Abstract
The distinctive morphology of neurons, with complex dendritic arbors and extensive axons, presents spatial challenges for intracellular signal transduction. The endosomal system provides mechanisms that enable signaling molecules initiated by extracellular cues to be trafficked throughout the expanse of the neuron, allowing intracellular signals to be sustained over long distances. Therefore endosomes are critical for many aspects of neuronal signaling that regulate cell survival, axonal growth and guidance, dendritic branching, and cell migration. An intriguing characteristic of neuronal signal transduction is that endosomal trafficking enables physiological responses that vary based on the subcellular location of signal initiation. In this review, we will discuss the specialized mechanisms and the functional significance of endosomal signaling in neurons, both during normal development and in disease.
Using the endosomal system, neurons can transduce signals over long distances and convey spatial information. These signals are important for cell survival, axonal growth and guidance, dendritic branching, and cell migration.
Endocytosis is a basic cellular process that has been conserved and adapted from single cell eukaryotes through humans (reviewed in Mellman 1996; Mukherjee et al. 1997). The fundamentals of endosomal recycling and degradation are the same in neurons as in other cell types (reviewed in Yap and Winckler 2012). However, the endocytic machinery is particularly important in neurons, as specialized vesicles are engaged in releasing neurotransmitters and in subsequent membrane retrieval (reviewed in Saheki and De Camilli 2012; von Zastrow and Williams 2012). Furthermore, endocytosis of neuronal growth factor receptors regulates where and when signaling cascades are initiated (reviewed in Hupalowska and Miaczynska 2012). Here we will discuss how the endocytic process in neurons is adapted so that vesicles can travel through the extensive span of neuronal axons and dendrites, and convey spatial information.
MECHANISMS OF ENDOCYTOSIS
The basic endocytic process for growth factor receptors is similar in neurons and in other cell types. Endocytosis can be broadly classified as being clathrin dependent or clathrin independent (Fig. 1B). In clathrin-mediated endocytosis, receptors and their ligands are recruited to clathrin-coated pits via adaptor proteins. These include the BAR domain proteins that are critical for early membrane deformation, and the sorting adaptor AP2 that recognizes and sorts the diverse variety of cargoes for endocytosis (reviewed in Rao et al. 2012). The large GTPase dynamin is required for fission of the clathrin-coated vesicles from the plasma membrane to form endosomes. There also exist many clathrin-independent mechanisms that are generally less well understood (reviewed in Doherty and McMahon 2009; Mooren et al. 2012). One example is macropinocytosis for internalization of larger areas of membrane, a process that involves ruffling of the plasma membrane and requires rac1 and the actin cytoskeleton. The existence of independent endocytic mechanisms is one way in which the nervous system can selectively regulate the internalization of some receptors but not others to control signaling events in time and space.
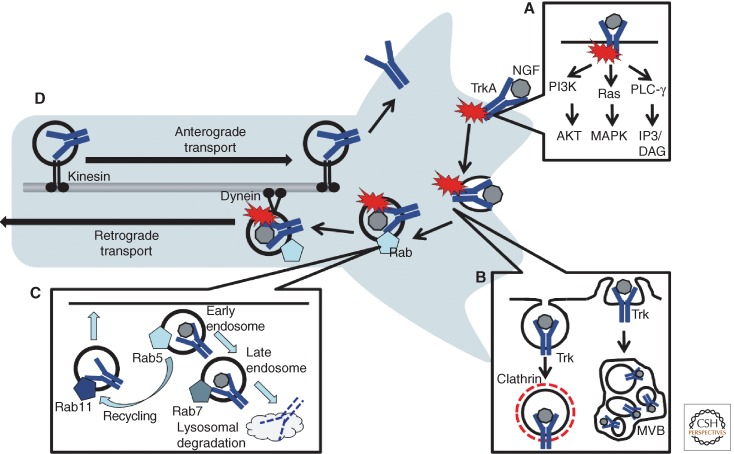
Figure 1.
Endocytosis in neurons. (A) Upon ligand binding, Trk receptors are activated and recruit PI3 kinase, Ras/MAPK, and PLC-γ to initiate downstream signaling pathways. (B) Internalization of neurotrophin/Trk complexes can occur via clathrin-dependent mechanisms to give rise to early endosomes, or via macropinocytosis, which gives rise to multivesicular bodies (MVBs). (C) The endosomal pathway involves maturation from early endosomes associated with Rab5 to late endosomes associated with Rab7. Vesicles are degraded in lysosomes or can be recycled back to the membrane through association with Rab11. (D) In neurons, endosomes are trafficked long distances along the axon by dynein-mediated retrograde transport toward the cell body, or by kinesin-mediated anterograde transport toward the distal axon.
The members of the large Rab family of small GTPases are critical components involved in regulation of endocytosis (reviewed in Kelly et al. 2012). Rabs control the progression of growth factor receptor endocytosis, beginning with initial internalization at the plasma membrane, and continuing through successive steps of membrane maturation and cargo transport (reviewed in Horgan and McCaffrey 2011). Regulated vesicle trafficking requires coordinated association and dissociation of individual Rabs in an orderly and sequential manner (Fig. 1C). For example, Rab5 is a critical mediator of vesicle formation and is associated with early endosomes, whereas subsequent endosome maturation involves detachment of Rab5 from vesicles and accumulation of Rab7. Thus, Rab7 is associated with late endosomes. As they are GTPases, Rabs are activated and deactivated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), respectively. It is thought that particular GEFs and GAPs mediate the regulated sequential cascade of Rab binding that occurs during trafficking steps. The cellular function of individual Rabs is determined by interactions with specific effector proteins. In this way, different Rabs can control distinct trafficking events and, potentially, control the resultant biological response.
A major question still to be addressed is how different Rabs are targeted to distinct endosomes containing particular cargoes. One possibility is that the complement of proteins recruited by an activated receptor at the membrane provides the structural basis that determines Rab binding and activation. The importance of a small number of Rabs has been documented in the nervous system, including Rab5 and Rab7 (reviewed in Ng and Tang 2008), although the specialized functions and regulatory networks of Rabs that contribute to neuronal endosomal signaling are just beginning to be understood.
