Abstract
Background:
Computer analysis of high-resolution CT (HRCT) scans may improve the assessment of structural lung injury in children with cystic fibrosis (CF). The goal of this cross-sectional pilot study was to validate automated, observer-independent image analysis software to establish objective, simple criteria for bronchiectasis and air trapping.
Methods:
HRCT scans of the chest were performed in 35 children with CF and compared with scans from 12 disease control subjects. Automated image analysis software was developed to count visible airways on inspiratory images and to measure a low attenuation density (LAD) index on expiratory images. Among the children with CF, relationships among automated measures, Brody HRCT scanning scores, lung function, and sputum markers of inflammation were assessed.
Results:
The number of total, central, and peripheral airways on inspiratory images and LAD (%) on expiratory images were significantly higher in children with CF compared with control subjects. Among subjects with CF, peripheral airway counts correlated strongly with Brody bronchiectasis scores by two raters (r = 0.86, P < .0001; r = 0.91, P < .0001), correlated negatively with lung function, and were positively associated with sputum free neutrophil elastase activity. LAD (%) correlated with Brody air trapping scores (r = 0.83, P < .0001; r = 0.69, P < .0001) but did not correlate with lung function or sputum inflammatory markers.
Conclusions:
Quantitative airway counts and LAD (%) on HRCT scans appear to be useful surrogates for bronchiectasis and air trapping in children with CF. Our automated methodology provides objective quantitative measures of bronchiectasis and air trapping that may serve as end points in CF clinical trials.
A thin-section CT scan, the best noninvasive tool for assessing bronchiectasis and structural lung injury, is commonly used to monitor cystic fibrosis (CF)-related lung disease because it provides morphologic information that is complementary to the functional information provided by lung function tests. Structural changes detected by CT scans often precede functional changes in children with CF,1‐5 and CT scanning may be a more sensitive method than measurement of lung function for detecting disease progression.6,7 Furthermore, CT scanning is a potential outcome measure for CF clinical trials.8,9
To provide quantitative information, CT images must be converted into numeric data to allow statistical comparison between scans. Both qualitative visual scoring by expert readers and quantitative computer analysis have been used successfully to interpret CT scans in CF. In numerous studies, expert reader CT scan scores have been shown to correlate with other CF lung disease parameters, including clinical status and lung function.10‐17 Although expert visual scoring such as the Brody scoring system16 includes more features of CF lung disease than can be evaluated by automated software, it is time consuming and limited by the number of expert readers available. In contrast, automated computer analysis does not require specially trained observers, avoids observer bias and variability, offers better standardization, and is a more sensitive method for detecting subtle changes.18‐21
The most studied metric of quantitative analysis from CT scans is air trapping.22 Air trapping, a result of small airways obstruction, is indicated by areas of abnormally low lung attenuation on expiratory CT images. Air trapping has been quantified in CF and other lung diseases using a variety of lung-density approaches.19,23‐26
Although air trapping is an important early indicator of peripheral airways disease in children with CF, the hallmark of airway pathology is bronchiectasis. The key feature of bronchiectasis on CT scanning is an enlarged airway lumen with bronchi that appear larger than the accompanying artery; however, this definition may underestimate the extent of airway damage.27 Other radiographic findings include the failure of the larger airways to taper while progressing to the lung periphery and the identification of airways in the most peripheral areas of the lung, within 2 cm of the costal or paravertebral pleura.28 Therefore, the number of visible airways, especially ones in the periphery, may be a surrogate of bronchiectasis on CT scans. There has been one previous report of counting visible airways in children with difficult-to-treat asthma.29
The objectives of this cross-sectional pilot study were to develop and validate automated, observer-independent image analysis software to quantify airway counts and the degree of air trapping on high-resolution CT (HRCT) scans in children with CF; to compare automated CT scan measures between children with CF and age-matched disease control subjects; and to examine relationships among automated CT scan measures, Brody CT scan scores, pulmonary function measures, and sputum markers of inflammation in children with CF.
Materials and Methods
Study Subjects
During routine outpatient clinic visits, we randomly recruited 35 children between the ages of 6 and 15 years who had CF. These children were studied during times of clinical stability, defined by both clinical impression and having had no hospitalizations or changes in antibiotic regimen during the 1 month prior to being studied. Twelve disease control subjects of similar ages who had undergone an HRCT scan for a variety of clinical indications (asthma, n = 4; chronic cough, n = 2; suspected interstitial lung disease, n = 2; exercise-induced dyspnea and/or hypoxia, n = 3; and pulmonary hypertension, n = 1) were identified. Inclusion criteria for the control group were having a similar age range to that of the CF group, CT scans showing no evidence of parenchymal or airways disease, and minimal air trapping on expiratory images. The criteria used for defining the control group did not include lung function or anthropometric measurements.
The protocol, No. 03-759, was approved by the Colorado Multiple Institutional Review Board, and informed consent was obtained from each of the subjects with CF, their parents, or both. Institutional approval was obtained to analyze deidentified HRCT scans from the control subjects.
Study Design
Each subject with CF underwent an HRCT scan of the chest, pulmonary function testing, and sputum induction during one outpatient clinic visit. All HRCT scans were performed between 2003 and 2005 on an Asteion S4 scanner (Toshiba Medical Systems Europe) using a pediatric protocol with a tube potential of 120 kVp, a current of 140 mA, and a 1-s scan time. Slice thickness was 1 mm, and scans were reconstructed with a high-frequency algorithm. The image matrix was 512 × 512 pixels, and the field of view was determined based on the subject’s size. Inspiratory images were obtained at 10-mm intervals, extending from the lung apices to below the costophrenic angles, at full voluntary inspiration. During a breath hold after full voluntary exhalation, six equally spaced expiratory images were obtained. The total effective radiation dose was estimated to be < 6.23 mSv, with variation based on patient size. The HRCT scans were evaluated independently by two individuals (rater 1 and rater 2) using the Brody scoring system16 to generate overall lung disease scores and subscores (standardized, expressed as percent: 0% to 100%) for the presence and severity of bronchiectasis and air trapping.
Spirometry was performed according to American Thoracic Society guidelines30,31 using a Sensormedics Vmax22 system (CareFusion). Lung volumes were obtained by plethysmography. The functional indexes measured included FEV1, FVC, midvolume forced expiratory flow (FEF25-75), total lung capacity (TLC), residual volume (RV), and RV/TLC. Percent predicted values were based on Wang et al32 and Hankinson et al.33
Sputum was induced by having the subject inhale 3% hypertonic saline for six 2-min sessions, as described previously.34 The sputum was collected into two containers, which were both transported on ice to the laboratory for processing within 20 min. One specimen was processed for quantitative cultures, according to the findings of a consensus conference on CF microbiology.35 Pseudomonas aeruginosa and Staphylococcus aureus infection status was defined based on the result of this single sputum culture. The other specimen was processed for cytology and measurement of the inflammatory mediators’ free neutrophil elastase activity and matrix metalloprotease (MMP)-9, as described previously.34
Image Processing and Analysis
Automated image analysis software was developed to detect and count visible airways on inspiratory CT images and to estimate the amount of air trapping on expiratory images. CT scan data were stored in DICOM format, with each pixel assigned a Hounsfield unit (HU) density value. A histogram of the HUs present on the inspiratory CT images was created and smoothed using a low-pass filter. The histogram had two large peaks, one close to −1,000 HU (indicative of air density) and one close to 0 HU (indicative of more dense structures, including soft tissue and bone) (e-Fig 1 (7.4MB, pdf) ). The software determined an air threshold value (ATV) defined as the minimum HU between these two peaks, and lung fields were segmented and isolated by selecting all pixels with an HU below the ATV. Next, the lung fields were smoothed, segmented, and divided into the left and right lungs. The total area of each lung was calculated. Discrete white objects within the lung segments, including interstitium, airway walls, mucus, and blood vessels, were retained in the lungs to create a lung mask.
