Abstract
Patients with mild-to-chronic kidney disease (CKD) exhibit a variety of haemostatic disorders, ranging from an increased clotting tendency and reductions in the levels of natural inhibitors of coagulation to defective fibrinolysis. In addition, platelet (PLT) abnormalities are common. In this minireview, we report on aspects of haemodialysis (HD)-induced PLT activation. It is demonstrated that PLTs from HD patients are exhausted due to repeated stimulation of HD treatment and recurrent release of PLT degranulation products. During HD, additional aberrations of the haemostatic process occur. Besides deviations of coagulation and fibrinolysis, PLT activation and a reduction in their granule content have been observed during HD treatment. As HD treatment is carried out three times per week, month after month, chronic HD patients may suffer persistently from coagulation defects and PLT disorders on top of the alterations induced by the uraemic state itself. PLT activation occurs together with thrombin and fibrin generation. However, macro fibrin depositions in clot devices are not demonstrated, microaggregates occur not only in the extracorporeal circuit (ECC) but are also present in the blood circulation. As vascular access thrombosis is a frequent complication in patients with HD treatment, it is believed that hypercoagulability could result from vascular changes combined with PLTs and activation of coagulation factors.
Keywords: coagulation, end-stage kidney disease, extracorporeal blood circulation, platelet activation
Introduction
In this review, aspects of platelet (PLT) disturbances and haemodialysis (HD) treatment are considered.
The haemostasis system contributes to a wide range of body defense systems which are essential for normal life. It impedes both the loss of blood and the disturbance of blood flow, but also provides for the repair of injured vasculature and tissue. Attacks by microorganisms are prevented by the formation of a temporary PLT and fibrin plug, which is dissolved at a later stage. In the case of haemostasis, processes are initiated to induce the formation of connective tissue and vessel wall revascularization. The main reactants implicate PLTs, vessel wall, coagulation factors, fibrinolysis factors, inhibitors of coagulation and fibrinolysis, calcium ions and phospholipids.
Patients with chronic kidney disease (CKD) exhibit a variety of haemostatic disorders, ranging from increased clotting tendency and reductions in the levels of natural inhibitors of coagulation to defective fibrinolysis [1–3]. Not only increased PLT activation has been described, but also defective PLT function occurs. Clinically, these disturbances are associated with the paradoxical observation of a thrombotic diathesis as well as an increased bleeding tendency. The pathogenesis of bleeding in uraemia is considered multifactorial. However, the major defects involve primary haemostasis because abnormalities in PLT–PLT and PLT–vessel wall interactions appear to be of crucial importance. Changes in PLT function are partially due to uraemic toxins present in circulating blood. Despite decreased PLT function, abnormalities of blood coagulation and fibrinolysis are less consistent and are more indicative of a hypercoagulable state carrying the risk of cardiovascular and thrombotic complications [4–6].
HD treatment improves PLT abnormalities and reduces, but does not eliminate, the risk of haemorrhage. The interaction between blood and artificial dialyser membrane surfaces and the use of anticoagulants may induce chronic activation of PLTs, leading to PLT exhaustion and aberrations in PLT function. The risk of bleeding may be minimized by using a low dose of high-molecular weight heparin (HMWH), the use of low-molecular weight heparin (LMWH) or regional anticoagulation with citrate to prevent clotting in the extracorporeal circuit (ECC) [7].
Despite their haemorrhagic tendency, uraemic patients have an activated coagulation system that is more prominent in those who are treated by HD. Uraemic subjects on HD are exposed to thrombotic complications related to their vascular access. Percutaneous cannulation, central vein catheters and native vein or prosthetic arteriovenous fistula are all associated with thrombotic occlusion. The risk factors for a hypercoagulable state include enhanced PLT aggregability, increased concentrations of plasma fibrinogen, thrombin–antithrombin complexes (TAT), prothrombin fragment F1 + 2, FVIIIc and von Willebrand Factor (vWF). It is suggested that coagulation aberrations and frequent PLT activation in the case of HD treatment contribute to increased risk of cardiovascular disease [2, 8]. Cardiovascular events related to thrombosis are a predominant cause of death and account for an important morbidity in patients with CKD.
