Abstract
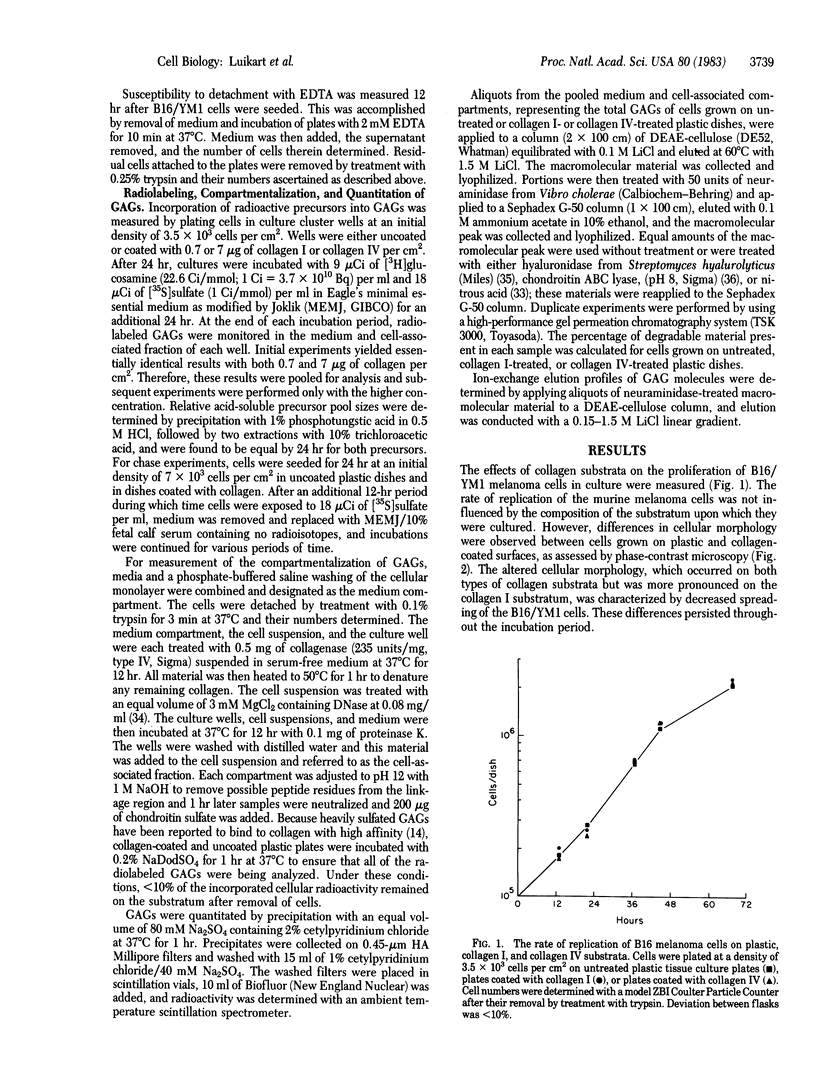
A cloned metastatic murine melanoma cell line exhibited similar growth characteristics when propagated on either type I collagen, type IV collagen, or plastic. However, cells grown on both types of collagen exhibited an altered cellular morphology and on type IV collagen only, an increased substrate adhesiveness, relative to those maintained on a plastic substratum. Incorporation of [3H]glucosamine and [35S]sulfate into glycosaminoglycans (GAGs) of cells grown on collagen substrates was 20% and 40% less, respectively, than cells grown on plastic, whereas degradation of cell-associated [35S]sulfate-labeled GAGs was similar in cells grown on collagen or plastic. Although the composition of GAGs was similar in all cultures, consisting of approximately 60% chondroitin and 40% heparin or heparan sulfate, the degree of sulfation of the heparin or heparan sulfate molecules was markedly decreased in cultures grown on collagen. The results indicate that the composition of the extracellular matrix influences the biological behavior of B16 melanoma cells, in part by altering the amount and nature of the GAG molecules produced.