The inspiratory CT scan series contained from 12 to 30 slices, depending on patient size. Slices were excluded in an automated fashion if they did not include lung fields or if the lung area they contained was too small for processing. Artifacts representing air cavities such as the stomach were removed automatically based on their location in the scan. Each inspiratory CT scan series was divided into three vertical regions defined as follows: upper slices (the top 25% of the lungs by height), center slices (slices below the top 25% and above the bottom 30% based on lung height), and lower slices (representing the remaining bottom 30% of lung slices).
The scans were further divided into peripheral and central regions. The peripheral region was defined as the outer 2 to 3 cm of lung parenchyma (“edge” of the lung), which is generally devoid of visible bronchi in healthy individuals.28 From a software programming perspective, the peripheral region was first determined using the inspiratory CT scan slice closest to the carina. The peripheral region was set as the outer 10% of the maximal width of both segmented lungs through the carina (white dotted line in e-Fig 2 (7.4MB, pdf) ). Because the large airways lie close to the carina, the central regions of the center vertical slices were connected between the left and right lung (black dotted line in e-Fig 2 (7.4MB, pdf) ), creating C-shaped peripheral regions rather than ring-shaped regions. Additionally, the areas of the peripheral regions increased in proportion to the distance from the carina, because the main bronchi that emanate from the pulmonary hilum are concentrated in the center vertical slices. Therefore, the upper and lower vertical slices contained larger peripheral areas than did the center vertical slices.
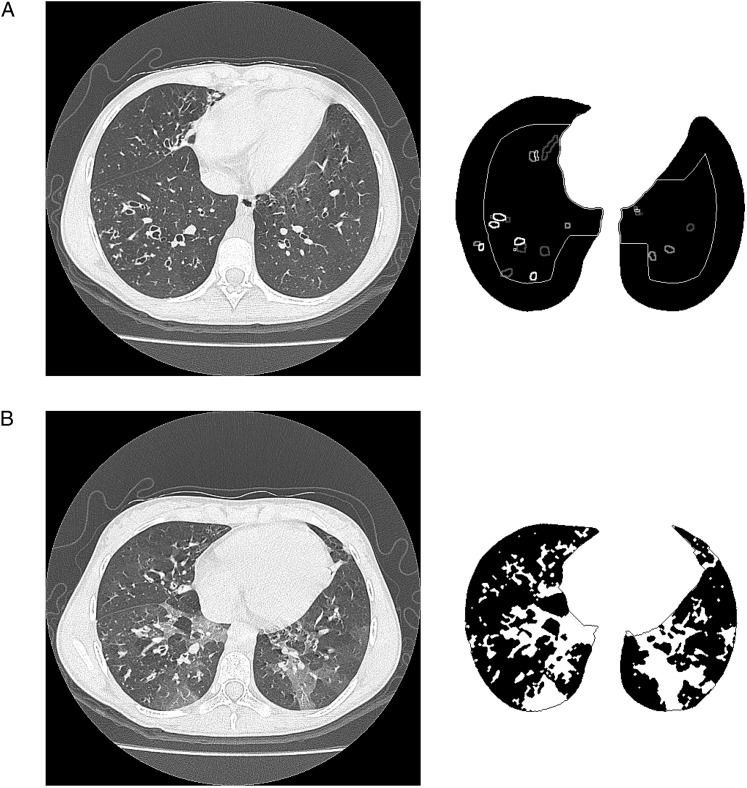
Airways were selected using the following iterative algorithm. Initially, regions of interest (ROI) were selected to be areas with a density below −1,050 HU completely surrounded by higher attenuation pixels. Iterations increased the threshold by 10 HU, and new ROIs were selected and added to previous ones. Iterations were stopped once the attenuation value reached the ATV. The resulting ROIs were validated by analyzing an outline of 3 pixels surrounding each ROI. If the majority of the outline pixels had an attenuation value higher than the ATV and if they completely enclosed the ROI without gaps, then the ROI was accepted as an airway. Airways were labeled by location within the central and peripheral regions and were counted (Fig 1A). The airway count was divided by the number of inspiratory CT scan slices per scan so that the final outcome measure was the average airway count per slice.
Figure 1.
Airway counts on inspiratory slices and low attenuation density (%) on expiratory slices. A, Lung segmentation with airways in peripheral and central regions counted on inspiratory images. B, Lung segmentation with low-density areas in black on expiratory images.
On expiratory images, lung fields were isolated and segmented from the six equally spaced CT images. The software recognized air trapping by identifying areas of low attenuation next to regions with more normal attenuation. Lung attenuation histograms for the segmented lung regions were calculated. Low attenuation limits defining air trapping were set based on the attenuation in the corresponding inspiratory CT scan slices, specifically the inspiratory CT scan slice nearest to the carina. To account for variation in technique, an HU representing air trapping was calculated for each subject’s CT scan. For each patient, the lower peak of the inspiratory histogram was assumed to be the HU of fully inflated lung tissue (e-Fig 3 (7.4MB, pdf) ). The peak of the expiratory histogram shifted to higher HU density values because the lungs contained less air after exhalation. The software determined the HU density that was 10% lower than the peak of the inspiratory histogram and designated this as the threshold or cutoff value for air trapping (e-Fig 3 (7.4MB, pdf) ). The software then identified pixels on the six expiratory CT scan slices that had an HU below this cutoff value. A low attenuation density (LAD) index, defined as the total amount of air trapped in the patient’s lung as a percentage of the total lung area on the six slices, was calculated (Fig 1B). For comparison, the percentage of pixels below a fixed threshold of −850 HU was also calculated for the six expiratory scans.
Statistical Analysis
Data were analyzed using a commercially available statistical package (SAS version 9.2; SAS Institute Inc). Descriptive statistics and scatter plots were used to summarize HRCT scan scores (airway counts and LAD [%]) and lung function by study group. A log10 transformation was used for data with nonnormal distributions (free neutrophil elastase activity, MMP-9 activity). Independent Student t tests were used for across-group comparisons. To compare lung zones within the CF cohort, paired comparisons were reported. Linear regression and Pearson’s correlation coefficient (r) with corresponding P values and 95% CIs were used to assess the relationship between continuous variables. A two-sided α level of 0.05 was used to determine statistical significance. Bland-Altman figures and intraclass correlation coefficients (ICCs) were generated to assess agreement across the two Brody score raters.36,37
Results
Clinical characteristics of the children with CF (43% girls; mean age 11.1 years [range, 6-15 years]) are displayed in Table 1. The control subjects were relatively age-matched children (50% girls; mean age 10.5 years [range, 8-13 years]) who were undergoing an HRCT scan for the clinical indications listed previously.