NEUROTROPHIN AND TRK SIGNALING
In neurons, the most extensively studied endosomal signaling system involves neurotrophin responses. Neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 and neurotrophin-4 (NT3 and NT4) bind and activate specific Trk-family receptor tyrosine kinases (reviewed in Segal 2003). The neurotrophin-bound Trk is internalized into “signaling endosomes” that have been found to include activated components of the Ras-MAP kinase, PLC-γ, and PI3-kinase pathways (Delcroix et al. 2003). These vesicles therefore can carry complete signaling complexes capable of activating multiple intracellular cascades (reviewed in Cosker et al. 2008; Ascano et al. 2012; Harrington and Ginty 2013). Neurons that innervate peripheral targets, such as sensory innervation of skin, or sympathetic innervation of blood vessels, respond to neurotrophins produced by the peripheral targets. Therefore these signaling endosomes are frequently generated at the nerve terminal adjacent to the peripheral target. Subsequently, microtubule-dependent dynein motors retrogradely transport signaling endosomes from the distal end of a long axon to the cell body and so enable neurotrophins to influence long-range signaling and transcriptional events.
Signaling Properties
The endocytic mechanism that gives rise to neurotrophin signaling endosomes can involve clathrin-dependent or clathrin-independent internalization. In experiments using the NGF-responsive pheochromocytoma cell line (PC12), isolated clathrin-coated vesicles were found to contain both Trk and NGF together with a variety of downstream signaling components (Howe et al. 2001). However, a separate study in PC12 cells showed that a macropinocytotic process could promote NGF-stimulated Trk endocytosis, in a process dependent on the large GTPase Pincher (Shao et al. 2002). Furthermore, Pincher-mediated endocytosis was also shown to be specifically required for retrograde neurotrophin signaling in both sympathetic and hippocampal neurons (Valdez et al. 2005, 2007).
There may be several alternative mechanisms that enable endocytosis of neurotrophins and receptors, and formation of signaling endosomes. Various studies have claimed that a Trk-containing signaling endosome is a Rab5-positive early endosome, a Rab7-positive late endosome, or a multivesicular body (reviewed in Harrington and Ginty 2013). One problem is that although the Rabs can be used as markers for distinct endosomal compartments, specific Rabs can be found in more than one compartment owing to the fluid nature of endosomal trafficking. This feature of Rabs may explain some of the controversy surrounding the precise nature of signaling endosomes. Another possibility is that Trk can be internalized and signal from more than one type of endosome. This is supported by the observation in sciatic nerve segments that activated TrkA, seen by ultrastructural visualization, localizes to several, structurally distinct types of vesicles (Bhattacharyya et al. 2002). Further investigation is required to determine what features define a signaling endosome. It is important to consider that these attributes may differ based both on the receptor, and the cellular context.
It is not known what regulatory mechanisms determine which of the diverse modes of endocytosis generates a Trk-containing signaling endosome. It is likely that signaling events that accompany receptor activation specify the endocytic pathway used (Fig. 1A). The PI3-kinase/AKT pathway and the PLC-γ pathway are both directly stimulated by activated Trk receptor tyrosine kinases. In fibroblasts, PI3 kinase has been implicated in macropinocytosis, and the phosphoinositide products regulated by this lipid kinase are involved in regulating dynamin, Rab5, and AP2 (Christoforidis et al. 1999; Abe et al. 2008; Krag et al. 2010). In neurons, PI3-kinase activity is required for retrograde signaling by neurotrophins, as inhibition of PI3 kinase prevents accumulation of NGF-TrkA endosomes in the cell bodies (Kuruvilla et al. 2000). However, it is not clear whether PI3 kinase regulates TrkA endocytosis and formation of a signaling endosome, or subsequent vesicle maturation and transport.
NGF binding to Trk also recruits and activates PLC-γ; activated PLC-γ generates the downstream effectors diacylglycerol (DAG) and inositol triphosphate (IP3). In turn, IP3 promotes the release of Ca2+ from intracellular stores. A recent study by Kuruvilla and colleagues showed that in sympathetic neurons, NGF activation of TrkA results in IP3-induced Ca2+ release and the subsequent activation of the calcium-responsive phosphatase calcineurin (Fig. 2B) (Bodmer et al. 2011). Surprisingly, calcineurin selectively interacts with and dephosphorylates the neuron-specific splice variant of dynamin (dynamin1). The investigators used a phosphosite mutant peptide of dynamin1 (dyn1769–784AA) to show that dephosphorylation of dynamin1 is critical for NGF-dependent TrkA internalization. Further studies will be needed to delineate additional mechanisms whereby PI3K and PLC-γ activation affect Trk internalization. These mechanisms are likely to include some that are specifically required in neurons, and others that play a more general role in the recruitment of proteins necessary for endosome formation.
Figure 2.
Internalization of Trk receptors is required for axon formation, elongation, and dendritic branching. (A) BDNF binding to TrkB activates cAMP and PKA to induce BDNF release and membrane insertion of TrkB. The increased BDNF-TrkB signaling activates PI3K to induce anterograde transport of TrkB to the membrane, creating a feed-forward autocrine loop that locally enhances BDNF signaling and promotes formation of an axon. (B) NGF-induced axon growth involves TrkA internalization. Signaling through PLC-γ, calcineurin, and dynamin1 promotes axon elongation independent of retrograde signaling and transcriptional responses. (C) BDNF induces dendritic branching that requires Rab11-dependent recycling of TrkB receptors.
