Abstract
The endosomal network comprises an interconnected network of membranous compartments whose primary function is to receive, dissociate, and sort cargo that originates from the plasma membrane and the biosynthetic pathway. A major challenge in cell biology is to achieve a thorough molecular description of how this network operates, and in so doing, how defects contribute to the etiology and pathology of human disease. We discuss the increasing body of evidence that implicates an ancient evolutionary conserved complex, termed “retromer,” as a master conductor in the complex orchestration of multiple cargo-sorting events within the endosomal network.
Retromer plays a key role in exporting cargo from the endosomal system and is implicated in other facets of endosome biology. One of its subunits, the cargo-selective complex, has been strikingly conserved throughout evolution.
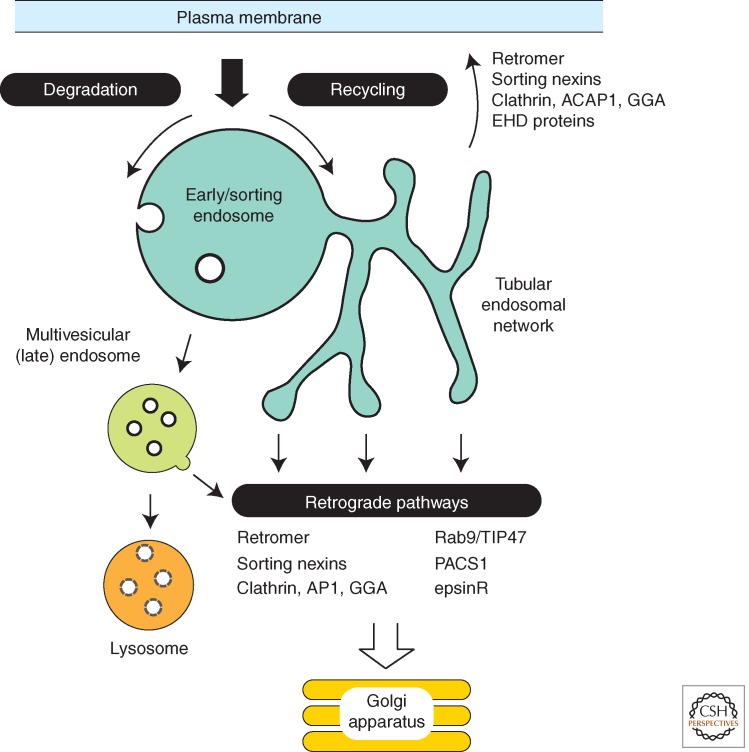
On entering the endosomal network, integral membrane protein cargoes have two fates: either they are sorted from the limiting endosomal membrane into intraluminal vesicles for delivery into the lysosomal degradative pathway (Fig. 1), or they are exported from the endosome via transport carriers that bud from the endosome membrane into the cytoplasm. The endosome export pathways ultimately deliver cargo to the trans-Golgi network (TGN) via retrograde pathways, or to the plasma membrane via recycling pathways (Huotari and Helenius 2011). The principal cargoes of the retrograde pathways are sorting receptors, SNAREs, and other molecules whose functions depend on continual retrieval from the endosomal system back to the biosynthetic pathway (Bonifacino and Rojas 2006; Johannes and Popoff 2008; Burd 2011). Plasma membrane recycling cargoes are especially diverse in structure, as they include numerous nutrient transporters, mitogenic signaling receptors, and cell adhesion receptors (Maxfield and McGraw 2004; Hsu et al. 2012). Although much has been learned regarding signals and sorting mechanisms that confer sorting into the degradative pathway, these aspects of endosome export pathways are poorly understood. There are therefore significant gaps in our understanding of how the endosomal system is integrated with cell physiology and the development of multicellular organisms, and how deficiencies in endosomal sorting contribute to human disease. Here, rather than give a broad overview of the field (we refer the interested reader to many excellent recent reviews: Grant and Donaldson 2009; Henne et al. 2011; Huotari and Helenius 2011; Johannes and Wunder 2011; Hsu et al. 2012), we discuss the role of an evolutionary conserved complex, termed “retromer,” that plays a key role in exporting cargo from the endosomal system and is increasingly implicated in many other facets of endosome function.
Figure 1.
Overview of endosomal sorting pathways. Cargo in the endosome is sorted either into the degradative pathway, leading to its eventual delivery to the lysosome, or into an export pathway that returns it to another organelle for reuse. Recycling export pathways deliver cargo to the plasma membrane, and retrograde export pathways return cargo to the trans-Golgi network. Sorting devices that act in each of these export pathways are listed. Transport carriers of the export pathways bud from the tubular endosomal network, whereas sorting into the lysosomal degradation pathway results in packaging of cargo into vesicles that bud into the lumen of multivesicular endosomes.
CARGO EXPORT FROM THE ENDOSOME
Early studies of endocytosis and lysosomal turnover indicated that the turnover rates for cell-surface receptors and their ligands vary substantially, with that of transmembrane receptors being relatively long-lived, and ligands short-lived. This led to the discovery of plasma membrane recycling pathways, whereby internalized receptor–ligand complexes dissociate in the acidic lumen of the endosome and the receptor then exits the endosome with the bulk flow of membrane lipids that are returned to the plasma membrane (Maxfield and McGraw 2004; Hsu et al. 2012). For the plasma membrane recycling of the transferrin receptor (TfnR), one of the most intensively investigated recycling cargoes, initial ideas evoked a sorting signal-independent model in which iterative formation of an extensive network of endosomal tubules ensured that bulk membrane flow occurred through exit from the endocytic network (Mayor et al. 1993). Central to this proposal was the geometric consideration that the relatively large ratio of membrane surface area-to-luminal volume of the tubular endosomal network (TEN), when compared with the vacuolar domain of the endosome, effectively serves to segregate integral membrane proteins from luminal content by default (Maxfield and McGraw 2004). It is now clear, however, that the majority of endocytic recycling is mediated in a signal-dependent manner through the engagement of specific sorting signals by a variety of sorting devices (Hsu et al. 2012). Signal-mediated sorting ensures robust endosome function and allows for detailed regulation in response to changes in the cellular environment.
