M, LeBlanc M, Paquette J, et al. 2011. Mutations in origin recognition complex gene ORC4 cause Meier–Gorlin syndrome. Nat Genet 43: 360–364 [DOI] [PubMed] [Google Scholar]
- Harada H, Nakagawa H, Takaoka M, Lee J, Herlyn M, Diehl JA, Rustgi AK 2008. Cleavage of MCM2 licensing protein fosters senescence in human keratinocytes. Cell Cycle 7: 3534–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Araki K, Osaki M, Nakamura H, Tomita K, Shimizu E, Ito H 2004. MCM2 and Ki-67 expression in human lung adenocarcinoma: Prognostic implications. Pathobiology 71: 193–200 [DOI] [PubMed] [Google Scholar]
- Hiraiwa A, Fujita M, Adachi A, Ono H, Nagasaka T, Matsumoto Y, Ohashi M, Tomita Y, Ishibashi M 1998. Specific distribution patterns of hCDC47 expression in cutaneous diseases. J Cutan Pathol 25: 285–290 [DOI] [PubMed] [Google Scholar]
- Huang S, Lee L, Hanson NB, Lenaerts C, Hoehn H, Poot M, Rubin CD, Chen DF, Yang CC, Juch H, et al. 2006. The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat 27: 558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Chinnery PF 2006. Mitochondrial DNA polymerase-γ and human disease. Hum Mol Genet 15: R244–R252 [DOI] [PubMed] [Google Scholar]
- Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, Costigan C, Clark AJ, Metherell LA 2012. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest 122: 814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DP, Freeman A, Morris LS, Burnet NG, Bird K, Davies TW, Laskey RA, Coleman N 2002. Early recurrence of benign meningioma correlates with expression of mini-chromosome maintenance-2 protein. Br J Neurosurg 16: 10–15 [DOI] [PubMed] [Google Scholar]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME 2004. For the Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 351: 2704–2714 [DOI] [PubMed] [Google Scholar]
- Jam K, Fox M, Crandall BF 1999. RAPADILINO syndrome: A multiple malformation syndrome with radial and patellar aplasia. Teratology 60: 37–38 [DOI] [PubMed] [Google Scholar]
- Kato H, Toki T, Shimizu M, Shiozawa T, Fujii S, Nikaido T, Konishi I 2003. A new proliferation marker, minichromosome maintenance protein 2, is associated with tumor aggressiveness in esophageal squamous cell carcinoma. J Surg Oncol 84: 24–30 [DOI] [PubMed] [Google Scholar]
- Kodani I, Osaki M, Shomori K, Araki K, Goto E, Ryoke K, Ito H 2003. Minichromosome maintenance 2 expression is correlated with mode of invasion and prognosis in oral squamous cell carcinomas. J Oral Pathol Med 32: 468–474 [DOI] [PubMed] [Google Scholar]
- Kruger S, Thorns C, Stocker W, Muller-Kunert E, Bohle A, Feller AC 2003. Prognostic value of MCM2 immunoreactivity in stage T1 transitional cell carcinoma of the bladder. Eur Urol 43: 138–145 [DOI] [PubMed] [Google Scholar]
- Larizza L, Roversi G, Volpi L 2010. Rothmund–Thomson syndrome. Orphanet J Rare Dis 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey RA 2004. DNA and cancer. In DNA: Changing science and society (ed. Krude T), p. 88 Cambridge University Press, Cambridge [Google Scholar]
- Longley MJ, Clark S, Yu Wai Man C, Hudson G, Durham SE, Taylor RW, Nightingale S, Turnbull DM, Copeland WC, Chinnery PF 2006. Mutant POLG2 disrupts DNA polymerase γ subunits and causes progressive external ophthalmoplegia. Am J Hum Genet 78: 1026–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madine MA, Swietlik M, Pelizon C, Romanowski P, Mills AD, Laskey RA 2000. The roles of the MCM, ORC, and Cdc6 proteins in determining the replication competence of chromatin in quiescent cells. J Struct Biol 129: 198–210 [DOI] [PubMed] [Google Scholar]
- Mandel H, Szargel R, Labay V, Elpeleg O, Saada A, Shalata A, Anbinder Y, Berkowitz D, Hartman C, Barak M, et al. 2001. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat Genet 29: 337–341 [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Meng MV, Grossfeld GD, Williams GH, Dilworth S, Stoeber K, Mulley TW, Weinberg V, Carroll PR, Tlsty TrD 2001. Minichromosome maintenance protein 2 expression in prostate: Characterization and association with outcome after therapy for cancer. Clin Cancer Res 7: 2712–2718 [PubMed] [Google Scholar]
- Mills AD, Coleman N, Morris LS, Laskey RA, 2000. Detection of S phase cells in tissue sections by in situ DNA replication. Nat Cell Biol 2: 244–245 [DOI] [PubMed] [Google Scholar]