The extent of signal transduction following Trk internalization can be controlled at the level of receptor turnover by ubiquitination. Other receptor tyrosine kinases including EGF and PDGF receptors are rapidly degraded after ligand binding by ubiquitination of the activated receptor (Thien and Langdon 2001). Chao and colleagues identified Nedd4-2, an E3 ubiquitin ligase that binds directly to the TrkA receptor and controls degradation of the receptor following activation by NGF (Arevalo et al. 2006). Nedd4-2 binds specifically to TrkA, and it does not associate with TrkB or TrkC, or affect levels of these receptors. The decreased levels of activated TrkA observed with Nedd4-2 overexpression resulted in increased apoptosis of sensory neurons, indicating that Trk ubiquitination alters biological responses to NGF. Identification of other specific E3 ubiquitin ligases will provide further potential mechanisms to control Trk receptor levels and thereby regulate neurotrophin signal transduction.
Intriguingly, the nature of the endosomes formed following neurotrophin binding depends on the specific ligand as well as the particular receptor. Although NGF and NT3 both bind and activate TrkA, they elicit different cellular outcomes in developing sympathetic neurons (Kuruvilla et al. 2004). During development of sympathetic neurons, signaling by TrkA is required for sequential stages of axon growth and for innervation of the final target tissue. In vivo, the vasculature along which the sympathetic axons grow constitutes the intermediate target of the sympathetic neurons and is known to secrete NT3 (Francis et al. 1999). In contrast, NGF is secreted by the final target, namely, the glandular tissue (Crowley et al. 1994). Although NT3 promotes axon extension, NGF can promote both axon extension and neuronal survival. This difference in biological response correlates with differences in formation of signaling endosomes; activation of TrkA following NGF binding generates signaling endosomes, whereas activation of TrkA by NT3 binding does not (Kuruvilla et al. 2004). Ginty and colleagues discovered that NGF-TrkA complexes remain stable after early endosome formation and vesicle acidification. In contrast, NT3-TrkA binding is compromised at low pH, resulting in more transient signaling after endocytosis. The more acid-stable NGF-TrkA complex is able to recruit and activate Rac1 and cofilin. Furthermore, the actin-severing action of cofilin is necessary for retrograde transport of signaling endosomes and therefore is required for the long-distance survival signaling of NGF. Thus, the actin cytoskeleton may create a barrier for maturation of an early endosome into a signaling endosome (Harrington et al. 2011). In this way, different growth factors binding to the same receptor can achieve distinct downstream signaling events and cellular outcomes.
Motor Proteins
Motor proteins are responsible for moving vesicles along actin or microtubule filaments. Although motor proteins are important for moving and localizing endosomes in all cell types, the need for active transport is particularly evident in neurons, owing to their expansive morphology (Fig. 1D). In axons, microtubules are all oriented in the same direction with the minus end at the terminals; in contrast, in dendrites, microtubules are not uniformly oriented. In axons, kinesin motor proteins bring vesicles toward the axon terminals, whereas dynein motors are responsible for retrograde movement of axonal cargoes. Therefore, it is not surprising that following target-derived neurotrophin stimulation, dynein is responsible for the retrograde movement of signaling endosomes through the long axons of neurons, and for the resultant biological responses (Heerssen et al. 2004).
How do signaling endosomes recruit the required motors to allow correct and rapid movement? Dnhc1, the motor-domain-containing dynein heavy chain, requires association with intermediate chains, light intermediate chains, light chains, and the dynactin complex to form a transport-competent motor protein. In neurons, dynactin has a specialized role in formation of motor-cargo complexes that promote the initiation of axonal transport (Moughamian and Holzbaur 2012). A major component of dynactin is the p150Glued subunit, which interacts directly with dynein and also associates with microtubules via the CAP-Gly domain. Mutations in the CAP-Gly domain cause selective neurodegenerative diseases, including Perry syndrome and distal hereditary motor neuropathy 7B (HNM7B); these phenotypes imply that the CAP-Gly domain has a neuron-specific role (Puls et al. 2003; Farrer et al. 2009). Work from Holzbaur and colleagues show that the specific CAP-Gly mutations that cause Perry syndrome (G71R, Q74P) lead to disrupted initiation of vesicular transport from the distal ends of axons. This disruption is owing to an inability of the mutant dynactin to accumulate at the distal axon terminals where motor-cargo complexes are initiated. Interestingly, in non-neuronal cells, the CAP-Gly domain of dynactin is not required for dynein-mediated vesicular transport (Kim et al. 2007; Dixit et al. 2008). Thus, the CAP-Gly domain is required to stably enrich dynactin at neurite tips and so serves a specialized function for efficient initiation of axonal transport in neurons.
Unlike kinesins, which belong to a large family of more than 45 distinct kinesin genes and so provide a high degree of selectivity with regard to cargo, retrograde axonal transport is achieved by the actions of a single dynein gene product (dnhc1) (reviewed in Vale 2003). This same motor protein is involved in axonal and dendritic transport, and also in cellular processes such as mitosis and Golgi trafficking. However, there are diverse intermediate and light-chain gene products that associate with dnhc1, and so distinguish the nature of the motor complex. Pfister and colleagues set out to identify dynein variants, as defined by the intermediate chain (IC) isoforms that transport signaling endosomes. They found that dynein complexes containing IC-1B, an isoform of the intermediate chain specifically expressed in the nervous system, selectively bind to and transport neuronal TrkB-signaling endosomes. In contrast, dynein complexes containing the highly related, ubiquitously expressed IC-2C intermediate isoform do not do so (Ha et al. 2008). Furthermore, TrkA signaling endosomes are not transported by IC-IB-containing dynein complexes, but bind instead to dynein complexes containing IC-2C. Thus, the neuron-specific IC-1B isoform provides cargo specificity in the dynein motor for long-distance trafficking of endosomes.
The same group showed that phosphorylation at a newly identified site within ICs by the MAP kinase Erk1/2 provides a novel mechanism for regulating dynein-endosome interactions (Mitchell et al. 2012). Live-cell imaging with fluorescently tagged IC mutants showed that the IC mutant (IC-1B S80A), which is unable to undergo phosphorylation at these sites, showed significantly reduced colocalization with Trks and with the late endosomal marker Rab7. Furthermore, NGF-dependent survival of sympathetic neurons was significantly reduced by the overexpression of the dephosphomimetic mutant IC. Likewise, inhibition of Erk1/2 reduced the motility of Rab7- and TrkB-containing endosomes and the extent of their colocalization with dynein in axons. These results show that neurotrophin binding to Trk initiates the recruitment of cytoplasmic dynein to signaling endosomes through Erk1/2 phosphorylation of ICs for subsequent retrograde transport of the Trk receptors in axons (Mitchell et al. 2012).