Endosomal cargo export pathways originate from multiple, functionally distinct gateways along the entire endosome maturation pathway. Plasma membrane recycling is mediated by clathrin-containing coat complexes, including clathrin-ACAP1 (that recognizes LF, RF, KR, and PLSLL sequences) and clathrin-GGA3 (Prorich motifs), that recognize cell adhesion receptors and nutrient transporters (Dai et al. 2004; Li et al. 2007; Parachoniak et al. 2011). Similarly, other, less well described sorting factors such as sorting nexin-17 (SNX17) (NPxY, NxxY, and NxxF sequences), EHD proteins (NPF motif), and sorting nexin-27 (SNX27) (PDZ ligands) also contribute to plasma membrane recycling (Burden et al. 2004; Joubert et al. 2004; Braun et al. 2005; van Kerkhof et al. 2005). Endosome-to-TGN retrograde sorting is similarly complex, with contributions by clathrin in conjunction with AP1, PACS1, and epsinR (Meyer et al. 2000; Crump et al. 2001; Saint-Pol et al. 2004; Johannes and Popoff 2008; Shiba et al. 2010; Johannes and Wunder 2011). In addition, export of the mannose-6-phosphate receptor from the late endosome appears to be regulated by the TIP47/Rab9 assembly (Lombardi et al. 1993; Díaz and Pfeffer 1998; Carroll et al. 2001; also see Bulankina et al. 2009).
THE RETROMER COMPLEX
Pioneering studies of protein sorting in the yeast endolysosomal system led to the identification of an endosomal coat protein complex named “retromer” that was found to be required for retrieval of a TGN sorting receptor (Vps10) from the endosome to the TGN (Seaman et al. 1998). The initial biochemical characterization of yeast retromer showed that it is composed of five proteins that can be dissociated into two subcomplexes (Horazdovsky et al. 1997; Seaman et al. 1997, 1998). One subcomplex contains a heterodimer of the Vps5 and Vps17 sorting nexins (SNX), which possess Bin/Amphiphysin/Rvs (BAR) domains (SNX-BAR proteins) that can induce and/or sense the formation of membrane tubules (Carlton et al. 2004; van Weering et al. 2012a). The other retromer subunits constitute a trimeric complex of Vps26, Vps29, and Vps35 that does not have intrinsic membrane-binding activity and relies on association with the Vps5-Vps17 for endosome recruitment (Seaman et al. 1998; Haft et al. 2000; Liu et al. 2012), or, independent of Vps5-Vps17, on association with the Rab7 ortholog, Ypt7, for recruitment to the vacuole membrane (Liu et al. 2012). The VPS26:VPS29:VPS35 heterotrimer has been shown to recognize cargo proteins and is therefore termed the cargo-selective complex (abbreviated “CSC”) (Seaman et al. 1998; Nothwehr et al. 2000; Norwood et al. 2011; Fjorback et al. 2012).
Whereas there is considerable divergence of sorting nexin proteins between species, CSC is an ancient protein assembly that emerged before the last common eukaryotic ancestor and has been strikingly conserved throughout eukaryotic evolution (Koumandou et al. 2011). Hence, the CSC trimer is considered to constitute the core functional component of retromer. Structural and sequence-based analysis predicts that human VPS35 is composed of 17 two-helix repeats that fold into an α-solenoid that is typical of vesicle coat proteins (Hierro et al. 2007). Mammals express two Vps26 orthologs, VPS26A and VPS26B (Kerr et al. 2005), that possess an arrestin fold and bind to the highly conserved amino-terminal region of VPS35 (Shi et al. 2006; Collins et al. 2008). VPS29 possesses a metallophosphoesterase fold (Collins et al. 2005; Wang et al. 2005) and it binds to the carboxy-terminal portion of VPS35 (Collins et al. 2008; Norwood et al. 2011) where it is proposed to scaffold the helical solenoid of VPS35. Very little is known regarding the manner by which CSC recognizes cargo. Most retromer cargoes possess at least one simple hydrophobic motif, F/W-L-M/V, required for retromer-dependent sorting (Seaman 2007), and Vps35 has long been considered to provide the sole interface for cargo recognition. However, very recently, VPS26 was shown to bind a FANSHY sorting signal in the cytoplasmic domain of sorLA (Fjorback et al. 2012), and other proteins that associate with CSC, including sorting nexin-3 (SNX3), SNX27, and the retromer SNX-BARs, have been implicated in cargo recognition (Parks et al. 2001; Heydorn et al. 2004; Strochlic et al. 2007; Temkin et al. 2011; Steinberg et al. 2013). It is now appreciated that there exist multiple direct and indirect mechanisms by which CSC recognizes cargo.
RETROMER AND THE ENDOSOME MATURATION PATHWAY
By promoting the export of cargo from the endosome, retromer serves to “rescue” proteins from lysosome-mediated turnover and, hence, retromer function is intrinsically coupled to the endosome maturation pathway. Endosomal maturation itself is defined by an increasing number of intraluminal vesicles as the early endosome matures into the late endosome/multivesicular body, an increase in luminal acidification, endosome movement from the cell periphery toward a juxtanuclear localization, and the switching of two key endosome identity cues (Jean and Kiger 2012): the Rab5-positive, phosphatidylinositol-3-monophosphate (PtdIns(3)P)-enriched early endosome switches to become a Rab7-positive, phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2)-enriched late endosome (Huotari and Helenius 2011).