Table 1.
—Clinical Characteristics of Subjects With CF
| Characteristic | Mean (SD) | No. (%) |
| Female | … | 15 (42.9) |
| Age, y | 11.1 (2.6) | … |
| CF genotype | ||
| F508del/F508del | … | 14 (40) |
| F508del/other | … | 15 (43) |
| Other/other | … | 6 (17) |
| FVC % predicted | 95.5 (17.6) | … |
| FEV1 % predicted | 95.3 (15.0) | … |
| FEF25-75 % predicted | 84.7 (25.2) | … |
| RV % predicteda | 128.5 (49.8) | … |
| RV/TLCa | 27.1 (9.4) | … |
| Weight, percentile | 37.7 (24.9) | … |
| Height, percentile | 34.9 (25.7) | … |
| BMI | 17.3 (2.3) | … |
| BMI, percentile | 42.3 (25.0) | … |
| Pseudomonas aeruginosab | … | 6 (17) |
| Staphylococcus aureusb | … | 16 (46) |
CF = cystic fibrosis; FEF25-75 = midvolume forced expiratory flow; RV = residual volume; TLC = total lung capacity.
RV and RV/TLC were available from 28 of 35 subjects.
Respiratory culture data were available from 32 of 35 subjects (91%) who expectorated induced sputum samples for microbial analysis at their baseline visit.
Children With CF vs Control Subjects
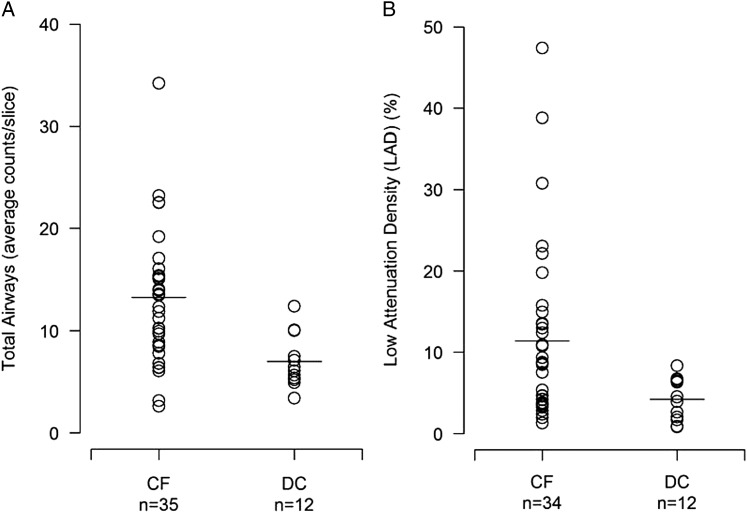
All inspiratory HRCT scans were analyzed using the automated system. The average number of total, central, and peripheral airways counted on each inspiratory slice was significantly higher in the children with CF compared with the disease control subjects (Fig 2A, Table 2). One CF expiratory HRCT scan could not be analyzed using the automated system because of poor image quality. LAD (%) was significantly increased in the remaining subjects with CF compared with the disease control subjects (Fig 2B, Table 2). The largest difference in LAD (%) between the two groups was seen in the upper lung zones, although significant differences were present in all lung zones. In the CF cohort, LAD (%) was highest in the upper lung zone compared with the middle (mean difference, 5.6%; 95% CI, 2.3-8.8; P = .002) and lower (mean difference, 3.7%; 95% CI, 0.0-7.5; P = .052) lung zones (Table 2). There was no significant difference between the variable threshold LAD index and the fixed threshold measurement.
Figure 2.
A, Total airway counts averaged over the number of inspiratory slices. B, LAD (%) averaged over six expiratory slices. Both are increased in CF vs DC. Horizontal lines represent the group median. CF = patients with cystic fibrosis; DC = disease control subjects; LAD = low attenuation density.
Table 2.
—Automated Airway Counts and LAD (%) by Study Group
| Automated CT Scan Measure | CF | Disease Control | P Value |
| Inspiratory airway counta | |||
| Inspiratory slices per scan, No. | 16.1 (3.2) | 15.9 (2.4) | .82 |
| Total | 13.2 (6.5) | 7.0 (2.6) | < .0001 |
| Central | 11.4 (4.7) | 6.6 (2.2) | < .0001 |
| Peripheral | 1.8 (2.0) | 0.4 (0.5) | .0003 |
| Expiratory LAD (%)b | |||
| Total | 11.4 (10.6) | 4.2 (2.6) | < .001 |
| Upper lung zone | 15.0 (15.6) | 3.7 (4.6) | < .001 |
| Middle lung zone | 9.4 (9.9) | 3.1 (2.7) | .002 |
| Lower lung zone | 11.3 (10.6) | 5.5 (3.5) | .008 |
| Expiratory fixed (%)c | |||
| Total | 11.4 (10.4) | 4.0 (2.4) | < .001 |
| Upper lung zone | 14.7 (15.0) | 2.6 (2.6) | < .0001 |
| Middle lung zone | 9.1 (9.2) | 3.3 (2.6) | .002 |
| Lower lung zone | 11.8 (11.0) | 5.5 (3.3) | .004 |
Data are presented as mean (SD). HRCT = high-resolution CT; LAD = low attenuation density. See Table 1 legend for expansion of other abbreviation.
CF: n = 35; disease control: n = 12. The number of visible airways (total, central, and peripheral) averaged over all inspiratory HRCT scan slices in an individual.
CF: n = 34; disease control: n = 12. Total LAD (%) averaged over six expiratory HRCT scan slices in an individual subject. Upper lung zones are composed of expiratory scan levels 1 and 2; middle lung zones are expiratory scan levels 3 and 4; and lower lung zones are expiratory scan levels 5 and 6.
CF: n = 34; disease control: n = 12. Air trapping score calculated using fixed −850 Hounsfield unit threshold.
Automated Measures and Brody CT Scan Scores in the CF Cohort
Prior to examining the relationship between the automated CT scan measures and the Brody scores in the population with CF, the agreement between the two raters was examined. Total Brody scores ranged from 0.5% to 35.1%, air trapping subscore from 0% to 72.2%, and bronchiectasis subscore from 0% to 24.7%. Bland-Altman plots of the differences vs the average scores indicate that rater agreement was quite good for the bronchiectasis subscore (ICC = 0.88), although in four HRCT scans, the bronchiectasis subscore differed by more than five points (e-Fig 4A (7.4MB, pdf) ). The air-trapping subscores were less concordant, with the ICC = 0.58 (e-Fig 4B (7.4MB, pdf) ). For the Brody overall lung disease score, one rater tended to score higher than the other, but most of the variability in the overall score was among patients (ICC = 0.80) and was not caused by the raters (e-Fig 4C (7.4MB, pdf) ).