Recently, a mouse model was generated in which the IC-1B isoform was tagged with green fluorescent protein (GFP) and tandem repeats of the FLAG epitope (Zhang et al. 2013). This mouse will allow GFP-dynein to be visualized when expressed at physiologic levels in neurons. In addition, this new mouse reagent will facilitate biochemical purification of this specialized form of dynein, to identify proteins that are bound to this particular motor complex. As axonal transport has been implicated in diverse neurologic disorders, greater understanding of the cargoes and regulators will give insight into the mechanisms that cause such neurological disease.
FUNCTIONAL SIGNIFICANCE OF SIGNALING ENDOSOMES
Endosomes are a critical element of signal transduction owing to the morphology of neurons. The functions of signaling endosomes are also intertwined with the distinct aspects of neuronal architecture, as these vesicles have a role in axon growth and guidance, dendritic arborization, and target-derived survival responses.
Axon and Dendritic Growth and Cell Migration
During development, neurons extend axons that navigate a complex path to their correct targets. During axonal extension, the growth cone at the tip encounters extracellular growth factors that stimulate and guide the axon. The growth cone is a specialized structure with a highly dynamic actin cytoskeleton that enables changes in shape and direction of growth in response to critical cues. Neurotrophins are important for axon formation and directed outgrowth, and endocytic signaling has been implicated in these critical events.
In hippocampal neurons, BDNF promotes axonal outgrowth by activating TrkB receptors. Intriguingly, there is an autocrine, feed-forward loop that functions in this process. When a nascent, TrkB-positive neurite encounters BDNF, activation of TrkB results in local increases in cAMP and in protein kinase A (PKA) activity, which triggers local secretion of BDNF. BDNF may also trigger Ca2+-dependent secretion of BDNF, providing a second potential mechanism for BDNF-dependent secretion of BDNF (Sadakata and Furuichi 2010). In addition to increased BDNF secretion, BDNF also promotes local membrane insertion of the receptor TrkB, and the BDNF/TrkB-induced PI3-kinase cascade promotes anterograde, kinesin-dependent transport of TrkB into the nascent axon, thereby further enhancing local BDNF/TrkB signaling (Fig. 2A) (Cheng et al. 2011). Thus, both secretion of BDNF and internalization of BDNF/TrkB in signaling endosomes amplify the signal in a feed-forward loop that polarizes one neurite so that it differentiates into an axon.
The endocytic machinery involved in the above feed-forward loop is not known. However, regulated dynamin function has been implicated in NGF-dependent axonal elongation, after the period in which the axon is determined. In sympathetic neurons, NGF promotes axon outgrowth. Studies by Kuruvilla and colleagues indicate that the phosphatase calcineurin is able to dephosphorylate the endocytic GTPase, dynamin1. Dephosphorylated dynamin1 colocalizes with TrkA and regulates dynamin-dependent endocytosis of these receptors. Thus, calcineurin signaling is required locally in sympathetic axons to support NGF-mediated growth, and conditional deletion of calcineurin in sympathetic neurons disrupts NGF-dependent innervation of peripheral target tissues (Fig. 2B). These studies emphasize the importance of regulated endocytosis of neurotrophin-receptor complexes for appropriate signaling (Bodmer et al. 2011).
Growth and branching of dendrites are often regulated by neurotrophin signaling, and these morphologic changes also depend on regulated endocytic processes. In particular, the actions of Rab components have been implicated in BDNF-TrkB dendritic arborization. A recent study showed that BDNF induces dendritic arborization through accumulation of TrkB in Rab11-positive endosomes (Lazo et al. 2013). Rab11 is usually associated with recycling endosomes; Rab11 and the actin-based motor protein myosin Vb mediate the rapid recycling of TrkB receptors back to the plasma membranes of dendrites. In this way, Rab11 increases TrkB localization in growing dendrites and so enhances local signaling and arborization in response to BDNF (Fig. 2C). Bronfman and colleagues also provide evidence that overexpression of a constitutively active Rab11 increases levels of phosphorylated CREB, a BDNF-responsive transcription factor. Because Rab11 can associate with dynein for microtubule-based vesicular transport, an interesting unresolved question is whether Rab11 plays an additional role in long-distance endosomal signaling for BDNF-TrkB-induced dendritic branching.
Many attributes of regulated cell migration resemble features of axon outgrowth or dendritic arborization. Neurotrophins can promote directed migration of neuronal precursors; as is the case for axon or dendritic growth, this process of directed movement involves Trk endocytosis. During neuronal migration, endocytic activation preferentially takes place in the leading processes (Fletcher and Rappoport 2010; Shieh et al. 2011). The potential importance of localized endocytosis was shown in studies on migration from the Segal laboratory. During cerebellar development, endocytic trafficking of TrkB receptor is required for neuronal precursor cell chemotaxis toward a BDNF gradient (Zhou et al. 2007). As is the case in axonal growth, BDNF initiates a feed-forward loop in which activation of TrkB stimulates BDNF release, and thus functions in an autocrine manner to promote precursor migration. Furthermore, local accumulation of activated TrkB in the leading processes during migration requires endocytosis. The protein Numb, which binds to α2 adaptor proteins that are implicated in endocytosis, also binds to activated TrkB to enhance receptor endocytosis following stimulation. Thus, Numb promotes the formation and accumulation of signaling endosomes in the leading processes of migrating cells. As Numb itself is phosphorylated and activated in response to TrkB activation, there is a cascade of enhanced endocytosis that is triggered by BDNF. The resultant pool of signaling endosomes provides an intracellular gradient of activated receptor to polarize the cell and promote directed migration (Zhou et al. 2011).