The CSC does not itself bind to endosomes; instead, association to early and maturing late endosomes occurs through a variety of indirect mechanisms. The specific binding of SNX3 to PtdIns(3)P targets the CSC to early endosomes (Harterink et al. 2011; Vardarajan et al. 2012), whereas the ability of VPS35 to bind Rab7-GTP associates the CSC with late endosomes (Nakada-Tsukui et al. 2005; Rojas et al. 2008; Seaman et al. 2009; Balderhaar et al. 2010; Liu et al. 2012; Zelazny et al. 2013). Rab7 (Ypt7 in yeast) is a principal regulator of late endosome dynamics, and the interaction with VPS35 appears to serve a dual function in recruiting and/or stabilizing CSC on the endosome membrane and in coordinating the timing of cargo export with endosome maturation. In yeast cells, depletion of Ypt7-GTP results in a deficiency in cargo export, even though CSC is assembled with SNX-BARs on the endosome membrane, suggesting that Ypt7 potentiates retromer-mediated cargo export (Liu et al. 2012). In further support for a role in Rab7 regulating retromer activity, in mammalian cells SNX-BAR-coated tubules bud predominantly from endosomes that have recently acquired Rab7 via Rab conversion (van Weering et al. 2012b). In yeast, CSC has been shown to inhibit Ypt7-regulated fusion of late endolysosomal organelles, suggesting that it can also influence endosome maturation indirectly by modulating the amount of Ypt7-GTP (Liu et al. 2012). In mammalian cells, CSC limits Rab7 signaling through the recruitment of TBC1D5, a potential Rab7 GAP (Mukhopadhyay et al. 2007), to the endosome via direct binding to Vps29 (Seaman et al. 2009; Harbour et al. 2010). Interestingly, TBC1D5 also directly associates with the small ubiquitin-like protein LC3, a marker for autophagic membranes (Kabeya et al. 2000) and during starvation-induced autophagy TBC1D5 is relocalized from endosomes to LC3-positive autophagosomes (Popovic et al. 2012). As Rab7-GTP is required for autophagosome maturation, it is tempting to speculate that competitive binding of TBC1D5 to CSC or LC3 might serve to reprogram endosomal sorting to accommodate the ensuing autophagic flux to the lysosome.
THE DIVERSITY OF RETROMER FUNCTION IN CELL FUNCTION AND ORGANISM PHYSIOLOGY
Biochemical and genetic studies have identified a wide array of cargoes and cellular processes that require the CSC including the establishment of cell polarity through trafficking of Crumbs and Scribble (Pocha et al. 2011; Zhou et al. 2011; Lohia et al. 2012), regulation of the morphology and geometry of epithelial tubes during Drosophila development through transport of Serpentine (Dong et al. 2013), formation of Wnt morphogenic gradients through transport of the Wnt sorting receptor Wntless (Belenkaya et al. 2008; Franch-Marro et al. 2008; Pan et al. 2008; Port et al. 2008; Yang et al. 2008), transcytosis of the polymeric immunoglobulin receptor (Vergés et al. 2004), apoptotic cell clearance through sorting of the Caenorhabditis elegans phagocytic receptor CED-1 (Chen et al. 2010), cell polarity and organ initiation through trafficking of the Arabidopsis thaliana PIN auxin efflux carriers (Jaillais et al. 2007), and mitochondria to peroxisome transport through association with the mitochondrial-anchored protein ligase MAPL (Braschi et al. 2010). Moreover, there is increasing realization that perturbed CSC function is linked to a number of human diseases. CSC deficiency plays a role in nonamyloidogenic versus amyloidogenic processing of amyloid precursor protein (APP) (He et al. 2005; Small et al. 2005; Muhammad et al. 2008; Wen et al. 2011; Choy et al. 2012) and the pathogenesis of Alzheimer’s disease (Siegenthaler and Rajendran 2012). In addition, a specific VPS35 mutation, Asp620Asn, has been linked to late-onset autosomal-dominant familial Parkinson’s disease (Vilariño-Güell et al. 2011; Zimprich et al. 2011), the loci for human VPS26A has been genetically associated with type 2 diabetes in South Asians (Kooner et al. 2011), and the endosomal transport of various pathogens (Salmonella, Herpesvirus saimiri, Coxiella burnetii, human papillomavirus, Legionella pneumophila) and toxins (Shiga toxin) is also mediated in part by the CSC (Bujny et al. 2007, 2008; Popoff et al. 2007; Kingston et al. 2011; Finsel et al. 2013; Lipovsky et al. 2013; McDonough et al. 2013).
FUNCTIONALLY DISTINCT RETROMER COMPLEXES
It is now appreciated that retromer CSC functions as a component of multiple distinct sorting devices. The first indication of the diversity of retromer in multicellular organisms came from a biochemical and genetic study of the role of retromer in regulating the retrograde endosome-to-TGN transport of Wntless, and hence the formation of Wnt morphogenic gradients in C. elegans and Drosophila (Belenkaya et al. 2008; Franch-Marro et al. 2008; Pan et al. 2008; Port et al. 2008; Yang et al. 2008). Here the functional retromer is composed of the direct binding of the CSC to the early endosome associated non-BAR domain-containing SNX3, an association that is independent of the SNX-BAR retromer subunits (Harterink et al. 2011; Zhang et al. 2011). Genetic loss of SNX3 or CSC in C. elegans and Drosophila, leads to the missorting of internalized Wntless into the lysosomal degradative pathway (this is also observed in mammalian cell culture upon RNAi-mediated suppression), a reduction in the steady-state level of Wntless, and a defect in Wnt secretion and hence Wnt gradient formation (Harterink et al. 2011). Thus, although the SNX3 retromer and SNX-BAR retromer both mediate endosome-to-TGN transport, they constitute distinct, cargo-specific pathways. Indeed, in C. elegans, the retromer-mediated sorting of CED-1 is SNX-BAR-retromer dependent but is independent of the SNX3 retromer (Chen et al. 2010; Harterink et al. 2011; Lu et al. 2011). Furthermore, the morphological profile of each transport carrier is distinct: SNX-BAR retromer residing on nonbranched tubules 170–230 nm in length and 20–50 nm in diameter (Mari et al. 2008), whereas SNX3 retromer is associated with small clathrin-coated vesicular profiles (Harterink et al. 2011). These results indicate that there are distinct forms of retromer that are defined via CSC associations with different sorting nexins and that these retromers act on distinct sets of cargo.