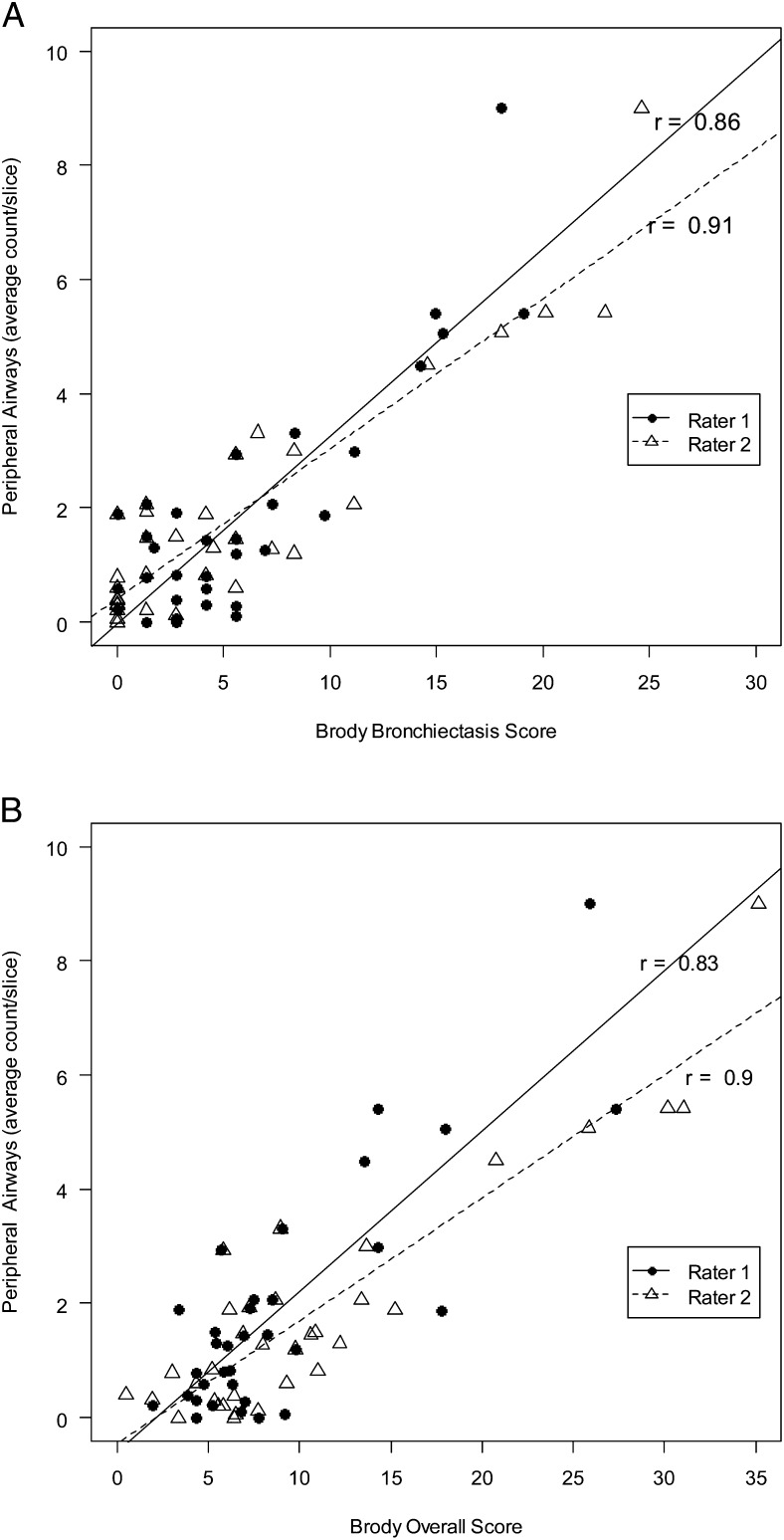
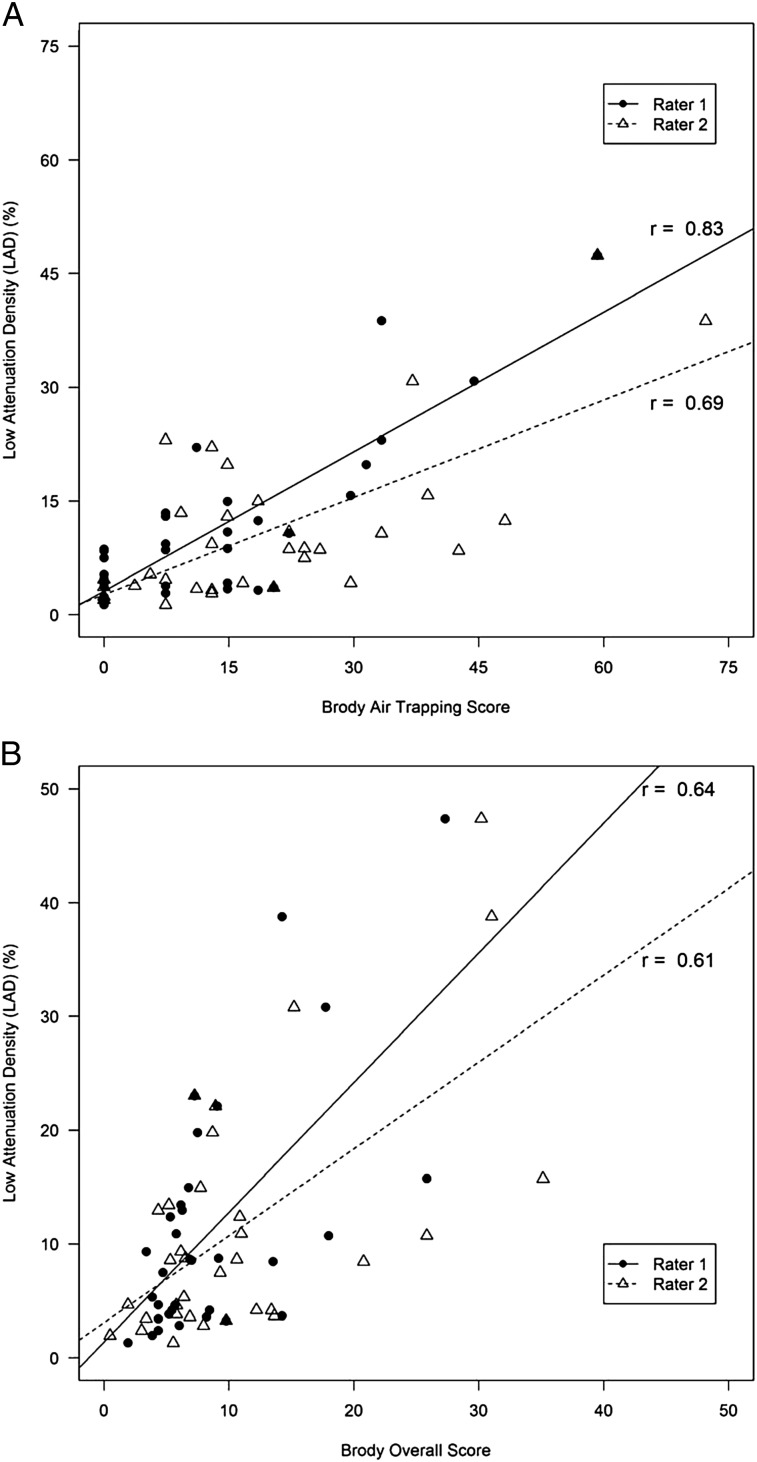
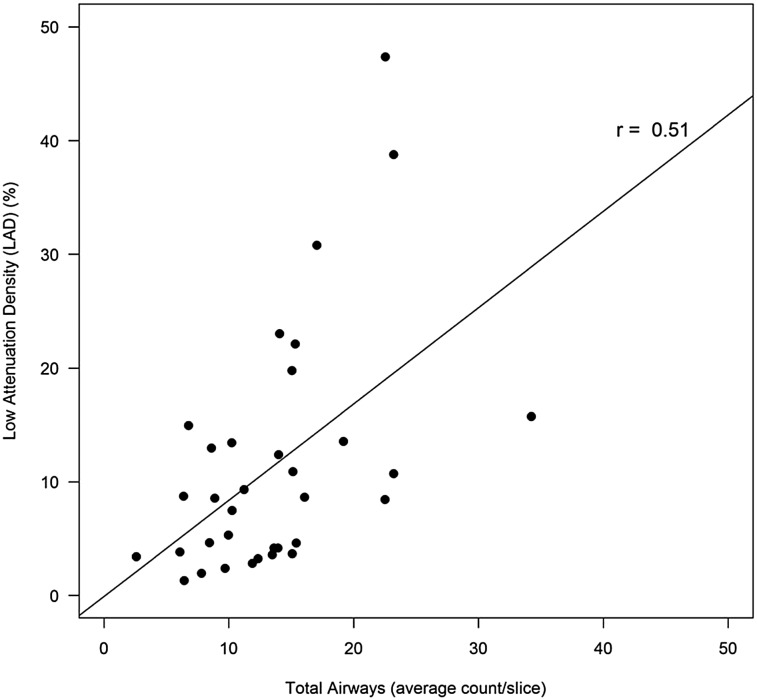
In the CF cohort, total and peripheral airway counts were highly correlated with bronchiectasis subscores and overall lung disease scores for both raters (Fig 3, Table 3). LAD (%) correlated significantly with Brody air trapping subscores and overall lung disease scores for both raters (Fig 4, Table 3), although the strength of the association was less than that observed between airway counts and Brody scores. LAD (%) correlated weakly with total airway counts in the CF cohort (r = 0.51; 95% CI, 0.20-0.72) (Fig 5).
Figure 3.
Peripheral airway counts correlate with Brody bronchiectasis and overall Brody scores in the CF cohort. A, Brody bronchiectasis. B, Overall Brody scores. See Figure 2 legend for expansion of abbreviation.
Table 3.
—Correlations (r) Among Automated Airway Counts, LAD (%), and Brody Scores in Subjects With CF
| Automated CT Scan Measure | Rater | Air Trapping Score |
Bronchiectasis Score |
Overall Score |
|||
| r | 95% CI | r | 95% CI | r | 95% CI | ||
| Total airways | 1 | … | … | 0.82 | 0.67-0.91 | 0.79 | 0.62-0.89 |