Full text
PDF
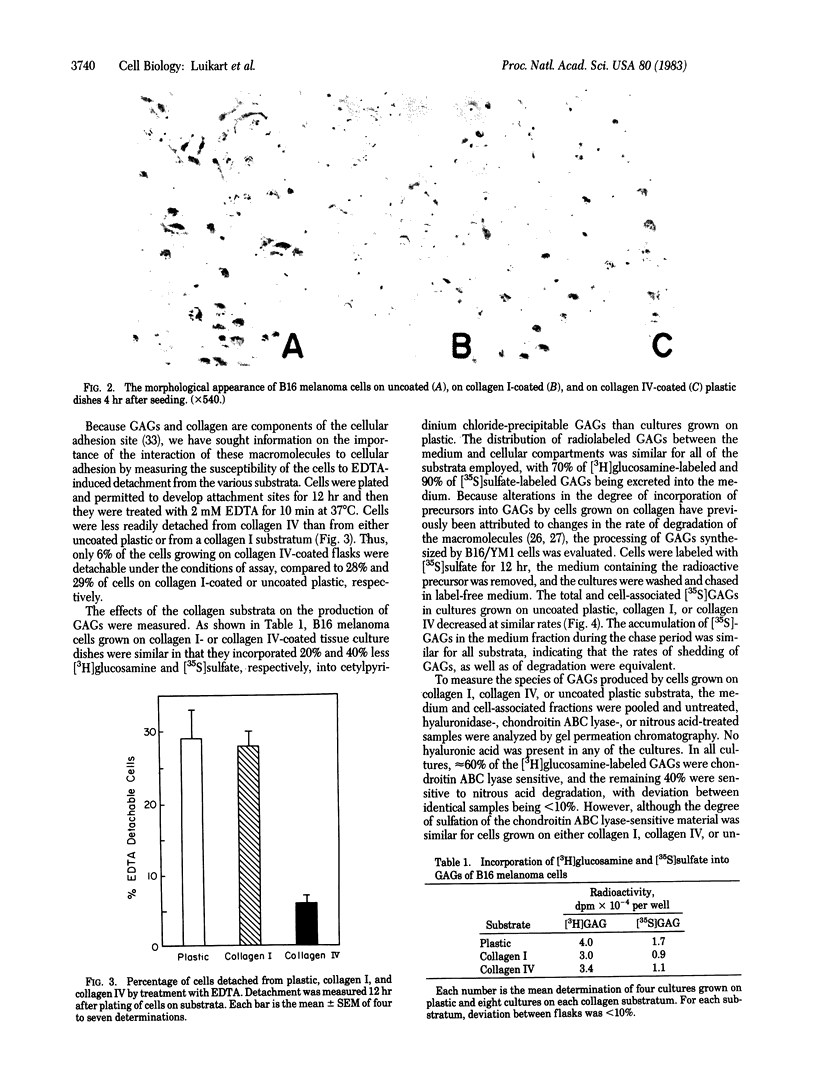
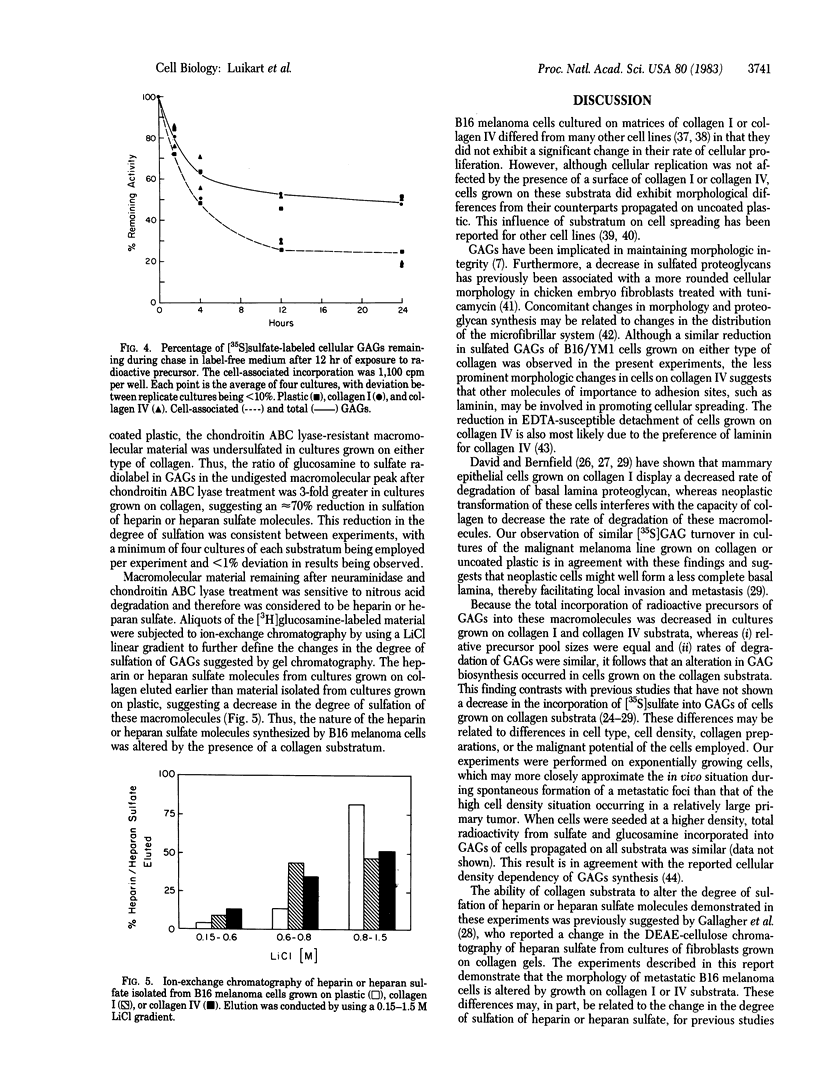

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angello J. C., Hauschka S. D. Hyaluronate-cell interaction. Effects of exogenous hyaluronate on muscle fibroblast cell surface composition. Exp Cell Res. 1980 Feb;125(2):389–400. doi: 10.1016/0014-4827(80)90133-0. [DOI] [PubMed] [Google Scholar]
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Burridge K., Murray A., Walsh M. L., Copple C. D., Bushnell A., McDougall J. K., Gallimore P. H. Modulation of cell surface glycocalyx: studies on large, external, transformation-sensitive protein. Ann N Y Acad Sci. 1978 Jun 20;312:366–381. doi: 10.1111/j.1749-6632.1978.tb16814.x. [DOI] [PubMed] [Google Scholar]
- Conrad G. W., Hart G. W. Heparan sulfate biosynthesis by embryonic tissues and primary fibroblast populations. Dev Biol. 1975 Jun;44(2):253–269. doi: 10.1016/0012-1606(75)90396-6. [DOI] [PubMed] [Google Scholar]
- Culp L. A., Rollins B. J., Buniel J., Hitri S. Two functionally distinct pools of glycosaminoglycan in the substrate adhesion site of murine cells. J Cell Biol. 1978 Dec;79(3):788–801. doi: 10.1083/jcb.79.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Bernfield M. Defective basal lamina formation by transformed mammary epithelial cells: a reduced effect of collagen on basal lamina (heparan sulfate-rich) proteoglycan degradation. J Cell Physiol. 1982 Jan;110(1):56–62. doi: 10.1002/jcp.1041100110. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. Type I collagen reduces the degradation of basal lamina proteoglycan by mammary epithelial cells. J Cell Biol. 1981 Oct;91(1):281–286. doi: 10.1083/jcb.91.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby M. A. Analysis of glycosaminoglycans within the extracellular environments encountered by migrating neural crest cells. Dev Biol. 1978 Oct;66(2):321–336. doi: 10.1016/0012-1606(78)90241-5. [DOI] [PubMed] [Google Scholar]
- Fisher M., Solursh M. The influence of the substratum on mesenchyme spreading in vitro. Exp Cell Res. 1979 Oct 1;123(1):1–13. doi: 10.1016/0014-4827(79)90416-6. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Gasiunas N., Schor S. L. Synthesis of glycosaminoglycans by human skin fibroblasts cultured on collagen gels. Biochem J. 1980 Aug 15;190(2):243–254. doi: 10.1042/bj1900243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K. D., Doller H. J., Hoar R. M. beta-D-xylosides cause abnormalities of growth and development in chick embryos. Nature. 1978 May 11;273(5658):151–154. doi: 10.1038/273151a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Handley C. J., Brooks P. R., Lowther D. A. Extracellular matrix metabolism by chondrocytes. VI. Concomitant depression by exogenous levels of proteoglycan of collagen and proteoglycan synthesis by chondrocytes. Biochim Biophys Acta. 1978 Dec 1;544(2):441–444. doi: 10.1016/0304-4165(78)90111-3. [DOI] [PubMed] [Google Scholar]
- Hronowski L., Anastassiades T. P. The effect of cell density on net rates of glycosaminoglycan synthesis and secretion by cultured rat fibroblasts. J Biol Chem. 1980 Nov 10;255(21):10091–10099. [PubMed] [Google Scholar]
- Jackson S. F. Environmental control of macromolecular synthesis in cartilage and bone: morphogenetic response to hyaluronidase. Proc R Soc Lond B Biol Sci. 1970 Sep 29;175(1041):405–453. doi: 10.1098/rspb.1970.0029. [DOI] [PubMed] [Google Scholar]
- Jones P. A., DeClerck Y. A. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980 Sep;40(9):3222–3227. [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Mourão P. A., Bonetti S. S. Quantitation of collagen - proteoglycan interaction in tissue sections. Connect Tissue Res. 1980;7(2):91–96. doi: 10.3109/03008208009152293. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosher R. A., Church R. L. Stimulation of in vitro somite chondrogenesis by procollagen and collagen. Nature. 1975 Nov 27;258(5533):327–330. doi: 10.1038/258327a0. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
- Laterra J., Ansbacher R., Culp L. A. Glycosaminoglycans that bind cold-insoluble globulin in cell-substratum adhesion sites of murine fibroblasts. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6662–6666. doi: 10.1073/pnas.77.11.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. Interaction between proteoglycan subunit and type II collagen from bovine nasal cartilage, and the preferential binding of proteoglycan subunit to type I collagen. Biochem J. 1976 Feb 1;153(2):259–264. doi: 10.1042/bj1530259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer T. F., Kochhar D. M. In vitro cartilage formation: effects of 6-diazo-5-oxo-L-norleucine (DON) on glycosaminoglycan and collagen synthesis. Dev Biol. 1979 Apr;69(2):517–528. doi: 10.1016/0012-1606(79)90309-9. [DOI] [PubMed] [Google Scholar]
- Markwald R. R., Fitzharris T. P., Smith W. N. Sturctural analysis of endocardial cytodifferentiation. Dev Biol. 1975 Jan;42(1):160–180. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Decker L. The effect of acid mucopolysaccharides and acid mucopolysaccharide-proteins on fibril formation from collagen solutions. Biochem J. 1968 Oct;109(4):517–526. doi: 10.1042/bj1090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Scott L. Interaction of epithelial cells and fibroblasts in culture: effect on glycosaminoglycan levels. Dev Biol. 1980 May;76(2):396–409. doi: 10.1016/0012-1606(80)90388-7. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Morris J. E., Hopwood J. J., Dorfman A. Biosynthesis of glycosaminoglycans in the developing retina. Dev Biol. 1977 Jul 15;58(2):313–327. doi: 10.1016/0012-1606(77)90094-x. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Liotta L., Rennard S. I., Martin G. R. Adhesion characteristics of murine metastatic and nonmetastatic tumor cells in vitro. Cancer Res. 1980 Feb;40(2):347–351. [PubMed] [Google Scholar]
- Obrink B. A study of the interactions between monomeric tropocollagen and glycosaminoglycans. Eur J Biochem. 1973 Mar 1;33(2):387–400. doi: 10.1111/j.1432-1033.1973.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Obrink B., Wasteson A. Nature of the interaction of chondroitin 4-sulphate and chondroitin sulphate-proteoglycan with collagen. Biochem J. 1971 Jan;121(2):227–233. doi: 10.1042/bj1210227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Larsen M. A., Johnston M. C. Migration of cranial neural crest cells in a cell-free hyaluronate-rich matrix. Dev Biol. 1975 Jun;44(2):298–305. doi: 10.1016/0012-1606(75)90400-5. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Yamada K. M., Olden K., Ohanian S. H., Hascall V. C. Tunicamycin-induced alterations in the synthesis of sulfated proteoglycans and cell surface morphology in the chick embryo fibroblast. Exp Cell Res. 1979 Feb;118(2):245–252. doi: 10.1016/0014-4827(79)90149-6. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Glycosaminoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Jan 9;18(1):141–148. doi: 10.1021/bi00568a022. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Sakashita S., Engvall E., Ruoslahti E. Basement membrane glycoprotein laminin binds to heparin. FEBS Lett. 1980 Jul 28;116(2):243–246. doi: 10.1016/0014-5793(80)80654-5. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Liotta L. A., Kidwell W. R. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981 Jan;78(1):382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Toole B. P. Binding and precipitation of soluble collagens by chick embryo cartilage proteoglycan. J Biol Chem. 1976 Feb 10;251(3):895–897. [PubMed] [Google Scholar]
- Toole B. P., Biswas C., Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6299–6303. doi: 10.1073/pnas.76.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Biswas C., Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6299–6303. doi: 10.1073/pnas.76.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley E. A., Roth S. Spontaneous glycosylation of glycosaminoglycan substrates by adherent fibroblasts. Cell. 1979 May;17(1):109–115. doi: 10.1016/0092-8674(79)90299-x. [DOI] [PubMed] [Google Scholar]
- Vertel B. M., Dorfman A. Simultaneous localization of type II collagen and core protein of chondroitin sulfate proteoglycan in individual chondrocytes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1261–1264. doi: 10.1073/pnas.76.3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Kimata K., Pratt R. M. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980 Jul 10;255(13):6055–6063. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]