Besides its well-established roles in endosome-to-TGN trafficking, it has recently emerged that retromer also plays a central role in endosome-to-plasma membrane recycling (Feinstein et al. 2011; Temkin et al. 2011). In mammalian cells, the endosomal recycling of the β2-adrenergic receptor (β2-AR) is dependent on the presence of a carboxy-terminal PDZ ligand that acts as a sorting signal for direct recycling to the plasma membrane (Cao et al. 1999). The PDZ domain of SNX27 binds directly to this signal, and by engaging the CSC mediates β2-AR recycling through SNX-BAR-retromer decorated tubules (Lauffer et al. 2010; Temkin et al. 2011). Indeed, the CSC constitutes a major endosome recycling hub. In HeLa cells, the endosome-to-plasma membrane recycling of >150 transmembrane spanning proteins would appear to be dependent on the CSC (Steinberg et al. 2013). Of these, >70 cargos require the presence of SNX27 for their recycling, many of which contain PDZ ligands, whereas others contain NPxY/NxxY sorting motifs (Steinberg et al. 2013). Because SNX27 harbors a functional FERM-like domain that can engage NPxY/NxxY-based sorting signals (Ghai et al. 2013), the cargo adaptor function of SNX27 in retromer-mediated recycling appears to embrace both PDZ ligand and NPxY/NxxY sorting motif recognition. Like the situation with the SNX3 retromer, loss of SNX27 or CSC leads to cargo missorting into lysosomal-mediated degradation (Temkin et al. 2011; Steinberg et al. 2013). This reinforces that a primary role of the CSC, and its associated cargo adaptors, is to prevent missorting of selected cargoes into the lysosomal degradative pathway.
Interestingly, retromer-mediated recycling of the β2-AR occurs through a specialized actin-stabilized tubular subdomain (Puthenveedu et al. 2010). Consistent with this, SNX27 lies at the core of a multiprotein complex defined by the binding to retromer, and the independent association with the retromer SNX-BARs and the Wiskott-Aldrich syndrome protein and SCAR homolog (WASH) (Temkin et al. 2011; Steinberg et al. 2013), which by regulating the activity of the Arp2/3 complex mediates the formation of branched actin filaments on the endosomal network (Derivery et al. 2009; Gomez and Billadeau 2009). Given the specific function of each individual component of this multiprotein SNX27-containing complex, one intriguing question is: How does retromer coordinate cargo capture and enrichment with the extensive membrane remodeling that is required to generate a cargo-enriched transport carrier?
COUPLING RETROMER SORTING TO CARGO EXPORT
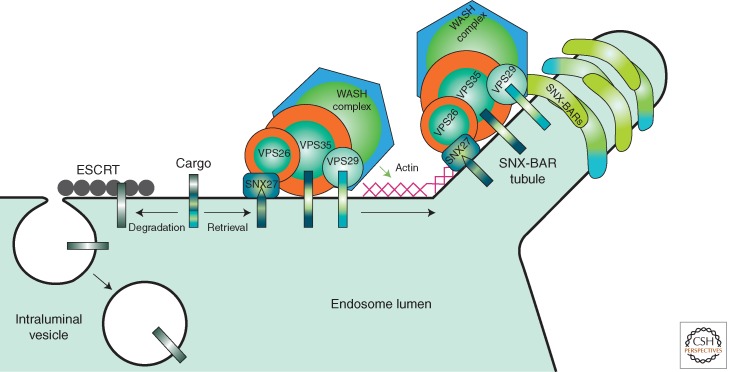
Export of cargo from the endosome by SNX-BAR retromer is initiated by the capture of cargo by CSC and other associated adaptors on the vacuolar portion of the endosome thereby preventing their entry into the lysosomal degradative pathway. A key consideration for understanding CSC function is that the functional units of membranes of the endocytic system are specialized microdomains, defined as assemblies of cargo proteins, lipids, and peripherally associated proteins, that partition into discrete networks within the plane of the membrane (Huotari and Helenius 2011). We speculate that CSC defines a subdomain(s) within the endosome membrane that promotes the efficient capture and enrichment of cargo, preventing their entry into the lysosomal degradative pathway and preparing them for subsequent inclusion into the TEN, or for the case of SNX3 retromer, vesicular transport carriers (Fig. 2). Cargo capture takes place on the vacuolar portion of the endosome, which is a region that is essentially flat (at the molecular scale), and subsequent events direct captured cargo into tubules of high relative membrane curvature that are coated with SNX-BAR retromer. Central to the formation of the TEN is the intrinsic ability of a subset of SNX-BAR proteins to self-assemble via interactions involving the BAR domain that can drive and/or stabilize the formation of membrane tubules (Carlton et al. 2004; Wassmer et al. 2007, 2009; van Weering et al. 2012a). In this respect, SNX-BAR proteins constitute an endosomal coat complex (Attar and Cullen 2010; Cullen and Korswagen 2012; Seaman 2012) and a major challenge for the field is to elucidate the mechanism(s) by which CSC orchestrates cargo capture and enrichment with packaging into the formation of these tubular transport carriers.
Figure 2.
SNX-BAR-retromer-mediated cargo exit from lysosomal-mediated degradation. The VPS26:VPS29: VPS35 CSC engage cargo in a signal-dependent manner. Through WASH-mediated actin polymerization a cargo-enriched endosome subdomain is assembled thereby avoiding ESCRT-mediated sorting into forming intraluminal vesicles. Subsequent transport into recycling pathways back to the plasma membrane or retrograde transport to the TGN is mediated through retromer SNX-BAR-mediated tubule formation. Actin polymerization may aid further membrane remodeling and the efficiency of tubule scission to form a cargo-enriched tubular transport carrier.