Neuronal Survival
The canonical role of neurotrophins is to promote the survival of neurons during critical phases of development. During development, neurons extend axons toward the targets that they will innervate; neurons whose axons reach appropriate targets encounter target-derived neurotrophins and survive. Neurons that do not receive these target-derived signals die. This neurotrophic hypothesis provides a mechanism that restricts survival to correctly innervated neurons and explains the high level of apoptosis that occurs during normal development. Neurotrophin-mediated survival therefore requires long-distance signal transmission from the site of stimulation at distal axons, to the cell body. The signaling endosome model is a widely accepted mechanism whereby activated Trks are internalized and retrogradely transported to activate Erk and PI3-kinase signaling to induce transcriptional changes in gene expression (Grimes et al. 1996, 1997; Riccio et al. 1997; Howe et al. 2001; Heerssen et al. 2004). This long-range, endosomal signaling has been shown to activate transcription factors in the nucleus such as CREB, MEF2, and c-fos, and thereby induce gene expression required for neuronal survival (Riccio et al. 1997; Watson et al. 1999; Pazyra-Murphy et al. 2009). A recent paper from the Segal laboratory showed that retrograde signaling pathways that control transcriptional events in the cell body can be coordinated with localized mechanisms that promote neurotrophin-dependent survival of axons. Target-derived neurotrophins induce transcription of the survival factor bclw and subsequent mRNA transport into the axon. The resulting increased levels of axonal Bclw protein locally protect axons from caspase-6 activation and axon degeneration (Cosker et al. 2013).
In addition to transcription-mediated survival pathways, neurotrophin signaling pathways have also been implicated in transcription-independent antiapoptotic changes (Putcha et al. 1999). Because components of the Erk and PI3-kinase pathways are associated with signaling endosomes as they are trafficked through axons, it is possible that signaling “en passant” occurs during vesicle transport. This would provide an additional mechanism in which the endocytic machinery enables sustained maintenance of the long axons characteristic of neurons.
ROLE IN DISEASE
Defects in endocytic membrane trafficking and vesicular transport have been implicated in many neurodegenerative disorders (Perlson et al. 2010; Wang et al. 2012). The question remains why neurons are particularly susceptible to faults in highly conserved cellular trafficking mechanisms that do not affect other cell types. One argument is that the distinct morphology of neurons makes these cells exceptionally vulnerable; perhaps specific endosomal signaling functions in neurons contribute to the pathology observed. Here we will discuss recent findings that implicate altered endosomal signaling mechanisms in the neuropathology observed in Charcot-Marie Tooth disease and Huntington’s disease.
Charcot-Marie Tooth Disease
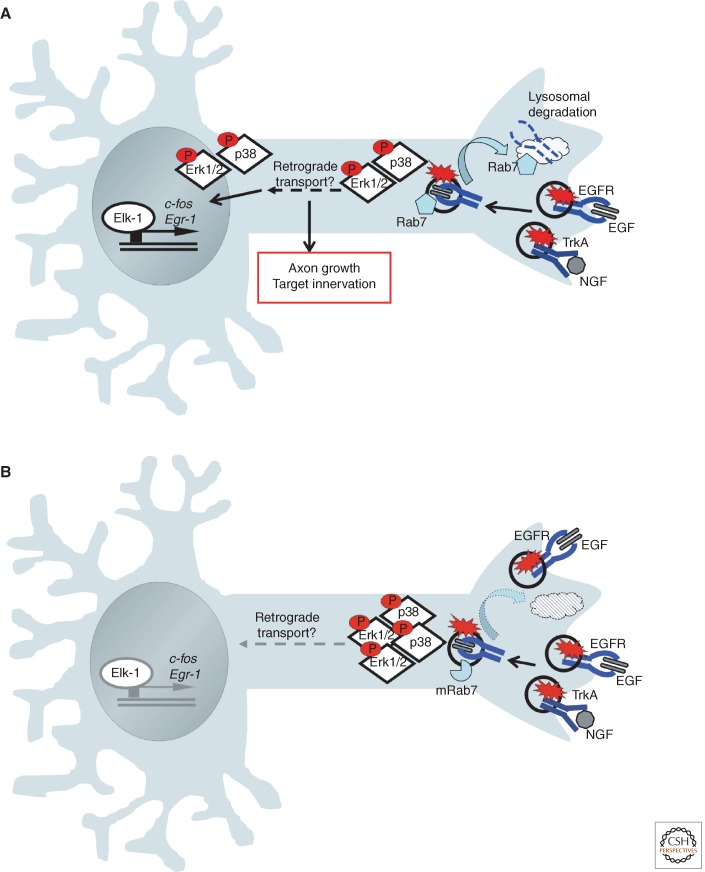
Charcot-Marie Tooth disease includes a large group of genetic disorders of the peripheral nervous system, characterized by progressive loss of motor and/or sensory innervation. Mutations that interfere with endosomal signaling are among the diverse abnormalities that can cause this disease. Autosomal dominant Charcot-Marie Tooth disease type 2B (CMT2B or hereditary motor and sensory neuropathy HMSN2B) is caused by mutations in the late endosomal Rab7 GTPase. Intriguingly, recent studies have linked the neuronal pathology to changes in receptor trafficking and resultant changes in intracellular signaling events (Fig. 3A,B). Expression of the CMT2B Rab7 mutants found in human patients (L129F, K157N, N161T, and V162M) leads to increased TrkA phosphorylation following NGF stimulation, and subsequently results in enhanced Erk1/2 activation (BasuRay et al. 2010). Similarly, mutant proteins delay trafficking of EGFR to the lysosome and so slow down the process of receptor degradation, causing enhanced EGFR signaling, and increased p38 and Erk1/2 activation (BasuRay et al. 2013). Interestingly, despite the increased receptor activation seen in response to NGF or EGF, the ability of the activated Erk1/2 to translocate into the nucleus was attenuated by the Rab7 mutants. It is not yet understood how mutant Rab7 affects nuclear translocation of Erk1/2, nor how the mutant Rab7 causes a subsequent decrease in expression of immediate early genes like c-fos and Egr-1. These studies provide a mechanistic link between alterations in signaling from endosomes and the neuronal pathology seen in CMT2B patients.