- Monnat RJ Jr 2010. Human RECQ helicases: Roles in DNA metabolism, mutagenesis and cancer biology. Semin Cancer Biol 20: 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuoglu M, Oshima J, von Kobbe C, Cheng WH, Leistritz DF, Bohr VA 2008. The clinical characteristics of Werner syndrome: Molecular and biochemical diagnosis. Hum Genet 124: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee G, Muralidhar B, Bafna UD, Laskey RA, Coleman N 2007. MCM immunocytochemistry as a first line cervical screening test in developing countries: A prospective cohort study in a regional cancer centre in India. Br J Cancer 96: 1107–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musahl C, Holthoff HP, Lesch R, Knippers R 1978. Stability of the replicative Mcm3 protein in proliferating and differentiating human cells. Exp Cell Res 241: 260–264 [DOI] [PubMed] [Google Scholar]
- Oshima J, Martin GM, Hisama FM 2012. Werner syndrome. In GeneReviews (ed. Pagon RA, et al. ). University of Washington, Seattle [Google Scholar]
- Ozaki S, Zen Y, Inoue M 2011. Biomarker expression in cervical intraepithelial neoplasia: Potential progression predictive factors for low-grade lesions. Hum Pathol 42: 1007–1012 [DOI] [PubMed] [Google Scholar]
- Ramnath N, Hernandez FJ, Tan DF, Huberman JA, Natarajan N, Beck AF, Hyland A, Todoro IT, Brooks JS, Bepler G 2001. MCM2 is an independent predictor of survival in patients with non-small-cell lung cancer. J Clin Oncol 19: 4259–4266 [DOI] [PubMed] [Google Scholar]
- Rhodes JM 2000. Colorectal cancer screening in the UK: Joint position statement by the British Society of Gastroenterology, the Royal College of Physicians, and the Association of Coloproctology of Great Britain and Ireland. Gut 46: 746–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodins K, Cheale M, Coleman N, Fox SB 2002. Minichromosome maintenance protein 2 expression in normal kidney and renal cell carcinomas: Relationship to tumor dormancy and potential clinical utility. Clin Cancer Res 8: 1075–1081 [PubMed] [Google Scholar]
- Saada A, Shaag A, Mandel H, Nevo Y, Eriksson S, Elpeleg O 2001. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet 29: 342–344 [DOI] [PubMed] [Google Scholar]
- Scarpini C, White V, Muralidhar B, Patterson A, Hickey N, Singh N, Mullerat J, Winslet M, Davies RJ, Phillips M-L, et al. 2008. Improved screening for anal neoplasia by immunocytochemical detection of minichromosome maintenance proteins. Cancer Epidemiol Biomarkers Prev 17: 2855–2864 [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J 2000. The Ki-67 protein: From the known and the unknown. J Cell Physiol 182: 311–322 [DOI] [PubMed] [Google Scholar]
- Scott IS, Morris LS, Bird K, Davies RJ, Vowler SL, Rushbrook M, Marshall AE, Laskey RA, Miller R, Arends MJ, et al. 2003. A novel immunohistochemical method to estimate cell-cycle phase distribution in archival tissue: Implications for the prediction of outcome in colorectal cancer. J Pathol 201: 187–197 [DOI] [PubMed] [Google Scholar]
- Scott IS, Morris LS, Rushbrook SM, Bird K, Vowler SL, Burne NG, Coleman N 2005. Immunohistochemical estimation of cell cycle entry and phase distribution in astrocytomas: Applications in diagnostic neuropathology. Neuropathol Appl Neurobiol 31: 455–466 [DOI] [PubMed] [Google Scholar]
- Scott IS, Odell E, Chatrath P, Morris LS, Davies RJ, Vowler SL, Laskey RA, Coleman N 2006. A minimally invasive immunocytochemical approach to early detection of oral squamous cell carcinoma and dysplasia. Br J Cancer 94: 1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima N, Alcaraz A, Liachko I, Buske TR, Andrews CA, Munroe RJ, Hartford SA, Tye BK, Schimenti JC 2007. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet 39: 93–98 [DOI] [PubMed] [Google Scholar]
- Siitonen HA, Sotkasiira J, Biervliet M, Benmansour A, Capri Y, Cormier-Daire V, Crandall B, Hannula-Jouppi K, Hennekam R, Herzog D, et al. 2009. The mutation spectrum in RECQL4 diseases. Eur J Hum Genet 17: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, Wanrooij S, Garrido N, Comi G, Morandi L, et al. 2001. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet 28: 223–231 [DOI] [PubMed] [Google Scholar]