Although retromer function opposes sorting into the degradative lysosomal pathway, the emerging principles of degradative sorting may be instructive for understanding CSC-dependent packaging of cargo into the TEN. In the degradative pathway, cargo is sorted into vesicles that bud into the lumen of the endosome. Central to our understanding of sorting into intraluminal vesicles is the notion that cargo is first captured in a signal-dependent manner and enriched within a subdomain of the endosomal limiting membrane from where the generation of negative membrane curvature (i.e., bending the endosomal membrane away from the cytosol) occurs concomitantly with the cargo entering intraluminal vesicles (ILVs) (Hurley and Hanson 2010; Henne et al. 2011). Degradative cargo is captured by a cargo-sorting receptor termed “endosomal sorting complex required for transport-0 (ESCRT-0)” and their lateral mobility restricted within an endosomal subdomain defined by ESCRT-0 (Wollert and Hurley 2010) and the formation of a clathrin bilayer coat (Sachse et al. 2002). Subsequently, ESCRT-III promotes the budding of the ILV (Teis et al. 2010; Wollert and Hurley 2010; Henne et al. 2012). Unlike cytoplasmic transport vesicles, ILVs are not surrounded by a proteinaceous coat, implying that once cargo is captured, it enters the forming ILV by default. The insights from endosomal sorting complex required for transport (ESCRT)-dependent sorting point to the key function of establishing a domain of entrapped cargo that serves to direct it into the budding transport carrier. CSC serves a similar function to ESCRT-0 in the signal-dependent capture of cargo that is subsequently transferred into a tubule/vesicle that buds from the endosome into the cytoplasm. In this view, the cargo burden of the transport carrier is defined chiefly by CSC and its associated adaptors (Fig. 3), rather than the specific sorting nexin that functions in sculpting the transport carrier.
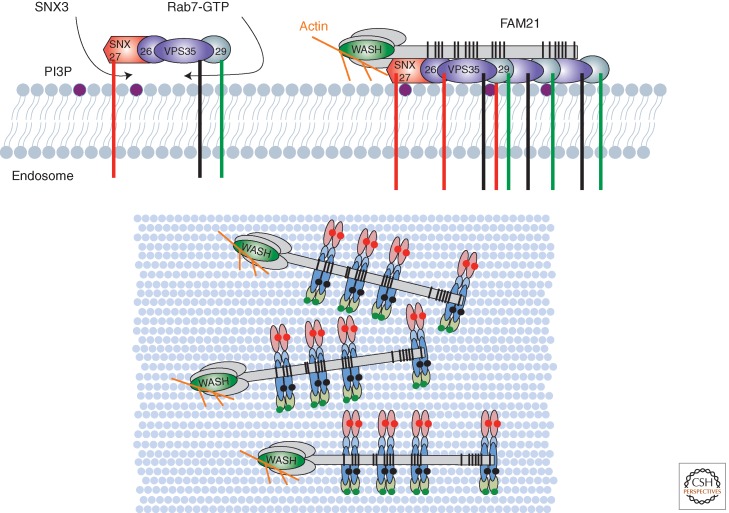
Figure 3.
A pictorial representation of the proposed CSC retrieval subdomain on the endosomal surface (based on data from Jia et al. 2012). The CSC is associated with the cytosolic face of the early endosome and the maturing late endosome through association with the phosphatidylinositol 3-monophosphate (PI3P)-binding SNX3 and Rab7-GTP, respectively. In addition to cargo selection mediated by the CSC, associated adaptors also engage cargo (the PI3P-binding SNX27 is depicted). Through association of the unstructured tail of FAM21, multiple CSCs, along with their associated cargoes, become enriched into a retrieval subdomain (retrieval zone) in which lateral mobility is restricted as a result of WASH-mediated actin polymerization. In the lower bird’s-eye view, two associated CSCs are shown to reflect evidence for dimeric assemblies (Norwood et al. 2011).
How does CSC mediate cargo sorting? One possibility is that it captures and concentrates cargo via a multivalent binding site to CSC proteins. Clear evidence for this is presently lacking, but it is in line with the lack of robust oligomerization of CSC in solution (Norwood et al. 2011). Emerging evidence suggests that actin dynamics on the endosome membrane, regulated by CSC, is a key factor that influences retromer-dependent sorting (Gomez and Billadeau 2009). The WASH complex is formed from the assembly of five proteins: strumpellin, FAM21, SWIP, ccdc53, and WASH (Derivery and Gautreau 2010). It is recruited to the endosome membrane through a complex mechanism that in part relies on the binding to the VPS35 CSC subunit (Gomez and Billadeau 2009; Harbour et al. 2010, 2012; Derivery et al. 2012; Jia et al. 2012; Helfer et al. 2013; Park et al. 2013). On the endosome membrane, the WASH complex activates the Arp2/3 complex, which mediates the nucleation of actin branching to a preexisting actin filament (Rotty et al. 2013). In cultured cells, loss of WASH results in collapse of the endolysosomal system into enlarged endosome-like compartments in which degradative cargo, recycling cargo, retrograde cargo, and lysosomal proteins accumulate (i.e., a missorting phenotype) (Duleh and Welch 2010; Derivery et al. 2012; Gomez et al. 2012; Piotrowski et al. 2013). Interestingly, the carboxy-terminal region of FAM21 possesses 21 copies of a motif distributed over nearly 1000 unstructured amino acids that is recognized by VPS35 (Jia et al. 2012). The multivalent nature of this interaction (i.e., one FAM21 can associate with multiple copies of CSC) has been suggested to effectively sense the density of CSC and associated cargo adaptors (and hence the density of captured cargo) thereby coordinating cargo capture and enrichment with the formation of a branched actin network that restricts lateral diffusion of the enriched cargo into other sorting pathways (Fig. 3) (Jia et al. 2012). The presence of actin subdomains on the endosome membrane is consistent with such a cargo “retrieval zone” (Sachse et al. 2002; Derivery et al. 2012), akin to the degradative sorting zone formed by ESCRT proteins. Whether these retrieval zones also act to modify the posttranslational status of captured cargo (e.g., ubiquitination and/or phosphorylation), is an interesting open question. Indeed, in higher metazoans VPS35 binds the E3 RING ubiquitin ligase, MAGE-L2-TRIM27, an interaction that localizes this enzyme to endosomes, and through K63-linked ubiquitination of WASH at lysine 220 relieves an autoinhibited WASH conformation leading to activation of endosomal actin polymerization (Hao et al. 2013). Loss of WASH also results in elongated SNX-BAR tubules, raising the possibility that a localized activation of WASH results in a burst of actin polymerization, spatially localized with the retrieval zone, that contributes to the efficiency of fission of sorting tubules coated with filamentous actin (Derivery et al. 2009; Gomez and Billadeau 2009; Puthenveedu et al. 2010). Clearly, understanding the interrelationship between retromer, cargo, and the activity of the WASH complex in regulating the dynamics of actin turnover is sure to generate exciting insight into the formation of sorting subdomains on the endosome membrane.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Although significant research will be required to further define the specific endosomal recycling and retrograde transport pathways, an increasingly important question becomes one of how degradative sorting versus recycling versus retrograde transport are coordinated to achieve a system capable of actively remodeling sorting itineraries in response to changing metabolic and environmental cues. By addressing this complex question much needed insight will be gained into the wiring between endosomal sorting and the varying physiological needs of cells in tissues and organs, and also a greater understanding of how deficiencies in endosomal sorting, either at the level of individual cargo or in the context of specific coat complexes and their associated protein assemblies, underlie a variety of human diseases. The problem is to identify those elements within the system that constitute the key “conductors” that broadly control endosome sorting. We consider that a growing body of evidence strongly points toward retromer, and in particular the CSC, as a vital evolutionary conserved conductor of sorting within the endosomal network. Exploring the transcriptional and translational control of this heterotrimer and the effects of posttranslation modification on its assembly, endosome association and wider protein:protein interactome are likely to generate significant new insight into the remodeling of endosomal sorting during cell function, organism development, and physiology, and perhaps most importantly, human health and disease.