Figure 3.
In Charcot-Marie Tooth disease type 2B, a mutant form of Rab7 impairs growth factor receptor endocytosis and signaling. (A) In PC12 cells, stimulation with EGF and NGF leads to activation of the Erk1/2 and p38 kinases and translocation of these kinases to the nucleus to activate the transcription factor Elk-1, and so promote gene expression of target genes required for axon growth and target innervation. (B) Expression of the CMT2B mutant form of Rab7 (mRab7) leads to increased pErk1/2 and p38 signaling owing to delayed trafficking of receptors to the lysosome for degradation. Mutant Rab7 attenuates the ability for Erk1/2 to translocate to the nucleus and so decreases expression of immediate early genes, c-fos and Egr-1.
Huntington’s Disease
The neurodegenerative disease Huntington’s is caused by polyglutamine (polyQ) expansions in the protein huntingtin. Therefore, the normal function of huntingtin protein has been a subject of great interest for many years. A clue to the pathogenesis of the disease came from the observation in mouse models that deletion of BDNF in the cortex results in striatal degeneration that resembles the pathology seen in Huntington’s disease (Baquet et al. 2004). Indeed, Jones and colleagues went on to further characterize these mice by gene expression profiling, and found that striatal gene expression observed in BDNF-depletion models resembled expression seen in human Huntington’s (Strand et al. 2007). Because cortical neurons synthesize the majority of BDNF for transport to the striatum (Altar et al. 1997), this suggested that degeneration in Huntington’s disease may be owing to a lack of trophic support by BDNF.
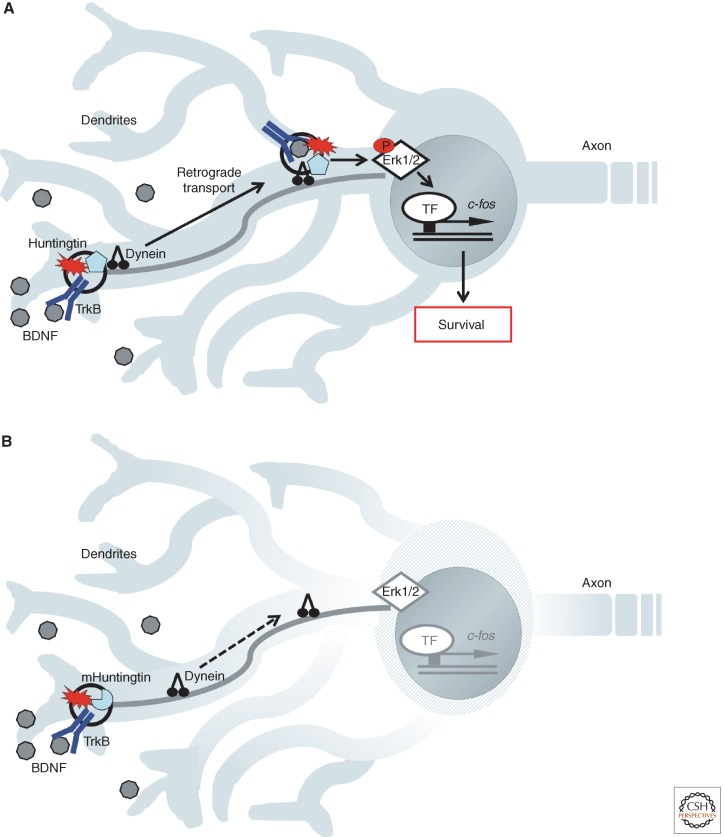
Huntingtin has been found to positively influence endosomal trafficking through interactions with the microtubule motors dynein and kinesin, and with actin-associated adaptor molecules (reviewed in Caviston and Holzbaur 2009; Caviston et al. 2011). Could it be that huntingtin is responsible for trafficking of BDNF and TrkB within a signaling endosome in cortical dendrites? A recent paper by Saudou and colleagues set out to study the role of huntingtin in retrograde BDNF-TrkB signaling within striatal dendrites as a mechanism for neuronal survival (Fig. 4A,B) (Liot et al. 2013). It is known that huntingtin mediates BDNF transport along microtubules through its association with huntingtin-associated protein (HAP-1) and the p150Glued subunit of dynactin (Gauthier et al. 2004). The investigators further found that TrkB binds to and colocalizes with huntingtin and with dynein. The polyQ expansion in huntingtin altered the binding of TrkB-containing vesicles to microtubules and reduced transport. In experiments using BDNF coupled to quantum dots in microfluidic devices, the investigators analyzed TrkB retrograde transport in response to BDNF applied to dendritic terminals of cortical neurons. They found that huntingtin is a critical regulator of TrkB retrograde trafficking and that signaling through ERK activation and c-fos induction were decreased in neurons from a Huntington’s disease mouse model (Liot et al. 2013). These studies show that huntingtin regulates not only the transport of BDNF itself, but also affects the trafficking of its ligand-bound receptor to ensure BDNF-TrkB-dependent survival signaling. Further investigation is needed to elucidate the precise nature of this retrograde dendritic signaling. It is possible that it is similar to the well-described retrograde survival signaling in axons. These two examples suggest that defects in signaling endosome mechanisms may be involved in many additional neurodegenerative diseases.
Figure 4.
Huntingtin mediates retrograde transport of TrkB-BDNF signaling endosomes in dendrites of striatal neurons. (A) Huntingtin is required for association of TrkB and BDNF with dynein motors and for appropriate retrograde signals that lead to Erk activation and c-fos induction. (B) Mutant huntingtin prevents retrograde transport of TrkB and BDNF and so leads to degeneration of striatal neurons as observed in Huntington’s disease.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
Endocytosis is an essential and highly conserved cellular mechanism. The importance of endocytosis is readily apparent in the nervous system, where endosomal trafficking and signaling are critical for organization, development, and function of morphologically complex neurons. In this review we have described a number of specialized adaptations of endosomal pathways that provide mechanisms for long-distance signaling in neurons and also contribute to the specificity of responses. Meanwhile, active investigation is still under way to further define the multiple cell-type-specific and cargo-specific mechanisms that underlie endosome trafficking. This includes expanding our understanding of the modes of endocytosis that contribute to formation of signaling endosomes in neurons and how precise sorting and trafficking of endosomes influence their spatial and temporal signaling profiles.