| 2 | … | … | 0.87 | 0.76-0.93 | 0.89 | 0.79-0.94 | |
| Central airways | 1 | … | … | 0.78 | 0.60-0.89 | 0.75 | 0.55-0.87 |
| 2 | … | … | 0.83 | 0.68-0.91 | 0.85 | 0.72-0.92 | |
| Peripheral airways | 1 | … | … | 0.75 | 0.55-0.87 | 0.83 | 0.69-0.91 |
| 2 | … | … | 0.85 | 0.72-0.92 | 0.9 | 0.81-0.95 | |
| LAD (%) | 1 | 0.83 | 0.68-0.91 | … | … | 0.64 | 0.38-0.81 |
| 2 | 0.69 | 0.45-0.83 | … | … | 0.61 | 0.33-0.79 | |
Figure 4.
LAD (%) correlates with Brody air trapping and overall Brody scores in the CF cohort. A, Brody air trapping. B, Overall Brody scores. See Figure 2 legend for expansion of abbreviations.
Figure 5.
Total airway count per slice correlates with LAD (%). Solid line is estimated regression. See Figure 2 legend for expansion of abbreviation.
Airway Counts in the CF Cohort
Among the subjects with CF, visible airway counts did not differ significantly between boys and girls (mean, female = 14.0 vs male = 12.7; P = .58). However, they tended to increase with age (total airways: 0.99 airways/y, r = 0.38, P = .02; central airways: 0.75 airways/y, r = 0.40, P = .014; peripheral airways: 0.24 airways/y, r = 0.30, P = .075).