ACKNOWLEDGMENTS
We thank members of our laboratories for helpful discussions and critical review of the manuscript, and Matthew Gallon for Figure 2. Work in the authors’ laboratories is supported by grants from the U.S. National Institutes of Health (GM061221 to C.B.), The Wellcome Trust (089928, 083474, and 083474 to P.J.C.), Biotechnology and Biological Sciences Research Council (BB/I011412/1 to P.J.C.), and Medical Research Council (MK/K018299/1 to P.J.C.).
Footnotes
Editors: Sandra L. Schmid, Alexander Sorkin, and Marino Zerial
Additional Perspectives on Endocytosis available at www.cshperspectives.org
REFERENCES
- Attar N, Cullen PJ 2010. The retromer complex. Adv Enzyme Regul 50: 216–236 [DOI] [PubMed] [Google Scholar]
- Balderhaar HJ, Arlt H, Ostrowicz C, Bröcker C, Sündermann F, Brandt R, Babst M, Ungermann C 2010. The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J Cell Sci 123: 4085–4094 [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X 2008. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 14: 120–131 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Rojas R 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 7: 568–579 [DOI] [PubMed] [Google Scholar]
- Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM 2010. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol 20: 1310–1315 [DOI] [PubMed] [Google Scholar]
- Braun A, Pinyol R, Dahlhaus R, Koch D, Fonarev P, Grant BD, Kessels MM, Qualmann B 2005. EHD proteins associate with syndapin I and II and such interactions play a crucial role in endosomal recycling. Mol Biol Cell 16: 3642–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujny MV, Popoff V, Johannes L, Cullen PJ 2007. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans-Golgi network. J Cell Sci 120: 2010–2021 [DOI] [PubMed] [Google Scholar]
- Bujny MV, Ewels PA, Humphrey S, Attar N, Jepson MA, Cullen PJ 2008. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole remodeling during Salmonella infection. J Cell Sci 121: 2027–2036 [DOI] [PubMed] [Google Scholar]
- Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Höning S 2009. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol 185: 641–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG 2011. Physiology and pathology of endosome-to-Golgi retrograde sorting. Traffic 12: 948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden JJ, Sun XM, García AB, Soutar AK 2004. Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J Biol Chem 279: 16237–16245 [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M 1999. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401: 286–290 [DOI] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ 2004. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol 14: 1791–800 [DOI] [PubMed] [Google Scholar]
- Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292: 1373–1376 [DOI] [PubMed] [Google Scholar]
- Chen D, Xiao H, Zhang K, Wang B, Gao Z, Jian Y, Qi X, Sun J, Miao L, Yang C 2010. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science 327: 1261–1264 [DOI] [PubMed] [Google Scholar]
- Choy RW, Cheng Z, Schekman R 2012. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid β (Aβ) production in the trans-Golgi network. Proc Natl Acad Sci 109: E2077–E2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, Skinner CF, Watson PJ, Seaman MN, Owen DJ 2005. Vps29 has a phosphoesterase fold that acts as a protein interaction scaffold for retromer assembly. Nat Struct Mol Biol 12: 594–602 [DOI] [PubMed] [Google Scholar]
- Collins BM, Norwood SJ, Kerr MC, Mahony D, Seaman MN, Teasdale RD, Owen DJ 2008. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic 9: 366–379 [DOI] [PubMed] [Google Scholar]
- Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J 20: 2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Korswagen HC 2012. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol 14: 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Li J, Bos E, Porcionatto M, Premont RT, Bourgoin S, Peters PJ, Hsu VW 2004. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell 7: 771–776 [DOI] [PubMed] [Google Scholar]
- Derivery E, Gautreau A 2010. Evolutionary conservation of the WASH complex, an actin polymerization machine involved in endosomal fission. Commun Integr Biol 3: 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A 2009. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 17: 712–723 [DOI] [PubMed] [Google Scholar]
- Derivery E, Helfer E, Henriot V, Gautreau A 2012. Actin polymerization controls the organization of WASH domains at the surface of endosomes. PLoS ONE 7: e39774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E, Pfeffer SR 1998. TIP47: A cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93: 433–443 [DOI] [PubMed] [Google Scholar]
- Dong B, Kakihara K, Otani T, Wada H, Hayashi S 2013. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat Commun 4: 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duleh SN, Welch MD 2010. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 67: 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga JP 2011. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol 7: 278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsel I, Ragaz C, Hoffmann C, Harrison CF, Weber S, van Rahden VA, Johannes L, Hilbi H 2013. The Legionella effector RidL inhibits retrograde trafficking to promote intracellular replication. Cell Host Microbe 14: 38–50 [DOI] [PubMed] [Google Scholar]
- Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, Militz D, Schmidt V, Madsen P, Nyengaard JR, et al. 2012. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci 32: 1467–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP 2008. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 10: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Bugarcic A, Liu H, Norwood SJ, Skeldal S, Coulson EJ, Li SS, Teasdale RD, Collins BM 2013. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci 110: E643–E652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD 2009. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17: 699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TS, Gorman JA, de Narvajas AA, Koenig AO, Billadeau DD 2012. Trafficking defects in WASH-knockout fibroblasts originate from collapsed endosomal and lysosomal networks. Mol Biol Cell 23: 3215–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG 2009. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10: 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI 2000. Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: Assembly into multimeric complexes. Mol Biol Cell 11: 4105–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YH, Doyle JM, Ramanathan S, Gomez TS, Jia D, Xu M, Chen ZJ, Billadeau DD, Rosen MK, Potts PR 2013. Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell 152: 1051–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN 2010. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci 123: 3703–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour ME, Breusegem SY, Seaman MN 2012. Recruitment of the endosomal WASH complex is mediated by the extended “tail” of Fam21 binding to the retromer protein Vps35. Biochem J 442: 209–220 [DOI] [PubMed] [Google Scholar]
- Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, van Weering JR, van Heesbeen RG, Middelkoop TC, Basler K, et al. 2011. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 13: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li F, Chang WP, Tang J 2005. GGA proteins mediate the recycling pathway of memapsin 2 (BACE). J Biol Chem 280: 11696–11703 [DOI] [PubMed] [Google Scholar]
- Helfer E, Harbour ME, Henriot V, Lakisic G, Sousa-Blin C, Volceanov L, Seaman MN, Gautreau A 2013. Endosomal recruitment of the WASH complex: Active sequences and mutations impairing interaction with the retromer. Biol Cell 105: 191–207 [DOI] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD 2011. The ESCRT pathway. Dev Cell 21: 77–91 [DOI] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Zhao Y, Emr SD 2012. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 151: 356–371 [DOI] [PubMed] [Google Scholar]
- Heydorn A, Søndergaard BP, Ersbøll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW 2004. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J Biol Chem 279: 54291–54303 [DOI] [PubMed] [Google Scholar]
- Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH 2007. Functional architecture of the retromer cargo-recognition complex. Nature 449: 1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD 1997. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell 8: 1529–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu VW, Bai M, Li J 2012. Getting active: Protein sorting in endocytic recycling. Nat Rev Mol Cell Biol 13: 323–328 [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A 2011. Endosome maturation. EMBO J 30: 3481–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Hanson PI 2010. Membrane budding and scission by the ESCRT machinery: It’s all in the neck. Nat Rev Mol Cell Biol 11: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miège C, Gaude T 2007. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Jean S, Kiger AA 2012. Coordination between Rab GTPase and phosphoinositide regulation and functions. Nat Rev Mol Cell Biol 13: 463–470 [DOI] [PubMed] [Google Scholar]
- Jia D, Gomez TS, Billadeau DD, Rosen MK 2012. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol Biol Cell 23: 2352–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L, Popoff V 2008. Tracing the retrograde route in protein trafficking. Cell 135: 1175–1187 [DOI] [PubMed] [Google Scholar]
- Johannes L, Wunder C 2011. Retrograde transport: Two (or more) roads diverged in an endosomal tree? Traffic 12: 956–962 [DOI] [PubMed] [Google Scholar]
- Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J 2004. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4(a) receptor splice variant: Roles in receptor targeting. J Cell Sci 117: 5367–5379 [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Bennetts JS, Simpson F, Thomas EC, Flegg C, Gleeson PA, Wicking C, Teasdale RD 2005. A novel mammalian retromer component, Vps26B. Traffic 6: 991–1001 [DOI] [PubMed] [Google Scholar]
- Kingston D, Chang H, Ensser A, Lee HR, Lee J, Lee SH, Jung JU, Cho NH 2011. Inhibition of retromer activity by herpesvirus saimiri tip leads to CD4 downregulation and efficient T cell transformation. J Virol 85: 10627–10638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, Been LF, Chia KS, Dimas AS, Hassanali N, et al. 2011. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet 43: 984–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumandou VL, Klute MJ, Herman EK, Nunez-Miguel R, Dacks JB, Field MC 2011. Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei. J Cell Sci 124: 1496–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M 2010. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol 190: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, Kandror KV, Hsu VW 2007. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol 178: 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, Kuruvilla L, Guie MA, Poffenberger AC, Nelson CD, Atwood WJ, et al. 2013. Genome-wide siRNA screen identified the retromer as a cellular entry factor for human papillomavirus. Proc Natl Acad Sci 110: 7452–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Gomez TS, Sackey BK, Billadeau DD, Burd CG 2012. Rab GTPase regulation of retromer-mediated cargo export during endosome maturation. Mol Biol Cell 23: 2505–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohia M, Qin Y, Macara IG 2012. The Scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS ONE 7: e51130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR 1993. Rab9 functions in transport between late endosomes and the trans-Golgi network. EMBO J 12: 677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Shen Q, Mahoney TR, Liu X, Zhou Z 2011. Three sorting nexins drive the degradation of apoptotic cells in response to PtdIns(3)P signaling. Mol Biol Cell 22: 354–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Bujny MV, Zeuschner D, Geerts WJ, Griffith J, Petersen CM, Cullen PJ, Klumperman J, Geuze HJ 2008. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 9: 380–393 [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE 2004. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132 [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR 1993. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol 121: 1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough JA, Newton HJ, Klum S, Swiss R, Agaisse H, Roy CR 2013. Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening. MBio 4: e00606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P 2000. μ1A-adaptin-deficient mice: Lethality, loss of AP1 binding and rerouting of mannose 6-phosphate receptors. EMBO J 19: 2193–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, et al. 2008. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Aβ accumulation. Proc Natl Acad Sci 105: 7327–7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Pan X, Lambright DG, Tissenbaum HA 2007. An endocytic pathway as a target of tubby for regulation of fat storage. EMBO Rep 8: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T 2005. A retromer-like complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol Biol Cell 16: 5294–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood SJ, Shaw DJ, Cowieson NP, Owen DJ, Teasdale RD, Collins BM 2011. Assembly and solution structure of the core retromer protein complex. Traffic 12: 56–71 [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Ha SA, Bruinsma P 2000. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol 151: 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G 2008. C. elegans AP2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 14: 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachoniak CA, Luo Y, Abella JV, Keen JH, Park M 2011. GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Dev Cell 20: 751–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Thomason PA, Zech T, King JS, Veltman DM, Carnell M, Ura S, Machesky LM, Insall RH 2013. Cyclical action of the WASH Complex: FAM21 and capping protein drive WASH recycling, not initial recruitment. Dev Cell 24: 169–181 [DOI] [PubMed] [Google Scholar]