Given the level of regulation required for endosomal signaling, from sequential maturation of vesicles, to cargo selection and motor transport, it is not surprising that defects affecting even just one of these events can have devastating consequences on the health and functioning of neurons. We have described two disease situations in which mutations in distinct proteins involved in membrane trafficking both lead to neurodegeneration and disease. However, the scope of the role of endocytosis in nervous system disease has yet to be fully elucidated. New evidence for mutations in NHE6 and NHE9, which are involved in endocytotis, has been found in autistic patients and may provide insights into endocytic mechanisms required for proper brain function (Schwede et al. 2013). Furthermore, trafficking defects have been described in Alzheimer’s disease, with early endosomal compartments being affected in degenerating cholinergic neurons (Choi et al. 2013). In addition, the role of endocytosis following neuronal injury is relatively unexplored, and could provide great therapeutic potential for functional repair. There remain many questions and challenges still to be addressed for a full understanding of the scope and intricacies of endosomal signaling within the nervous system. Further understanding of the regulatory roles of endocytosis and endosomal trafficking in proper nervous system development and functioning will provide insights into both neurodevelopment and neurodegenerative diseases that may lead to targeted approaches for treatment.
ACKNOWLEDGMENTS
We apologize to those authors whose work was not included in this review owing to space restraints. Our thanks go to Sara Fenstermacher, Sarah Pease, Maria Pazyra-Murphy, and Mark McClintock for helpful comments and editing. We thank the National Institutes of Health (R01 NS050674) for funding.
Footnotes
Editors: Sandra L. Schmid, Alexander Sorkin, and Marino Zerial
Additional Perspectives on Endocytosis available at www.cshperspectives.org
REFERENCES
- Abe N, Inoue T, Galvez T, Klein L, Meyer T 2008. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J Cell Sci 121: 1488–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ 1997. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389: 856–860 [DOI] [PubMed] [Google Scholar]
- Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV 2006. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron 50: 549–559 [DOI] [PubMed] [Google Scholar]
- Ascano M, Bodmer D, Kuruvilla R 2012. Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol 22: 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR 2004. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci 24: 4250–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BasuRay S, Mukherjee S, Romero E, Wilson MC, Wandinger-Ness A 2010. Rab7 mutants associated with Charcot-Marie-Tooth disease exhibit enhanced NGF-stimulated signaling. PLoS ONE 5: e15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BasuRay S, Mukherjee S, Romero EG, Seaman MN, Wandinger-Ness A 2013. Rab7 mutants associated with Charcot-Marie-Tooth disease cause delayed growth factor receptor transport and altered endosomal and nuclear signaling. J Biol Chem 288: 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson FL, Pomeroy SL, Zhang YZ, Stiles CD, Segal RA 2002. High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. J Neurobiol 51: 302–312 [DOI] [PubMed] [Google Scholar]
- Bodmer D, Ascano M, Kuruvilla R 2011. Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth. Neuron 70: 1085–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL 2009. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol 19: 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Zajac AL, Tokito M, Holzbaur EL 2011. Huntingtin coordinates the dynein-mediated dynamic positioning of endosomes and lysosomes. Mol Biol Cell 22: 478–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM 2011. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci 108: 18430–18435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Kaur G, Mazzella MJ, Morales-Corraliza J, Levy E, Mathews PM 2013. Early endosomal abnormalities and cholinergic neuron degeneration in amyloid-β protein precursor transgenic mice. J Alzheimers Dis 34: 691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M 1999. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252 [DOI] [PubMed] [Google Scholar]
- Cosker KE, Courchesne SL, Segal RA 2008. Action in the axon: Generation and transport of signaling endosomes. Curr Opin Neurobiol 18: 270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Pazyra-Murphy MF, Fenstermacher SJ, Segal RA 2013. Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. J Neurosci 33: 5195–5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. 1994. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76: 1001–1011 [DOI] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC 2003. NGF signaling in sensory neurons: Evidence that early endosomes carry NGF retrograde signals. Neuron 39: 69–84 [DOI] [PubMed] [Google Scholar]
- Dixit R, Levy JR, Tokito M, Ligon LA, Holzbaur EL 2008. Regulation of dynactin through the differential expression of p150Glued isoforms. J Biol Chem 283: 33611–33619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT 2009. Mechanisms of endocytosis. Annu Rev Biochem 78: 857–902 [DOI] [PubMed] [Google Scholar]
- Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, et al. 2009. DCTN1 mutations in Perry syndrome. Nat Genet 41: 163–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher SJ, Rappoport JZ 2010. Moving forward: Polarised trafficking in cell migration. Trends Cell Biol 20: 71–78 [DOI] [PubMed] [Google Scholar]
- Francis NI, Farinas I, Brennan C, Rivas-Plata K, Backus C, Reichardt L, Landis S 1999. NT3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev Biol 210: 411–427 [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, et al. 2004. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118: 127–138 [DOI] [PubMed] [Google Scholar]
- Grimes ML, Zhou J, Beattie EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC 1996. Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci 16: 7950–7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes ML, Beattie E, Mobley WC 1997. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci 94: 9909–9914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK 2008. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol 181: 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Ginty DD 2013. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci 14: 177–187 [DOI] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD 2011. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell 146: 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen HM, Pazyra MF, Segal RA 2004. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci 7: 596–604 [DOI] [PubMed] [Google Scholar]