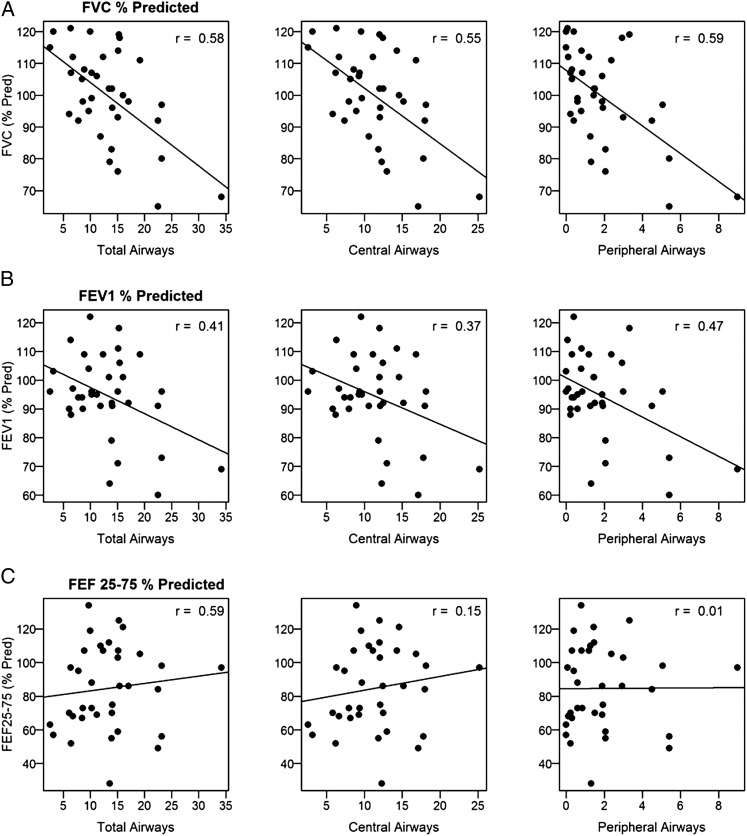
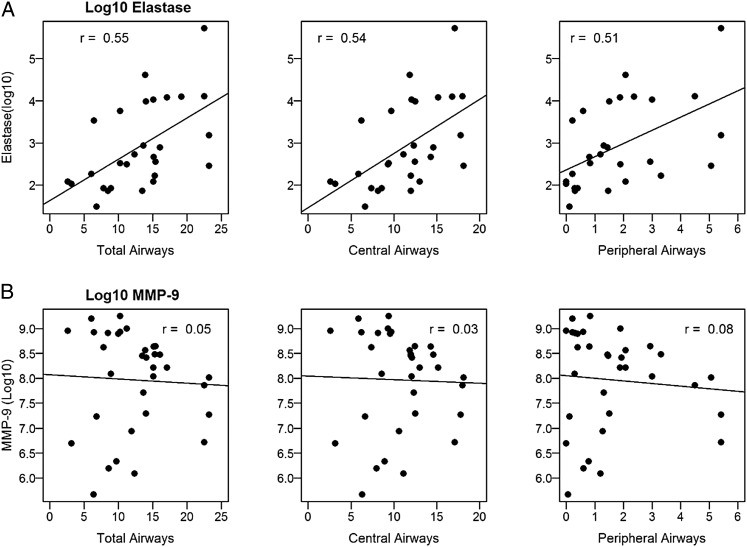
In subjects with CF, total airway counts were inversely related to FVC, with an estimated drop in FVC (% predicted) of 1.3 and 4.4 for each additional total or peripheral airway, respectively (Fig 6A, e-Table 1 (7.4MB, pdf) ). Similarly, airway counts correlated negatively with FEV1 % predicted, with peripheral airways having the strongest relationship (Fig 6B, e-Table 1 (7.4MB, pdf) ). Airway counts did not correlate with FEF25-75 (Fig 6C, e-Table 1 (7.4MB, pdf) ). Regarding sputum markers of inflammation, visible airway counts correlated with free neutrophil elastase activity (total airways: 0.10 log10 increase in neutrophil elastase per airway, r = 0.55, P < .01; central airways: 0.12 log10 increase per airway, r = 0.54, P < .01; peripheral airways: 0.31 log10 increase per airway, r = 0.51, P < .01) (Fig 7A, e-Table 1 (7.4MB, pdf) ). No significant relationships were observed between airway counts and MMP-9 activity (Fig 7B, e-Table 1 (7.4MB, pdf) ). Airway counts were not significantly different between subjects who were P aeruginosa-positive and subjects who were P aeruginosa-negative (total airways: mean difference, 1.7; 95% CI, −4.6-8.1; P = .58; central airways: 1.3, 95% CI, −3.2-5.9; P = .55; peripheral airways: 0.4, 95% CI, −1.5-2.3; P = .67). Similarly, there were no significant differences in airway counts between subjects who were Staphylococcus aureus-positive and subjects who were Staphylococcus aureus-negative (total airways: mean difference, 2.2; 95% CI, −3.6-8.1; P = .44; central airways: 2.2, 95% CI, −2.0-6.4; P = .30; peripheral airways: 0.06, 95% CI, −1.7-1.9; P = .95).
Figure 6.
A, Airway counts correlate with FVC % predicted. B, Airway counts correlate with FEV1 % predicted. C, Peripheral airway counts do not correlate with FEF 25-75. Solid line is estimated regression. FEF 25-75 = midvolume forced expiratory flow; pred = predicted.
Figure 7.
A, Airway counts correlate with Log10 of neutrophil elastase. B, Airway counts do not correlate with Log10 of MMP-9. Solid line is estimated regression. MMP-9 = matrix metalloprotease-9.
LAD (%) in the CF Cohort
In subjects with CF, LAD (%) tended to increase with age, with an estimated slope of 1.3% per year (r = 0.31, P = .07), and was marginally higher in girls (female mean = 15.7%, male mean = 8.3%, P = .08). There were no correlations found between LAD (%) (total, upper, mid-, and lower lung zones) and the lung function tests, including RV and RV/TLC, or either of the sputum markers of inflammation.
Discussion
In this cross-sectional pilot study, we demonstrated the use of fully automated image analysis software that provided quantitative measures of visible airway counts and air trapping derived from HRCT scans. Both airway counts and LAD (%) were significantly increased in the children with CF compared with disease control subjects. In the CF cohort, airway counts correlated strongly with expert reader-derived bronchiectasis subscores, and a LAD index correlated well with expert reader-derived air trapping subscores, which suggests that visible airway counts and LAD (%) may be useful surrogates of bronchiectasis and air trapping, respectively. Further, we found that total and peripheral airway counts were significantly related to lung function measurements (FVC and FEV1) and sputum neutrophil elastase. Because neutrophil elastase has been implicated in CF lung disease pathogenesis and the development of bronchiectasis,38‐40 the observed relationship provides further support for the use of visible airways as a surrogate for bronchiectasis.
Previous CF studies using automated CT scanning software have focused primarily on measuring lung density.18,19,25,41 The method put forth by Goris et al25 defined different severities of air trapping using information derived from both expiratory and inspiratory histograms, similar to the approach we took. In their study of patients with mild CF, all CT scan measures of air trapping correlated weakly with functional air trapping as measured by RV/TLC but did not correlate with FVC or FEV1.25 The same analysis method was used in a clinical trial of dornase alfa in children with mild CF lung disease.18 After 3 and 12 months of therapy with dornase alfa or placebo, only quantitative air trapping by CT scan showed a difference that approached statistical significance. These investigators concluded that quantitative air trapping was a more sensitive outcome measure than lung function indexes and expert-reader CT scan scores in detecting treatment effects in children with CF with mild lung disease. Quantitative airway dimensions have been examined in other CF groups and significant correlations between bronchial dilatation and lung function have been demonstrated.42‐44 However, in contrast to our fully automated method, these studies involved semiautomated computer programs with input from expert readers to measure airway dimensions.
Building on these studies, we believe that automated image analysis can enhance the value of HRCT imaging both in CF clinical trials and in routine care. We propose that airway counts on inspiratory CT images represent large airway abnormalities, whereas an LAD index reflects small airway abnormalities. In addition, we speculate that airway counts are more likely to change over months to years, whereas an LAD index may change in a shorter time frame. A prospective study evaluating the change in these measures over time is being performed. In our study, we identified visible airway counts by their location in the central or peripheral lung zones. Peripheral airway counts correlated marginally better with Brody bronchiectasis scores and lung function than did total airway counts; however, total airway counts alone were an adequate surrogate for bronchiectasis, and this could serve to simplify the automated analysis.