- Parks WT, Frank DB, Huff C, Renfrew Haft C, Martin J, Meng X, de Caestecker MP, McNally JG, Reddi A, Taylor SI, et al. 2001. Sorting nexin 6, a novel SNX, interacts with the transforming growth factor-β family of receptor serine-threonine kinases. J Biol Chem 276: 19332–19339 [DOI] [PubMed] [Google Scholar]
- Piotrowski JT, Gomez TS, Schoon RA, Mangalam AK, Billadeau DD 2013. WASH knockout T cells demonstrate defective receptor trafficking, proliferation, and effector function. Mol Cell Biol 33: 958–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E 2011. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol 21: 1111–1117 [DOI] [PubMed] [Google Scholar]
- Popoff V, Mardones GA, Tenza D, Rojas R, Lamaze C, Bonifacino JS, Raposo G, Johannes L 2007. The retromer complex and clathrin define an early endosomal retrograde exit site. J Cell Sci 120: 2022–2031 [DOI] [PubMed] [Google Scholar]
- Popovic D, Akutsu M, Novak I, Harper W, Behrends C, Dikic I 2012. Rab GAPs in autophagy: Regulation of endocytic and autophagy pathways by direct binding to human ATG8 modifiers. Mol Cell Biol 32: 1733–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Bänziger C, Hausmann G, Basler K 2008. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 10: 178–185 [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M 2010. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS 2008. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol 183: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotty JD, Wu C, Bear JE 2013. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol 14: 7–12 [DOI] [PubMed] [Google Scholar]
- Sachse M, Urbé S, Oorschot V, Strous GJ, Klumperman J 2002. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell 13: 1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Pol A, Yélamos B, Amessou M, Mills IG, Dugast M, Tenza D, Schu P, Antony C, McMahon HT, Lamaze C, et al. 2004. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev Cell 6: 525–538 [DOI] [PubMed] [Google Scholar]
- Seaman MN 2007. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci 120: 2378–2389 [DOI] [PubMed] [Google Scholar]
- Seaman MN 2012. The retromer complex—Endosomal protein recycling and beyond. J Cell Sci 125: 4693–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Marcusson EG, Cereghino JL, Emr SD 1997. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol 137: 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD 1998. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 142: 665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, Harbour ME, Tattersall D, Read E, Bright N 2009. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci 122: 2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Rojas R, Bonifacino JS, Hurley JH 2006. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat Struct Mol Biol 13: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Römer W, Mardones GA, Burgos PV, Lamaze C, Johannes L 2010. AGAP2 regulates retrograde transport between early endosomes and the TGN. J Cell Sci 123: 2381–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler BM, Rajendran L 2012. Retromers in Alzheimer’s disease. Neurodegener Dis 10: 116–121 [DOI] [PubMed] [Google Scholar]
- Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, Honig L, Vonsattel JP, Kim TW 2005. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol 58: 909–919 [DOI] [PubMed] [Google Scholar]
- Steinberg F, Gallon M, Winfield M, Thomas E, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ 2013. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 15: 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strochlic TI, Setty TG, Sitaram A, Burd CG 2007. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol 177: 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis D, Saksena S, Judson BL, Emr SD 2010. ESCRT-II coordinates the assembly of ESCRT-III filaments for cargo sorting and multivesicular body vesicle formation. EMBO J 29: 871–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M 2011. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13: 715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G 2005. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J 24: 2851–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Sessions RB, Traer CJ, Kloer DP, Bhatia VK, Stamou D, Carlsson SR, Hurley JH, Cullen PJ 2012a. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J 31: 4466–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Verkade P, Cullen PJ 2012b. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 13: 94–107 [DOI] [PubMed] [Google Scholar]
- Vardarajan B, Bruesegem S, Harbour M, St George-Hyslop P, Seaman MNJ, Farrer LA 2012. Identification of Alzheimer disease associated variants in genes that regulate retromer function. Neurobiol Aging 33: e15–e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergés M, Luton F, Gruber C, Tiemann F, Reinders LG, Huang L, Burlingame AL, Haft CR, Mostov KE 2004. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol 6: 763–769 [DOI] [PubMed] [Google Scholar]
- Vilariño-Güell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, et al. 2011. VPS35 mutations in Parkinson disease. Am J Hum Genet 89: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Guo M, Liang Z, Fan J, Zhu Z, Zang J, Zhu Z, Li X, Teng M, Niu L, et al. 2005. Crystal structure of human vacuolar protein sorting protein 29 reveals a phosphodiesterase/nuclease-like fold and two protein-protein interaction sites. J Biol Chem 280: 22962–22967 [DOI] [PubMed] [Google Scholar]
- Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ 2007. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci 120: 45–54 [DOI] [PubMed] [Google Scholar]
- Wassmer T, Attar N, Harterink M, van Weering JR, Traer CJ, Oakley J, Goud B, Stephens DJ, Verkade P, Korswagen HC, et al. 2009. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell 17: 110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, Shen C, Jung JU, Xiong F, Lee DH, et al. 2011. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J Cell Biol 195: 765–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Hurley JH 2010. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464: 864–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC 2008. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 14: 140–147 [DOI] [PubMed] [Google Scholar]
- Zelazny E, Santambrogio M, Pourcher M, Chambrier P, Berne-Dedieu A, Fobis-Loisy I, Miege C, Jaillais Y, Gaude T 2013. Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis. J Biol Chem 288: 8815–8825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wu Y, Belenkaya TY, Lin X 2011. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res 21: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wu Y, Lin X 2011. Retromer regulates apical-basal polarity through recycling Crumbs. Dev Biol 360: 87–95 [DOI] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, et al. 2011. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 89: 168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]