- Stanley M 2010. HPV—Immune response to infection and vaccination. Infect Agent Cancer 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber K, Halsall I, Freeman A, Swinn R, Doble A, Morris L, Coleman N, Bullock N, Laskey RA, Hales CN, et al. 1999. Immunoassay for urothelial cancers that detects DNA replication protein Mcm5 in urine. Lancet 354: 1524–1525 [DOI] [PubMed] [Google Scholar]
- Stoeber K, Tlsty RTD, Happerfield L, Thomas GA, Romanov S, Bobrow L, Williams ED, Williams GH 2001. DNA replication licensing and human cell proliferation. J Cell Sci 114: 2027–2041 [DOI] [PubMed] [Google Scholar]
- Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, Marr J, Turner WH, Bullock N, Doble A, et al. 2002. Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst 94: 1071–1079 [DOI] [PubMed] [Google Scholar]
- Suomalainen A, Isohanni P 2010. Mitochondrial DNA depletion syndromes—Many genes, common mechanisms. Neuromuscul Disord 20: 429–437 [DOI] [PubMed] [Google Scholar]
- Sznajer Y, Siitonen HA, Roversi G, Dangoisse C, Scaillon M, Ziereisen F, Tenoutasse S, Kestila M, Larizza L 2008. Atypical Rothmund–Thomson syndrome in a patient with compound heterozygous mutations in RECQL4 gene and phenotypic features in RECQL4 syndromes. Eur J Pediatr 167: 175–181 [DOI] [PubMed] [Google Scholar]
- Tachibana KK, Gonzalez MA, Coleman N 2005. Cell cycle control of DNA replication and its relevance to cancer pathology. J Pathol 205: 123–129 [DOI] [PubMed] [Google Scholar]
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ 2001. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol 3: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambouret RH, Misdraji J, Wilbur DC 2008. Longitudinal clinical evaluation of a novel antibody cocktail for detection of high-grade squamous intraepithelial lesions on cervical cytology specimens. Arch Pathol Lab Med 132: 918–925 [DOI] [PubMed] [Google Scholar]
- Todorov IT, Werness BA, Wang HO, Buddharaju LN, Todorova PD, Slocum HK, Brooks JS, Huberman JA 1998. HsMCM2/BM28: A novel proliferation marker for human tumors and normal tissues. Lab Invest 78: 73–78 [PubMed] [Google Scholar]
- Toschi L, Bravo R 1988. Changes in cyclin/proliferating cell nuclear antigen distribution during DNA repair synthesis. J Cell Biol 107: 1623–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goethem G, Dermaut B, Lofgren A, Martin JJ, Van Broeckhoven C 2001. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 28: 211–212 [DOI] [PubMed] [Google Scholar]
- Vennos EM, Collins M, James WD 1992. Rothmund–Thomson syndrome: Review of the world literature. J Am Acad Dermatol 27: 750–762 [DOI] [PubMed] [Google Scholar]
- Wang LL, Levy ML, Lewis RA, Chintagumpala MM, Lev D, Rogers M, Plon SE 2001. Clinical manifestations in a cohort of 41 Rothmund–Thomson syndrome patients. Am J Med Genet 102: 11–17 [DOI] [PubMed] [Google Scholar]
- Wharton SB, Chan KK, Anderson JR, Stoeber K, Williams GH 2001. Replicative Mcm2 protein as a novel proliferation marker in oligodendrogliomas and its relationship to Ki67 labelling index, histological grade and prognosis. Neuropathol Appl Neurobiol 27: 305–313 [DOI] [PubMed] [Google Scholar]
- White V, Scarpini C, Barbosa-Morais NL, Ikelle E, Carter S, Laskey RA, Miller R, Coleman N 2009. Isolation of stool-derived mucus provides a high yield of colonocytes suitable for early detection of colorectal carcinoma. Cancer Epidemiol Biomarkers Prev 18: 2006–2013 [DOI] [PubMed] [Google Scholar]
- Williams GH, Romanowski P, Mills AD, Morris L, Stoeber K, Marr J, Laskey RA, Coleman NC 1998. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci 95: 14932–14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Dutta A 2000. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290: 2309–2312 [DOI] [PubMed] [Google Scholar]
- Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA 2010. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol 11: 174–183 [DOI] [PubMed] [Google Scholar]
- Young MJ, Longley MJ, Li FY, Kasiviswanathan R, Wong LJ, Copeland WC 2011. Biochemical analysis of human POLG2 variants associated with mitochondrial disease. Hum Mol Genet 20: 3052–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]