The correlations between LAD (%) and Brody air trapping and overall scores were weaker than those observed between visible airway counts and Brody bronchiectasis and overall scores. LAD (%) did not correlate with either lung function or sputum inflammatory markers. Our HRCT scan protocol minimized radiation because only six expiratory slices were obtained, which may have limited the observed correlations. Two studies have shown that CT scan air trapping estimates become less precise when fewer slices are used to calculate air trapping scores.20,25 Furthermore, an LAD index is an effort-dependent measurement, and changes in lung attenuation may result from intrasubject and intersubject variability in respiratory maneuvers. In our study, CT scans were performed without spirometric gating, which increases CT scan availability and simplifies the CT scan procedure. Controversy exists regarding the need for spirometric control of CT scans in research. In general, CT imaging studies using the Brody score have not used spirometric control. If studies are performed without spirometer control, they must be performed with attention to correct technique, including practiced and coached respiratory maneuvers by members of the radiology or pulmonology team.45,46
Alternatively, a LAD index may measure areas that represent a different pathophysiologic process than air trapping that is measured functionally. To circumvent the problem of lung density changes with growth and to control for variable inspiration and exhalation among subjects, a computer-assisted histogram was used to identify the threshold of air trapping, using each subject’s inspiratory lung density as the internal control for expiratory thresholding. More studies are needed in subjects with a wider age range to demonstrate an advantage of this approach compared with using a fixed threshold.
Our HRCT images were thin slices obtained at intervals, rather than thin volumetric CT scans. This technique does not allow three-dimensional reconstruction and, thus, limited our analysis to only the inspiratory and expiratory slices that were available. Volumetric scans would likely facilitate the detection of specific regional changes in longitudinal studies. Volumetric measurement of air trapping has been shown to be an improvement over limited sampling methods.47 Using a state-of-the-art CT scanner and current technique guidelines, a volumetric inspiratory and expiratory CT scan study can be performed in seconds using a dose < 2 mSv.48 Current CT scanning technology would allow longitudinal comparison of airway counts and LAD (%).
In terms of CT scan analysis, expert scoring systems are less dependent on subtle differences in scanning techniques and are able to recognize and quantify a broader range of structural abnormalities, including mucus plugging and atelectasis. Despite these advantages, reproducibility of the Brody expert observer system has shown less than ideal agreement, particularly when subscores are analyzed.16 Expert reader scoring systems are also limited by the number of experts available and the need to retain them throughout the study period to lessen interobserver variability, which may be particularly challenging in long-term prospective CF studies.
Conclusions
In conclusion, our automated approach offers two novel quantitative CT scan measures, visible airway counts and a LAD index, that correlate well with bronchiectasis and air trapping, respectively, which are two of the most important CT scan features of structural lung disease in school-aged children with CF. These quantitative CT scan scores can complement data derived from expert scoring systems and can augment the value of HRCT scanning in CF. Quantitative CT scan analysis may prove valuable not only in CF clinical trials, but also in routine clinical care, to monitor changes in structural lung disease.
Supplementary Material
Online Supplement
Acknowledgments
Author contribution: Dr DeBoer is the guarantor of the paper and takes responsibility for its content, including the data and analysis.
Dr DeBoer: contributed to the study design, analysis and interpretation of data, and writing of the manuscript.
Dr Swiercz: contributed to the development of the automated image analysis software to quantify airway counts and air trapping on CT scans and writing of the sections describing software.
Dr Heltshe: contributed to the editing and approval of the paper.
Ms Anthony: contributed to the research coordination and editing and approval of the paper.
Dr Szefler: contributed to the evaluation of the HRCT scans using the Brody scoring system (rater 1) and editing and approval of the paper.
Dr Klein: contributed to the evaluation of the HRCT scans using the Brody scoring system (rater 2) and editing and approval of the paper.
Dr Strain: contributed to ensuring standard operating procedures were followed, quality control of HRCT scans, and editing and approval of the paper.
Dr Brody: contributed to the development and validation of the Brody scoring system, training of raters 1 and 2, final editing, and approval of the paper.
Dr Sagel: contributed to the study design, data interpretation, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Heltshe has received funding from the Cystic Fibrosis Foundation, but not specifically for this project. Dr Sagel received funding from the Cystic Fibrosis Foundation and NIH to support this project. He has also received grants from the Colorado Department of Public Health and Environment related to CF Newborn Screening. Drs DeBoer, Swiercz, Szefler, Klein, Strain, and Brody and Ms Anthony have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the laboratory technicians in the Pediatric Clinical Translational Research Center Core Laboratory at Children’s Hospital Colorado for their outstanding technical assistance in this work, and Frank Accurso, MD, for his mentorship, invaluable feedback, and critical review of this manuscript.
Additional information: The e-Figures and e-Table can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- ATV
air threshold value
- CF
cystic fibrosis
- FEF25-75
midvolume forced expiratory flow
- HRCT
high-resolution CT
- HU
Hounsfield unit
- ICC
intraclass correlation coefficient
- LAD
low attenuation density
- MMP
matrix metalloprotease
- ROI
region of interest
- RV
residual volume
- TLC
total lung capacity
Footnotes
A prior abstract with preliminary data was presented at the 2006 North American Cystic Fibrosis Conference, November 2-5, 2006, Denver, CO, and part of this article has been presented in abstract form (Sagel SD, Swiercz W, Heltshe S, Kaess H, Anthony M. Pediatric Pulmonology 2006;41[S29]:387).
Funding/Support: This research was supported by the National Institutes of Health (NIH) [K23 RR018611] and by the NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Institute [Grant UL1 TR000154].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Sanders DB, Li Z, Brody AS, Farrell PM. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;184(7):816-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young patients with cystic fibrosis: Distribution of abnormalities and correlation with pulmonary function tests. J Pediatr. 2004;145(1):32-38 [DOI] [PubMed] [Google Scholar]
- 3.Sly PD, Brennan S, Gangell C, et al. ; Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146-152 [DOI] [PubMed] [Google Scholar]
- 4.Mott LS, Park J, Murray CP, et al. ; AREST CF Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67(6):509-516 [DOI] [PubMed] [Google Scholar]
- 5.Pillarisetti N, Linnane B, Ranganathan S; AREST CF Early bronchiectasis in cystic fibrosis detected by surveillance CT. Respirology. 2010;15(6):1009-1011 [DOI] [PubMed] [Google Scholar]