Haemostasis
The process of blood coagulation activation can be divided into several stages. Steps mediated by the interaction of PLTs, vessel wall and plasma coagulation proteins are indicated as primary haemostasis (Figure 1), whereas the stage of fibrin formation, mediated by activated coagulation factors, is indicated as secondary haemostasis (Figure 2). The haemostatic system is balanced in order to maintain blood in a fluid state under physiological conditions, and to stop blood loss in the case of vascular injury. At the site of injury, PLTs adhere to the vessel wall within a period of seconds. Within a period of minutes, PLTs aggregate and blood coagulation is initiated. Haemostatic plug formation occurs within a period of minutes due to the synergistic action of multiple plasma factors and cellular elements.
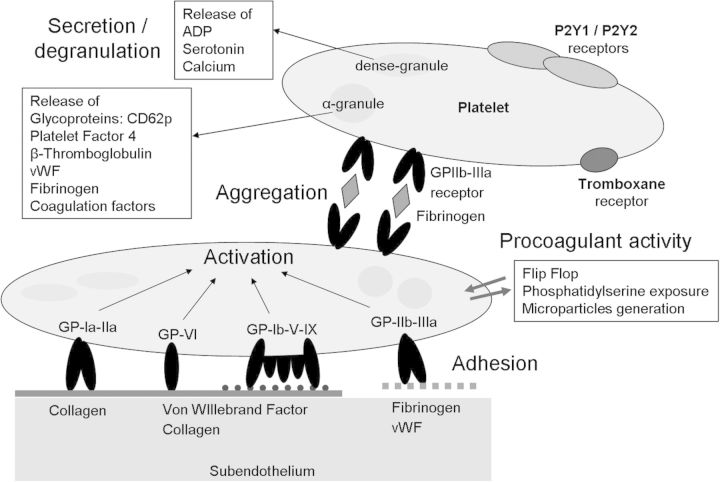
Fig. 1.
The role of PLT in primary haemostasis. Vascular wall injury results in the exposure of the subendothelial matrix including collagen. PLT adhesion is initially mediated by vWF, which serves as a bridge between collagen and the GPIb/IX/V complex on the PLT. Collagen binding to GPVI results in PLT activation. The cytoplasma of PLTs contain αlpha and dense granules. During PLT activation, the granules release their content, such as platelet factor 4, glycoproteins (GPs), ADP and serotonin. GP1b and GPIIb/IIIa serve as receptors and ligand-binding sites for vWF and fibrinogen. Both are important for firm PLT adhesion and aggregation via the activated GPIIb/IIIa receptor. PLT activation also results in a shape change of the PLTs through the formation of pseudopodia. The shape change and exposure of phosphatidyl serine and p-selectin (CD62p) on the PLT membrane result in a procoagulant PLT surface. Procoagulant activity results in the generation of thrombin and fibrin formation, and stabilization of the hemostatic plug.
Fig. 2.
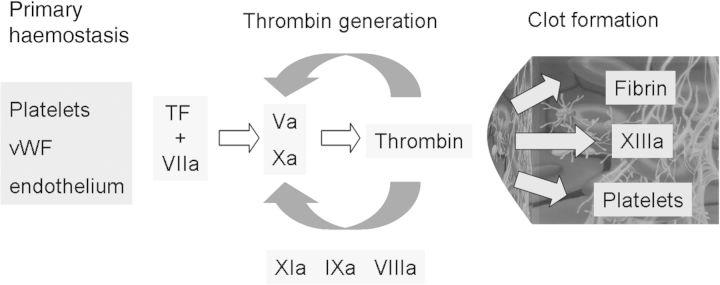
Three phases of coagulation from an analytical point of view. (i) Primary haemostasis: based on an interaction of platelets, vWF and endothelium. (ii) Thrombin generation: induced by tissue factor (TF) and Factor VIIa. The TF–VIIa complex activates the conversion of Factor X to Factor Xa and leads to the production of a small amount of thrombin. Thrombin is amplified by activation of intrinsic coagulation factors (VIIIa, IXa, XIa) and Factor V. The thrombin burst results in clot formation. (iii) Clot formation: the clot is formed by fibrin and activated PLTs and stabilized by Factor XIIIa. All three components are activated by thrombin, which is the key enzyme for clot formation.
PLT morphology, PLT activation and activation of the coagulation pathway
Laboratory parameters reflecting PLT characteristics, PLT activation and degranulation which are of relevance in detecting aberrations in haemostasis in subjects with HD treatment are demonstrated in Table 1. Evaluation of these markers is performed in order to assess poor dialyser membrane biocompatibility in the case of extracorporeal blood circulation [9–11]. PLT counts feature the balance between the activity of thrombopoiesis and the rate of PLT removal. The results of haemocytometric parameters demonstrate small changes in PLT count, MPV, platelet distribution width (PDW) and p-LCR during HD (Figure 3) [12]. In blood smears, the degree of PLT aggregation and morphological aspects of PLTs, more specific PLT granular density, can be classified by light microscopic evaluation [12]. Whereas granules containing PLT cytoplasm stain light purple or pink, PLT cytoplasma turns faintly grey after discharge of the granule content. In healthy individuals, >70% of PLTs reveals a staining density of >75% [13]. In HD patients, obviously decreased values are found [12].
Table 1.
Mini-overview of laboratory applications and physiological findings before, during and at the end of a HD treatment
| PLT characteristics | HD treatment | Physiological findings before, during, end HD | Reference |
|---|---|---|---|
| PLT, | PS, LMWH | n − ↓, n − ↓, n − ↓ | [12, 15] |
| PDW, MPV | PS, LMWH | n, n − ↓, n − ↓ | [12, 15] |
| % PLTs with <25% staining density | PS, LMWH | ↑, ↑ − ↑↑, ↑ | [15] |
| % PLTs with >75% staining density | PS, LMWH | ↓, ↓ − ↓↓, ↓ | [12, 15] |
| PLT activation and degranulation | |||
| CD62p% | PS, LMWH | n − ↑, ↑ − ↑↑ n − ↑ | [11, 12, 15, 18, 21] |
| PLT degranulation | |||
| PF4 (IU/mL) | PS, LMWH | n, ↑↑↑, ↑ − ↑↑ | [11, 12, 15, 18, 21] |
| β-BTG (IU/mL) | PS, LMWH | ↑, ↑↑, ↑ − ↑↑ | [15, 18] |
| Coagulation | |||
| TAT, F1 + 2, fibrinogen | PS, LMWH | n − ↑, n − ↑, ↑ − ↑↑ | [9, 10, 11, 15] |
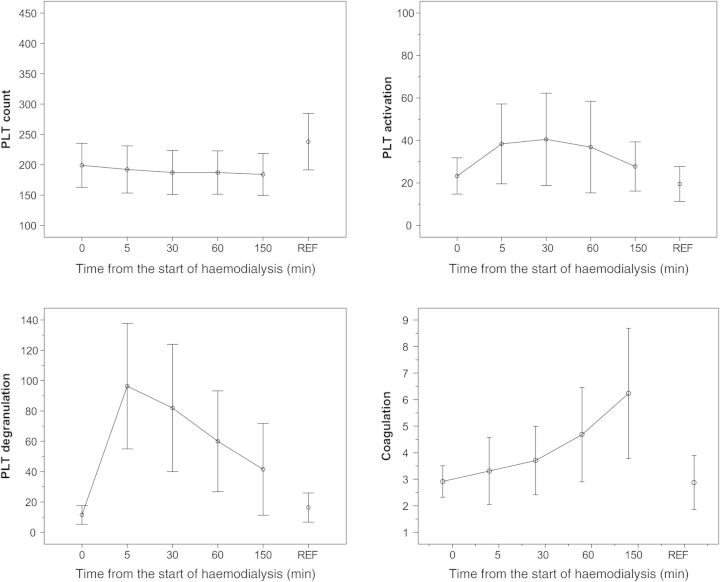
Fig. 3.
Overview of results for parameters reflecting PLT count (109/L), PLT activation, PLT degranulation and coagulation before and at 5, 30, 60 and 150 min after starting HD treatment. Anti-clotting agent: low-molecular weight heparin; membrane characteristics: polysulphone. For comparison, the laboratory data of a reference group of 20 healthy controls (age 20–50 years) (REF) are also demonstrated [12, 15]. HD, haemodialysis; LMWH, low-molecular weight heparin; PF4, platelet factor 4 (kIU/L); PLT, platelet; TAT, thrombin antithrombin (µg/L)
The degree of PLT activation is detected by longitudinally monitoring CD62p (Figure 3). PLT activation is indicated by an increase in the expression of CD62p on the PLT surface. CD62p (140 kD) is a granule membrane protein, exposed on the PLT surface membrane in the case of activation (Figures 1 and 3). The process of activation results in a partial release of PLT granules content in plasma, such as PLT Factor 4 (PF4) and β-thromboglobulin (β-TG) (Figures 1 and 3). Thus, PF4 and β-TG concentrations are markers concerning PLT degranulation which may be used in order to assess biocompatibility of extracorporeal blood treatment.