- Horgan CP, McCaffrey MW 2011. Rab GTPases and microtubule motors. Biochem Soc Trans 39: 1202–1206 [DOI] [PubMed] [Google Scholar]
- Howe CL, Valletta JS, Rusnak AS, Mobley WC 2001. NGF signaling from clathrin-coated vesicles: Evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32: 801–814 [DOI] [PubMed] [Google Scholar]
- Hupalowska A, Miaczynska M 2012. The new faces of endocytosis in signaling. Traffic 13: 9–18 [DOI] [PubMed] [Google Scholar]
- Kelly EE, Horgan CP, Goud B, McCaffrey MW 2012. The Rab family of proteins: 25 years on. Biochem Soc Trans 40: 1337–1347 [DOI] [PubMed] [Google Scholar]
- Kim H, Ling SC, Rogers GC, Kural C, Selvin PR, Rogers SL, Gelfand VI 2007. Microtubule binding by dynactin is required for microtubule organization but not for cargo transport. J Cell Biol 176: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag CE, Malmberg K, Salcini AE 2010. PI3KC2α, a class II PI3K, is required for dynamin-independent internalization pathways. J Cell Sci 123: 4240–4250 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Ye H, Ginty DD 2000. Spatially and functionally distinct roles of the PI3K effector pathway during NGF signaling in sympathetic neurons. Neuron 27: 499–512 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD 2004. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118: 243–255 [DOI] [PubMed] [Google Scholar]
- Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, Bronfman FC 2013. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci 33: 6112–6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liot G, Zala D, Pla P, Mottet G, Piel M, Saudou F 2013. Mutant Huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. J Neurosci 33: 6298–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I 1996. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12: 575–625 [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, Blasier KR, Jeffery ED, Ross MW, Pullikuth AK, Suo D, Park J, Smiley WR, Lo KW, Shabanowitz J, et al. 2012. Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport. J Neurosci 32: 15495–15510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren OL, Galletta BJ, Cooper JA 2012. Roles for actin assembly in endocytosis. Annu Rev Biochem 81: 661–686 [DOI] [PubMed] [Google Scholar]
- Moughamian AJ, Holzbaur EL 2012. Dynactin is required for transport initiation from the distal axon. Neuron 74: 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SR, Ghosh N, Maxfield FR 1997. Endocytosis. Physiol Rev 77: 759–803 [DOI] [PubMed] [Google Scholar]
- Ng EL, Tang BL 2008. Rab GTPases and their roles in brain neurons and glia. Brain Res Rev 58: 236–246 [DOI] [PubMed] [Google Scholar]
- Pazyra-Murphy MF, Hans A, Courchesne SL, Karch C, Cosker KE, Heerssen HM, Watson FL, Kim T, Greenberg ME, Segal RA 2009. A retrograde neuronal survival response: Target-derived neurotrophins regulate MEF2D and bcl-w. J Neurosci 29: 6700–6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu MM, Moughamian AF, Holzbaur EL 2010. Retrograde axonal transport: Pathways to cell death? Trends Neurosci 33: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. 2003. Mutant dynactin in motor neuron disease. Nat Genet 33: 455–456 [DOI] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM Jr 1999. BAX translocation is a critical event in neuronal apoptosis: Regulation by neuroprotectants, BCL-2, and caspases. J Neurosci 19: 7476–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Ruckert C, Saenger W, Haucke V 2012. The early steps of endocytosis: From cargo selection to membrane deformation. Eur J Cell Biol 91: 226–233 [DOI] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, Ginty DD 1997. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science 277: 1097–1100 [DOI] [PubMed] [Google Scholar]
- Sadakata T, Furuichi T 2010. Ca2+-dependent activator protein for secretion 2 and autistic-like phenotypes. Neurosci Res 67: 197–202 [DOI] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P 2012. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol 4: a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede M, Garbett K, Mirnics K, Geschwind DH, Morrow EM 2013. Genes for endosomal NHE6 and NHE9 are misregulated in autism brains. Mol Psychiatry 10.1038/mp.2013.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA 2003. Selectivity in neurotrophin signaling: Theme and variations. Annu Rev Neurosci 26: 299–330 [DOI] [PubMed] [Google Scholar]
- Shao Y, Akmentin W, Toldeo-Aral JJ, Rosenbaum J, Valdez G, Cabot JB, Hilbush BS, Halegoua S 2002. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. J Cell Biol 157: 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh JC, Schaar BT, Srinivasan K, Brodsky FM, McConnell SK 2011. Endocytosis regulates cell soma translocation and the distribution of adhesion proteins in migrating neurons. PLoS ONE 6: e17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, Beal MF, Jones L, Kooperberg C, Olson JM, et al. 2007. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci 27: 11758–11768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien CB, Langdon WY 2001. Cbl: Many adaptations to regulated protein tyrosine kinases. Nat Rev Mol Cell Biol 2: 294–307 [DOI] [PubMed] [Google Scholar]
- Valdez G, Akmentin W, Philippidou P, Kuruvilla R, Ginty DD, Halegoua S 2005. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J Neurosci 25: 5236–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Philippidou P, Rosenbaum J, Akmentin W, Shao Y, Halegoua S 2007. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proc Natl Acad Sci 104: 12270–12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD 2003. The molecular toolbox for intracellular transport. Cell 112: 467–480 [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Williams JT 2012. Modulating neuromodulation by receptor membrane traffic in the endocytic pathway. Neuron 76: 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Chan CC, Cherry S, Hiesinger PR 2012. Membrane trafficking in neuronal maintenance and degeneration. Cell Mol Life Sci 70: 2919–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA 1999. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci 19: 7889–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Winckler B 2012. Harnessing the power of the endosome to regulate neural development. Neuron 74: 440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Twelvetrees AE, Lazarus JE, Blasier KR, Yao X, Inamdar NA, Holzbaur EL, Pfister KK, Xiang X 2013. Establishing a novel knock-in mouse line for studying neuronal cytoplasmic dynein under normal and pathologic conditions. Cytoskeleton (Hoboken) 70: 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA 2007. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron 55: 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Alfaro J, Chang EH, Zhao X, Porcionatto M, Segal RA 2011. Numb links extracellular cues to intracellular polarity machinery to promote chemotaxis. Dev Cell 20: 610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]