- 6.de Jong PA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J. 2004;23(1):93-97 [DOI] [PubMed] [Google Scholar]
- 7.Sanders DB, Li Z, Rock MJ, Brody AS, Farrell PM. The sensitivity of lung disease surrogates in detecting chest CT abnormalities in children with cystic fibrosis. Pediatr Pulmonol. 2012;47(6):567-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiddens HA, de Jong PA. Imaging and clinical trials in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):343-346 [DOI] [PubMed] [Google Scholar]
- 9.de Jong PA, Tiddens HA. Cystic fibrosis specific computed tomography scoring. Proc Am Thorac Soc. 2007;4(4):338-342 [DOI] [PubMed] [Google Scholar]
- 10.Brody AS, Molina PL, Klein JS, Rothman BS, Ramagopal M, Swartz DR. High-resolution computed tomography of the chest in children with cystic fibrosis: support for use as an outcome surrogate. Pediatr Radiol. 1999;29(10):731-735 [DOI] [PubMed] [Google Scholar]
- 11.Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179(3):783-788 [DOI] [PubMed] [Google Scholar]
- 12.Nathanson I, Conboy K, Murphy S, Afshani E, Kuhn JP. Ultrafast computerized tomography of the chest in cystic fibrosis: A new scoring system. Pediatr Pulmonol. 1991;11(1):81-86 [DOI] [PubMed] [Google Scholar]
- 13.Maffessanti M, Candusso M, Brizzi F, Piovesana F. Cystic fibrosis in children: HRCT findings and distribution of disease. J Thorac Imaging. 1996;11(1):27-38 [DOI] [PubMed] [Google Scholar]
- 14.Helbich TH, Heinz-Peer G, Eichler I, et al. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology. 1999;213(2):537-544 [DOI] [PubMed] [Google Scholar]
- 15.Shah RM, Sexauer W, Ostrum BJ, Fiel SB, Friedman AC. High-resolution CT in the acute exacerbation of cystic fibrosis: evaluation of acute findings, reversibility of those findings, and clinical correlation. AJR Am J Roentgenol. 1997;169(2):375-380 [DOI] [PubMed] [Google Scholar]
- 16.Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21(1):14-21 [DOI] [PubMed] [Google Scholar]
- 17.Robinson TE, Leung AN, Northway WH, et al. Spirometer-triggered high-resolution computed tomography and pulmonary function measurements during an acute exacerbation in patients with cystic fibrosis. J Pediatr. 2001;138(4):553-559 [DOI] [PubMed] [Google Scholar]
- 18.Robinson TE, Goris ML, Zhu HJ, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease: a quantitative analysis. Chest. 2005;128(4):2327-2335 [DOI] [PubMed] [Google Scholar]
- 19.Bonnel AS, Song SM, Kesavarju K, et al. Quantitative air-trapping analysis in children with mild cystic fibrosis lung disease. Pediatr Pulmonol. 2004;38(5):396-405 [DOI] [PubMed] [Google Scholar]
- 20.Loeve M, de Bruijne M, Hartmann IC, van Straten M, Hop WC, Tiddens HA. Three-section expiratory CT: Insufficient for trapped air assessment in patients with cystic fibrosis? Radiology. 2012;262(3):969-976 [DOI] [PubMed] [Google Scholar]
- 21.Owens CM, Aurora P, Stanojevic S, et al. ; London Cystic Fibrosis Collaboration Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax. 2011;66(6):481-488 [DOI] [PubMed] [Google Scholar]
- 22.Brody AS, Tiddens HA, Castile RG, et al. ; CT Scanning in Cystic Fibrosis Special Interest Group Computed tomography in the evaluation of cystic fibrosis lung disease. Am J Respir Crit Care Med. 2005;172(10):1246-1252 [DOI] [PubMed] [Google Scholar]
- 23.Worthy SA, Brown MJ, Müller NL. Technical report: Cystic air spaces in the lung: change in size on expiratory high-resolution CT in 23 patients. Clin Radiol. 1998;53(7):515-519 [DOI] [PubMed] [Google Scholar]
- 24.Pifferi M, Caramella D, Ragazzo V, Pietrobelli A, Boner AL. Low-density areas on high-resolution computed tomograms in chronic pediatric asthma. J Pediatr. 2002;141(1):104-108 [DOI] [PubMed] [Google Scholar]
- 25.Goris ML, Zhu HJ, Blankenberg F, Chan F, Robinson TE. An automated approach to quantitative air trapping measurements in mild cystic fibrosis. Chest. 2003;123(5):1655-1663 [DOI] [PubMed] [Google Scholar]
- 26.Jain N, Covar RA, Gleason MC, Newell JD, Jr, Gelfand EW, Spahn JD. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 2005;40(3):211-218 [DOI] [PubMed] [Google Scholar]
- 27.Kapur N, Masel JP, Watson D, Masters IB, Chang AB. Bronchoarterial ratio on high-resolution CT scan of the chest in children without pulmonary pathology: need to redefine bronchial dilatation. Chest. 2011;139(6):1445-1450 [DOI] [PubMed] [Google Scholar]
- 28.Sagel SS. Lung. In: Lee JKT, Sagel SS, Stanley RJ, Heiken JP, eds. Computed Body Tomography with MRI Correlation. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:421-568 [Google Scholar]
- 29.Marchac V, Emond S, Mamou-Mani T, et al. Thoracic CT in pediatric patients with difficult-to-treat asthma. AJR Am J Roentgenol. 2002;179(5):1245-1252 [DOI] [PubMed] [Google Scholar]
- 30.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338 [DOI] [PubMed] [Google Scholar]
- 31.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511-522 [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75-88 [DOI] [PubMed] [Google Scholar]
- 33.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187 [DOI] [PubMed] [Google Scholar]
- 34.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186(9):857-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns JL, Emerson J, Stapp JR, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27(1):158-163 [DOI] [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310 [PubMed] [Google Scholar]
- 37.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428 [DOI] [PubMed] [Google Scholar]
- 38.Bruce MC, Poncz L, Klinger JD, Stern RC, Tomashefski JF, Jr, Dearborn DG. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985;132(3):529-535 [DOI] [PubMed] [Google Scholar]
- 39.Stone PJ, Konstan MW, Berger M, Dorkin HL, Franzblau C, Snider GL. Elastin and collagen degradation products in urine of patients with cystic fibrosis. Am J Respir Crit Care Med. 1995;152(1):157-162 [DOI] [PubMed] [Google Scholar]
- 40.Stick SM, Brennan S, Murray C, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009; 155(5):623-628.e1 [DOI] [PubMed] [Google Scholar]
- 41.Palumbo AA, Luccichenti G, Belgrano M, et al. Three-dimensional quantitative assessment of lung parenchyma in cystic fibrosis: preliminary results. Radiol Med (Torino). 2007;112(1):21-30 [DOI] [PubMed] [Google Scholar]
- 42.Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr. 2004;144(2):154-161 [DOI] [PubMed] [Google Scholar]
- 43.Martínez TM, Llapur CJ, Williams TH, et al. High-resolution computed tomography imaging of airway disease in infants with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(9):1133-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaudon M, Berger P, Cangini-Sacher A, et al. Bronchial measurement with three-dimensional quantitative thin-section CT in patients with cystic fibrosis. Radiology. 2007;242(2):573-581 [DOI] [PubMed] [Google Scholar]
- 45.Brody AS. Computed tomography scanning in cystic fibrosis research trials: practical lessons from three clinical trials in the United States. Proc Am Thorac Soc. 2007;4(4):350-354 [DOI] [PubMed] [Google Scholar]
- 46.Lynch DA, Newell JD. Quantitative imaging of COPD. J Thorac Imaging. 2009;24(3):189-194 [DOI] [PubMed] [Google Scholar]
- 47.Goris ML, Robinson TE. Sampling density for the quantitative evaluation of air trapping. Pediatr Radiol. 2009;39(3):221-225 [DOI] [PubMed] [Google Scholar]
- 48.Johnston JH, Podberesky DJ, Yoshizumi TT, et al. Comparison of radiation dose estimates, image noise, and scan duration in pediatric body imaging for volumetric and helical modes on 320-detector CT and helical mode on 64-detector CT. Pediatr Radiol. 2013;43(9):1117-1127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement