Abstract
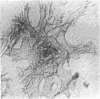
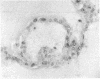
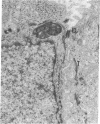
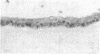
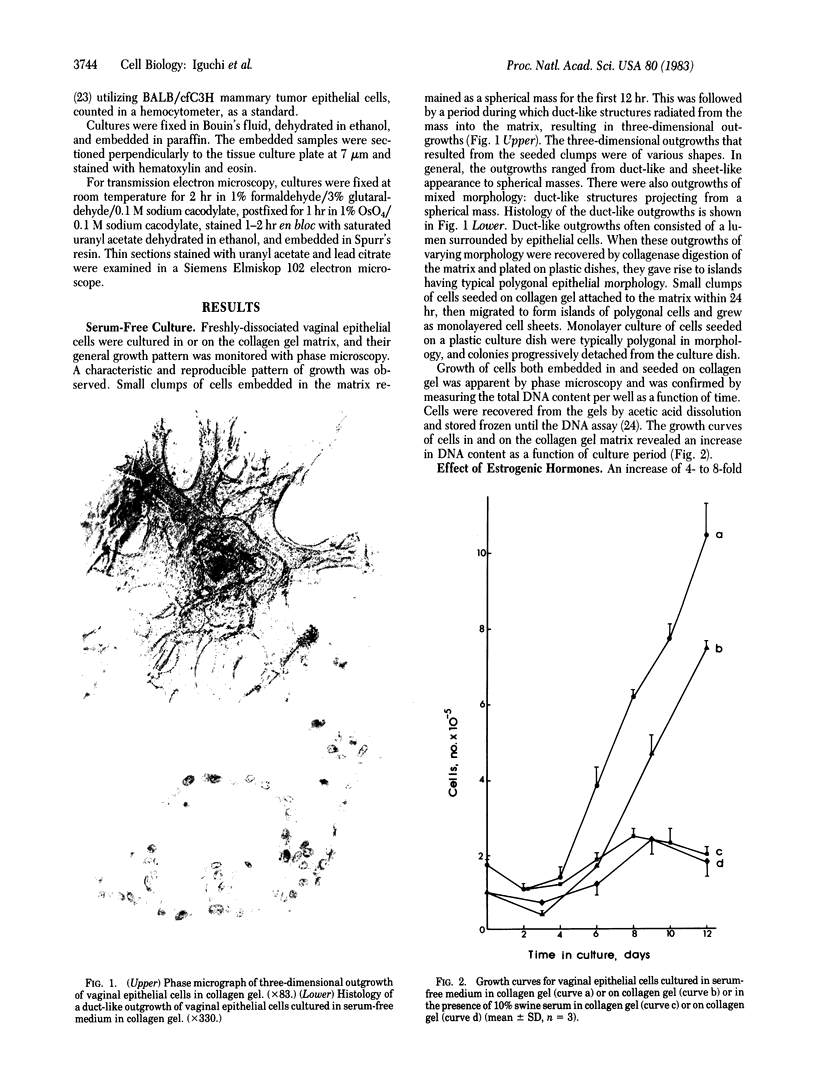
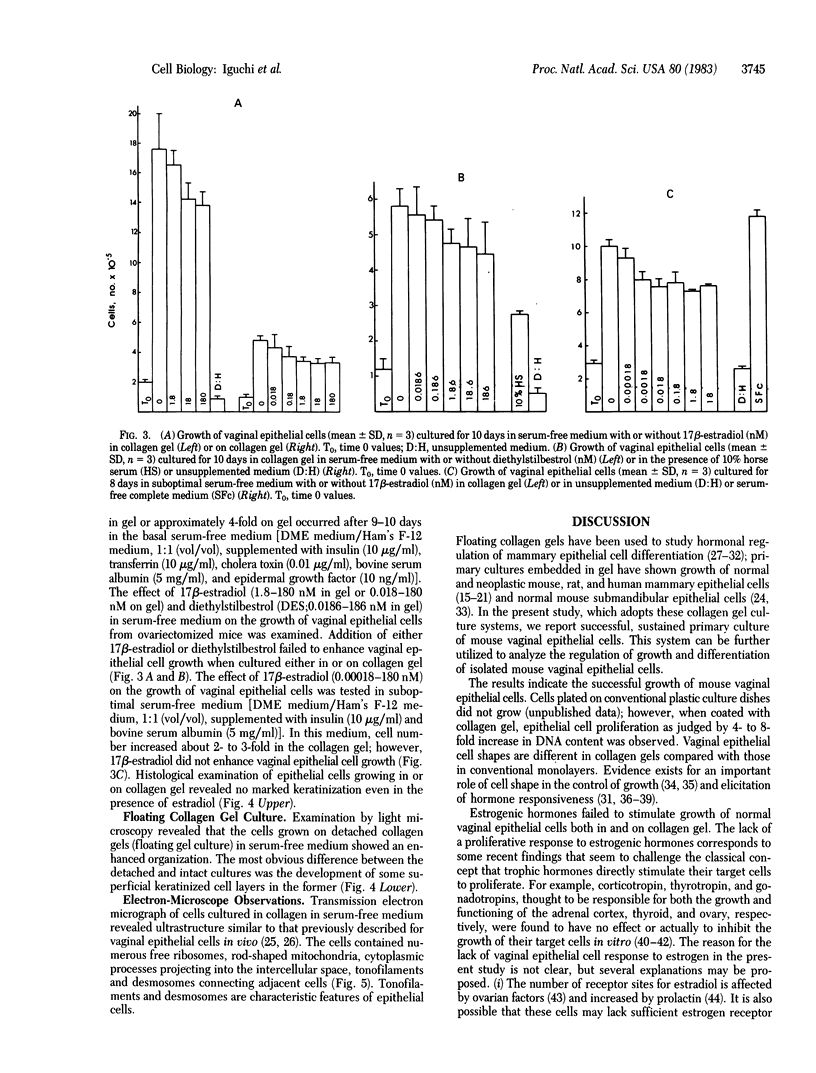
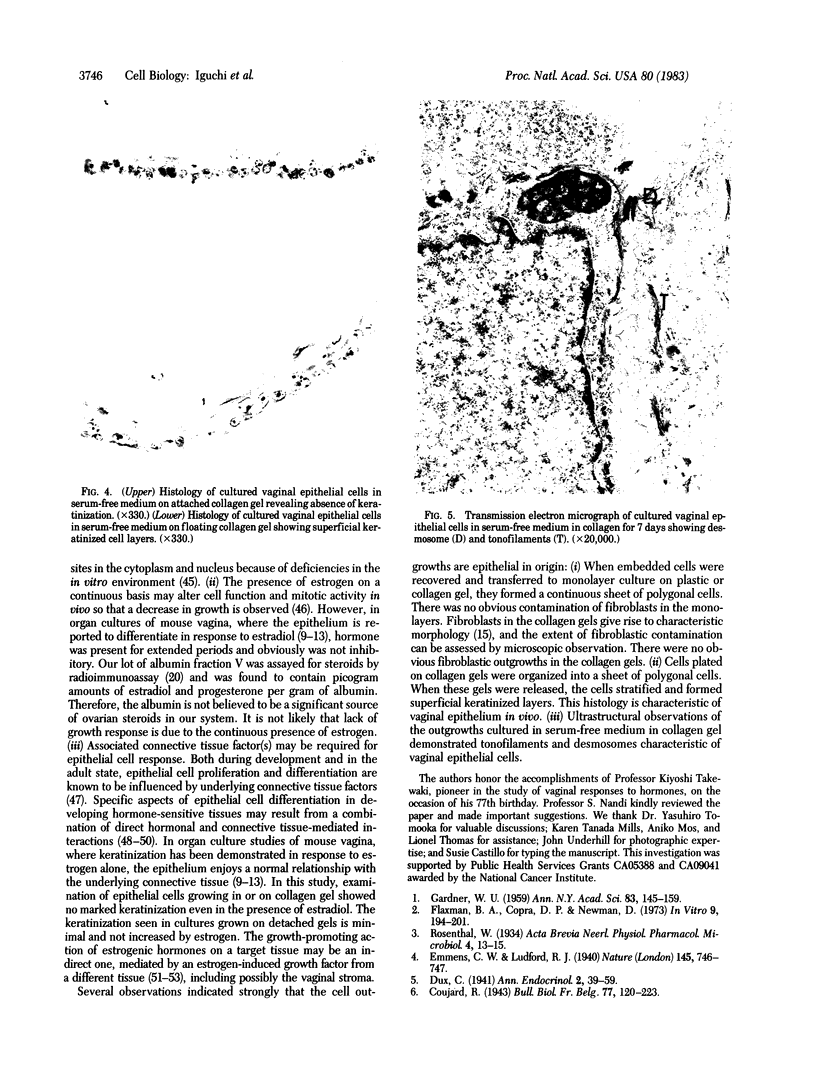
Sustained growth in primary culture of vaginal epithelial cells from ovariectomized adult BALB/cCrgl mice embedded within or seeded on collagen gel matrix was achieved in a serum-free medium composed of Ham's F-12 medium/Dulbecco's modified Eagle's medium, 1:1 (vol/vol), supplemented with insulin, bovine serum albumin fraction V, epidermal growth factor, cholera toxin, and transferrin. Three-dimensional growth of vaginal epithelial cells occurred inside the collagen gel matrix. Cell numbers increased 4- to 8-fold in collagen gel and about 4-fold on collagen gel after 9-10 days in culture. The effect of 17 beta-estradiol (0.00018-180 nM in gel or 0.018-180 nM on gel) and diethylstilbestrol (DES; 0.0186-186 nM in gel) on the growth of vaginal epithelial cells was examined. The addition of estrogen did not enhance the growth of vaginal epithelial cells during this time period either in the complete medium or in a suboptimal medium. Cultures on floating collagen gels in the serum-free medium are composed of 1-3 cell layers with superficial cornification. Estrogen does not appear to be a direct mitogen for vaginal epithelial cells, at least in this system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGERS J. D., CLARINGBOLD P. J., HARDY M. H. The action of oestrogens on the vagina of the mouse in tissue culture. J Physiol. 1956 Mar 28;131(3):497–515. doi: 10.1113/jphysiol.1956.sp005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwen S. J., Pitelka D. R. Secretory function of lactating mouse mammary epithelial cells cultured on collagen gels. Exp Cell Res. 1980 Apr;126(2):249–262. doi: 10.1016/0014-4827(80)90263-3. [DOI] [PubMed] [Google Scholar]
- Cunha G. R. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol. 1976;47:137–194. doi: 10.1016/s0074-7696(08)60088-1. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Shannon J. M., Neubauer B. L., Sawyer L. M., Fujii H., Taguchi O., Chung L. W. Mesenchymal-epithelial interactions in sex differentiation. Hum Genet. 1981;58(1):68–77. doi: 10.1007/BF00284152. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Burwen S. J., Pitelka D. R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11(1):109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Enami J., Nandi S. Secretion of casein in cultures of mouse mammary epithelial cells on floating collagen gels. J Dairy Sci. 1978 Jun;61(6):729–732. doi: 10.3168/jds.S0022-0302(78)83640-6. [DOI] [PubMed] [Google Scholar]
- Enami J., Yang J., Nandi S. Simultaneous production of casein and mammary tumor virus in mouse mammary epithelial cells grown on floating collagen gels. Cancer Lett. 1979 Feb;6(2):99–105. doi: 10.1016/s0304-3835(79)80007-5. [DOI] [PubMed] [Google Scholar]
- Flaxman B. A., Chopra D. P., Newman D. Growth of mouse vaginal epithelial cells in vitro. In Vitro. 1973 Nov-Dec;9(3):194–201. doi: 10.1007/BF02618437. [DOI] [PubMed] [Google Scholar]
- Fleming H., Blumenthal R., Gurpide E. Effects of cyclic nucleotides on estradiol binding in human endometrium. Endocrinology. 1982 Nov;111(5):1671–1677. doi: 10.1210/endo-111-5-1671. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Franks L. M., Riddle P. N., Carbonell A. W., Gey G. O. A comparative study of the ultrastructure and lack of growth capacity of adult human prostate epithelium mechanically separated from its stroma. J Pathol. 1970 Feb;100(2):113–119. doi: 10.1002/path.1711000206. [DOI] [PubMed] [Google Scholar]
- GARDNER W. U. Sensitivity of the vagina to estrogen: genetic and transmitted differences. Ann N Y Acad Sci. 1959 Nov 18;83:145–159. doi: 10.1111/j.1749-6632.1960.tb40889.x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. R., Birdwell C. R. Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro. I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology. 1977 Apr;100(4):1108–1120. doi: 10.1210/endo-100-4-1108. [DOI] [PubMed] [Google Scholar]
- HARDY M. H. Vaginal cornification of the mouse produced by oestrogens in vitro. Nature. 1953 Dec 26;172(4391):1196–1197. doi: 10.1038/1721196a0. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Ohta Y. Electron-microscopic study on the development of permanently proliferated and cornified vaginal epithelium in mice treated neonatally with androgen. Acta Anat (Basel) 1980;108(4):469–480. doi: 10.1159/000145346. [DOI] [PubMed] [Google Scholar]
- Imagawa W., Tomooka Y., Nandi S. Serum-free growth of normal and tumor mouse mammary epithelial cells in primary culture. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4074–4077. doi: 10.1073/pnas.79.13.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN R. H. Effect of oestrogen and of vitamin A on vaginal cornification in tissue culture. Nature. 1954 Aug 14;174(4424):317–317. doi: 10.1038/174317a0. [DOI] [PubMed] [Google Scholar]
- KAHN R. H. Vaginal keratinization in vitro. Ann N Y Acad Sci. 1959 Nov 18;83:347–355. doi: 10.1111/j.1749-6632.1960.tb40908.x. [DOI] [PubMed] [Google Scholar]
- LASNITZKI I. Effect of excess vitamin A on the normal and oestrone-treated mouse vagina grown in chemically defined medium. Exp Cell Res. 1961 Jun;24:37–45. doi: 10.1016/0014-4827(61)90244-0. [DOI] [PubMed] [Google Scholar]
- LASNITZKI I. The effect of radiation on the normal and oestrone-treated mouse vagina grown in vitro. Br J Radiol. 1961 Jun;34:356–361. doi: 10.1259/0007-1285-34-402-356. [DOI] [PubMed] [Google Scholar]
- McGuire J. L., Lisk R. D. Estrogen receptors in the intact rat. Proc Natl Acad Sci U S A. 1968 Oct;61(2):497–503. doi: 10.1073/pnas.61.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Sattler G. L., Pitot H. C. Hormonal regulation and the effects of glucose on tyrosine aminotransferase activity in adult rat hepatocytes cultured on floating collagen membranes. Cancer Res. 1978 Jun;38(6):1550–1555. [PubMed] [Google Scholar]
- Mori R., Nagahama Y., Bern H. A., Young P. N. Ultrastructural changes in vaginal epithelium of mice neonatally treated with estrogen and prolactin. Anat Rec. 1974 Jun;179(2):225–240. doi: 10.1002/ar.1091790206. [DOI] [PubMed] [Google Scholar]
- Pasco D., Quan A., Smith S., Nandi S. Effect of hormones and EGF on proliferation of rat mammary epithelium enriched for alveoli. An in vitro study. Exp Cell Res. 1982 Oct;141(2):313–324. doi: 10.1016/0014-4827(82)90219-1. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Bowman P. D., Yang J., McCormick K., Nandi S. Glucocorticoid regulation of prolactin receptors on mammary cells in culture. Endocrinology. 1979 May;104(5):1447–1449. doi: 10.1210/endo-104-5-1447. [DOI] [PubMed] [Google Scholar]
- Shafie S., Brooks S. C. Effect of prolactin on growth and the estrogen receptor level of human breast cancer cells (MCF-7). Cancer Res. 1977 Mar;37(3):792–799. [PubMed] [Google Scholar]
- Shannon J. M., Pitelka D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro. 1981 Nov;17(11):1016–1028. doi: 10.1007/BF02618428. [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A. Estrogen induction of growth factors specific for hormone-responsive mammary, pituitary, and kidney tumor cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3786–3790. doi: 10.1073/pnas.75.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VICENTE MARTIN J. [Apropos of 2 cases of abdominal ectopic pregnancy]. Rev Esp Obstet Ginecol. 1959 Nov-Dec;18:334–342. [PubMed] [Google Scholar]
- Westermark B., Karlsson F. A., Wålinder O. Thyrotropin is not a growth factor for human thyroid cells in culture. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2022–2026. doi: 10.1073/pnas.76.4.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Elias J. J., Petrakis N. L., Wellings S. R., Nandi S. Effects of hormones and growth factors on human mammary epithelial cells in collagen gel culture. Cancer Res. 1981 Mar;41(3):1021–1027. [PubMed] [Google Scholar]
- Yang J., Enami J., Nandi S. Regulation of mammary tumor virus production by prolactin in BALB/cfC3H mouse normal mammary epithelial cells in vitro. Cancer Res. 1977 Oct;37(10):3644–3647. [PubMed] [Google Scholar]
- Yang J., Flynn D., Larson L., Hamamoto S. Growth in primary culture of mouse submandibular epithelial cells embedded in collagen gels. In Vitro. 1982 May;18(5):435–442. doi: 10.1007/BF02796470. [DOI] [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Imagawa W., McCormick K., Nandi S. Growth factor- and cyclic nucleotide-induced proliferation of normal and malignant mammary epithelial cells in primary culture. Endocrinology. 1980 Jul;107(1):35–41. doi: 10.1210/endo-107-1-35. [DOI] [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Jentoft V., DeVault M. R., Wellings S. R., Nandi S. Primary culture of human mammary epithelial cells embedded in collagen gels. J Natl Cancer Inst. 1980 Aug;65(2):337–343. [PubMed] [Google Scholar]
- Yang J., Larson L., Nandi S. Three-dimensional growth and morphogenesis of mouse submandibular epithelial cells in serum-free primary culture. Exp Cell Res. 1982 Feb;137(2):481–485. doi: 10.1016/0014-4827(82)90057-x. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Richards J., Guzman R., Imagawa W., Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]