Activation of coagulation can be detected by monitoring the activity of coagulation factors in plasma and by means of coagulation markers such as TAT or prothrombin fragment F1 + 2 (Figure 3). During HD both the coagulation system and PLTs are activated (Figure 3). Activation of coagulation is a rather complicated multifactorial process initiated with interaction of PLTs, vWF and the vessel wall. PLTs are additionally activated due to contact with the artificial membrane during treatment with HD and heparin recruitment at the beginning of a dialysis session. PLT activation increases when thrombin generation is initiated by a release of the tissue factor from endothelium, Factor VIIa and Factor XIIa (Figure 2) [13, 14].
The coherence between PLT aggregation, PLT degranulation as detected by morphological evaluation and release of the PLT content as detected by establishment of concentrations of PF4 and β-TG is not yet elucidated [15]. Morphological PLT aberrations and PLT counts were considered simultaneously with HD-induced stimulation of markers for activation of PLTs and plasma coagulation factors. Surprisingly, interdependency indicating interaction could not be established [15]. In fact, neither deviations in PLT counts, CD62p nor any parameter of PLT degranulation correlated at any time interval with plasma concentrations of coagulation markers, such as TAT and prothrombin fragment F1 + 2 [15]. Within the ECC, endothelium is lacking and activation of PLTs and biomarkers concerning activation of coagulation are induced by mechanical triggers. It is conceivable that the highly unphysiological conditions within the ECC, adherence and release of activated PLTs from the dialyser membrane, explain the lack of a clear association between deviations in PLT activation and the coagulation system.
Aspects of extracorporeal blood circulation
Extracorporeal blood circulation is a complicated process because intravascular coagulation is induced [16]. Factors concerning thrombogenicity include reduction of blood flow, modifications in the blood vessel wall, changes in blood composition and biocompatibility of artificial membranes, respectively.
Dialysis procedure
The dialysis procedure itself will interfere with haemostasis. Compared with HD, both haemofiltration and haemodiafiltration (HDF) procedures demonstrate increased plasma concentrations of thrombin–antithrombin and D-dimers [17]. Recently, it was shown that PLT activation, as measured by upregulation of CD62p, is increased during HDF when compared with HD. Surprisingly, decreased β-TG levels are possibly due to loss of this substance through the high-flux dialyser membrane and excretion into the dialysate [18].
Artificial dialyser membranes
During HD treatment, blood of the subjects is exposed to foreign surfaces within the ECC, including the wall of blood lines and the artificial dialyser membrane, and also highly unphysiological mechanical forces of the roller pump. Furthermore, the effectiveness of the anticoagulation treatment results in an efficient HD process [11].
Acute-phase response parameters and a concomitantly increased degree of hypercoagulability are demonstrated to be exaggerated by definite characteristics of the dialyser membrane, for instance membrane permeability and chemical composition. Cuprophan and polyacrylonitrile membranes induce a higher degree of intradialytic PLT activation when compared with polysulphone and cellulose-triacetate membranes [9, 10, 19, 20].
Aspects of anti-clotting agents
PLT activation resulting in thrombus generation in the ECC is a multifactorial process, in which the dialysis membrane and other parts of the ECC, such as needle, pump and shunt, play a role. Probably the most important factor in PLT activation is the used anti-clotting agent like the kind of heparin (HMWH, LMWH) or trisodiumcitrate (TSC) [11, 16, 21]. To prevent clotting, in clinical practice a bolus of LMWH or unfractionated heparin is administered just before the start of HD. Despite adequate anticoagulation treatment, an increase of PLT activation and pro-coagulatory activity is shown in HD patients. During HD, PLTs are activated within the ECC, as illustrated by an increase in the expression of CD62p and release of β-TG within the ECC [18]. Previously, it was reported that PLT counts decline during HD. By taking blood samples at various time intervals from various sites, it is demonstrated that PLTs will aggregate and disaggregate across the entire length of the ECC, but only in small numbers and not sufficiently to explain a drop in PLT counts [21]. Apparently, activated PLTs stick to the artificial dialyser membrane. As a result of PLT activation during HD treatment micro PLT aggregates and thrombin generation occur. PLT aggregates are detected by microscopic evaluation on stained blood slides. Impaired PLT function is detected by means of a PLT function analyser (PFA-100™). The PFA-100™ system simulates in vitro haemodynamic conditions of PLT adhesion and aggregation in a vascular lesion. Within a group of HD patients, prolonged closure times with a PFA-100™ system are demonstrated as a result of PLT aggregates which do not fit tightly to the membrane (own observation, not published). Functionally, PLTs in the case of uraemia are less responsive to ADP-induced stimulation, as demonstrated with PLT aggregation tests in PRP plasma [22, 23]. Moreover, fibrinogen, which inhibits PLT function by competitive binding to the fibrinogen receptor GP IIb–IIIa on PLTs, is increased in uraemic plasma [24]. PLT aggregates block pores of the dialyser membrane, reducing exchange of harmful substances like urea. In practice, visible clotting in the air trap device is not observed.
It has been recently shown that the anti-clotting agent, which is administered during HD, affects the degree of PLT activation and degranulation [21]. In a comparative study between unfractionated heparin (UH), LMWH and TSC, it is demonstrated that UH and Fragmin® induce substantial increases in PLT activation, whereas PLT characteristics remain unaltered during HD with TSC [11, 21].
Consequences of repetitive PLT activation
PLT disturbancies are common in CKD patients and aggravate during HD treatment. Immediately after starting HD treatment, PLTs are being activated and release their content. PLT activation is illustrated by up-regulation of surface receptors and release of degranulation products. PLT activation results in increased turnover of PLTs. Usually the PLT count decreases slightly during the first hour of HD, but returns to initial values by the end of HD [25]. During HD treatment, statistically significant changes in PLT counts could not be detected. It is hypothesized that exhausted PLTs are continuously being removed from the circulation and new PLTs are released simultaneously [12]. The results of morphological analysis support the hypothesis that PLTs from HD patients are chronically exhausted [12, 15].
Alterations vary according to the stage of CKD, anticoagulant strategy and dialysis procedure. In spite of application of unfractionated heparin and LMWH during dialysis, haemostatic imbalance increases during the course of treatment [11, 16, 21]. Although significant activation of PLTs occurs during HD treatment, short-term and long-term effects of PLT activation on the microcirculation are mainly unknown [25]. It appears that during HD interactions between the anti-clotting agents, the haemostatic system and the endothelium exert a protective effect, at least against activation of the tissue factor coagulation pathway [26].
As PLT disturbances are already detected in CKD patients not yet on dialysis, although to a lesser extent, it is not elucidated whether HD-induced alterations of the haemostatic system will contribute to an increased risk of cardiovascular disease [12].
In clinical practice, alterations in PLT function are associated with a paradoxical observation of both a procoagulatory state, as demonstrated by recurrent vascular access failure, and increased bleeding tendency, as illustrated by a higher risk of gastrointestinal blood loss [27, 28]. Hypercoagulability is associated with cardiac disease, cerebral spill, pulmonary embolism as well as thrombus formation in vascular access for dialysis, particularly in the case of fistula with polytetrafluorethylene. Vascular complications represent 20–25% of hospitalizations of patients with HD treatment [29]. Stenosis is due to gradual hyperplasia of the intima and muscular layers of the vessels. Stenosis, in turn, results in blood flow reduction, which favours hypercoagulability. The majority of HD patients with recurrent problems of vascular access revealed higher amounts of circulating activated PLTs. Activated PLTs predispose to hypercoagulability yielding frequent occurrences of thrombosis in the vascular access [27]. Although PLTs play an important role in the explanation of HD-related symptoms and diseases, most data provide limited support to the above mentioned clinical effects, because of a lack of a clinically outcome study.
Conclusions
Three main conclusions can be drawn with respect to HD-induced PLT activation.
First, already before dialysis both activation markers on the PLT surface area and the PLT granule content are markedly decreased below the reference range. PLTs from chronic HD patients are chronically exhausted due to repeated stimulation and activation in the course of HD treatment and recurrent release of PLT degranulation products.
Second, during HD treatment, several alterations of the haemostatic process additionally occur. Besides deviations of coagulation parameters during HD, PLTs are activated (CD62p) and a concomitant reduction in the granule content is observed (% PLTs with >75% staining density, PF4, β-TG). Because changes occur three times a week, chronic HD patients reveal persistent coagulation defects and PLT activation in addition to alterations induced by a severe uraemic state itself.
Third, during HD treatment, PLT activation combined with thrombin and fibrin generation occurs. However, fibrinogen or fibrin depositions on clot devices could not be demonstrated. Nevertheless, microaggregates occur not only in the ECC but also in the blood circulation.
As vascular access thrombosis is a frequent complication in patients with HD treatment, it is believed that hypercoagulability could result from vascular changes combined with PLTs and coagulation factors activation. Unfortunately most data provide only limited support for linking repetitive PLT activation during HD treatment with HD-related symptoms and clinical outcomes. Treatment-related changes in the haemostatic balance might contribute to an in-vivo procoagulatory state. Hypercoagulability might be a risk factor for vascular access thrombosis and may contribute to an increased risk of cardiovascular disease. Much needs to be clarified in this interesting field before the contribution of dialysis-induced PLT alterations could be discussed in the light of clinically relevant end points, e.g. myocardial infarction, mortality, gastrointestinal bleeding.
Conflict of interest statement
None declared.
Footnotes
A version has been published to make this article Open Access.
References
- 1.Adams MJ, Irish AB, Watts GF, et al. Hypercoagulability in chronic kidney disease is associated with coagulation activation but not endothelial function. Thromb Res. 2008;123:374–380. doi: 10.1016/j.thromres.2008.03.024. doi:10.1016/j.thromres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Kushiya F, Wada H, Sakakura M, et al. Atherosclerotic and hemostatic abnormalities in patients undergoing hemodialysis. Clin Appl Thromb Hemost. 2003;9:53–60. doi: 10.1177/107602960300900107. doi:10.1177/107602960300900107. [DOI] [PubMed] [Google Scholar]
- 3.Undas A, Kolarz M, Kopec G, et al. Altered fibrin clot properties in patients on long-term haemodialysis: relation to cardiovascular mortality. Nephrol Dial Transplant. 2008;23:2010–2015. doi: 10.1093/ndt/gfm884. doi:10.1093/ndt/gfm884. [DOI] [PubMed] [Google Scholar]
- 4.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30:579–589. doi: 10.1055/s-2004-835678. doi:10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 5.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–322. doi: 10.1111/j.1525-139X.2006.00179.x. doi:10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009;22:279–286. doi: 10.1111/j.1525-139X.2008.00556.x. doi:10.1111/j.1525-139X.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 7.Lohr JW, Schwab SJ. Minimizing hemorrhagic complications in dialysis patients. J Am Soc Nephrol. 1991;2:961–975. doi: 10.1681/ASN.V25961. [DOI] [PubMed] [Google Scholar]
- 8.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. doi:10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 9.Bartels PCM, Schoorl M, Schoorl M, et al. Activation of coagulation during treatment with haemodialysis. Scand J Clin Lab Invest. 2000;60:283–290. doi: 10.1080/003655100750046440. doi:10.1080/003655100750046440. [DOI] [PubMed] [Google Scholar]
- 10.Bartels PC, Schoorl M, Schoorl M, et al. Deviations in coagulation activation due to treatment with different haemodialysis membranes. Scand J Clin Lab Invest. 2003;63:417–424. doi: 10.1080/00365510310002004. doi:10.1080/00365510310002004. [DOI] [PubMed] [Google Scholar]
- 11.Bartels PCM, Schoorl M, Schoorl M. Activatie van stolling tijdens hemodialyse is afhankelijk van de wijze van antistolling [Activation of coagulation during haemodialysis is dependent on the type of anti-coagulant] Ned Tijdschr Klin Chem Labgeneesk. 2005;30:282–284. [Google Scholar]
- 12.Schoorl M, Schoorl M, Bartels PC. Changes in platelet volume, morphology and RNA content in subjects treated with haemodialysis. Scand J Clin Lab Invest. 2008;68:335–342. doi: 10.1080/00365510701744481. doi:10.1080/00365510701744481. [DOI] [PubMed] [Google Scholar]
- 13.De Sanctis LB, Stefoni S, Cianciolo G, et al. Effect of different dialysis membranes on platelet function. A tool for biocompatibility evaluation. Int J Artif Organs. 1996;19:404–410. [PubMed] [Google Scholar]
- 14.Lang T, Depka von M. Possibilities and limitations of thromboelastometry/thromboelastography. Hämostaseologie. 2006;26:S21–S29. [Google Scholar]
- 15.Schoorl M, Schoorl M, Nub MJ, et al. Platelet depletion, platelet activation and coagulation during treatment with hemodialysis. Scand J Clin Lab Invest. 2011;71:240–247. doi: 10.3109/00365513.2011.558106. doi:10.3109/00365513.2011.558106. [DOI] [PubMed] [Google Scholar]
- 16.Opatrny K, Jr, Bouda M, Kohoutkova L, et al. A clinical study to assess the effect of heparin in dialyser rinsing solutions. Int J Artif Organs. 1997;20:112–118. [PubMed] [Google Scholar]
- 17.Klingel R, Schaefer M, Schwarting A, et al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol Dial Transplant. 2004;19:164–170. doi: 10.1093/ndt/gfg459. doi:10.1093/ndt/gfg459. [DOI] [PubMed] [Google Scholar]
- 18.Gritters-van den Oever M, Grooteman MP, Bartels PCM, et al. Post-dilution haemodiafiltration and low-flux haemodialysis have dissimilar effects on platelets: a side study of CONTRAST. Nephrol Dial Transplant. 2009;24:3461–3468. doi: 10.1093/ndt/gfp308. doi:10.1093/ndt/gfp308. [DOI] [PubMed] [Google Scholar]
- 19.Sirolli V, Ballone E, Di Stante S, et al. Cell activation and cellular-cellular interactions during hemodialysis: Effect of dialyzer membrane. Int J Artif Organs. 2002;25:529–537. doi: 10.1177/039139880202500607. [DOI] [PubMed] [Google Scholar]
- 20.Kuragano T, Kuno T, Takahashi Y, et al. Comparison of the effects of cellulose triacetate and polysulfone membrane on GPIIb/IIIa and platelet activation. Blood Purif. 2003;21:176–182. doi: 10.1159/000069157. doi:10.1159/000069157. [DOI] [PubMed] [Google Scholar]
- 21.Gritters M, Borgdorff P, Grooteman MP, et al. Platelet activation in clinical haemodialysis: LMWH as a major contributor to bio-incompatibility? Nephrol Dial Transplant. 2008;23:2911–2917. doi: 10.1093/ndt/gfn137. doi:10.1093/ndt/gfn137. [DOI] [PubMed] [Google Scholar]
- 22.Moal V, Brunet P, Dou L, et al. Impaired expression of glycoproteins on resting and stimulated platelets in uraemic patients. Nephrol Dial Transplant. 2003;18:1834–1841. doi: 10.1093/ndt/gfg185. doi:10.1093/ndt/gfg185. [DOI] [PubMed] [Google Scholar]
- 23.Bartels PCM, Heine SF, Schoorl M, et al. A new parameter for screening on platelet dysfunction in subjects treated with haemodialysis. Ned Tijdschr Klin Chem. 1998;23:73. [Google Scholar]
- 24.Thekkedath UR, Chirananthavat T, Leypoldt JK, et al. Elevated fibrinogen fragment levels in uremic plasma inhibit platelet function and expression of glycoprotein IIb-IIIa. Am J Hematol. 2006;81:915–926. doi: 10.1002/ajh.20720. doi:10.1002/ajh.20720. [DOI] [PubMed] [Google Scholar]
- 25.Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012;82:147–157. doi: 10.1038/ki.2012.130. doi:10.1038/ki.2012.130. [DOI] [PubMed] [Google Scholar]
- 26.Cardigan RA, Mackie IJ, Machin SJ. Hemostatic-endothelial interactions: A potential anticoagulant role of the endothelium in the pulmonary circulation during cardic surgery. J Cardiothorac Vasc Anesth. 1997;11:329–336. doi: 10.1016/s1053-0770(97)90103-8. doi:10.1016/S1053-0770(97)90103-8. [DOI] [PubMed] [Google Scholar]
- 27.Chuang YC, Chen JB, Yang LC, et al. Significance of platelet activation in vascular access survival of haemodialysis patients. Nephrol Dial Transplant. 2003;18:947–954. doi: 10.1093/ndt/gfg056. doi:10.1093/ndt/gfg056. [DOI] [PubMed] [Google Scholar]
- 28.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64:1455–1461. doi: 10.1046/j.1523-1755.2003.00225.x. doi:10.1046/j.1523-1755.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 29.Knoll GA, Wells PS, Young D, et al. Thrombophilia and the risk for hemodialysis vascular access thrombosis. J Am Soc Nephrol. 2005;16:1108–1114. doi: 10.1681/ASN.2004110999. doi:10.1681/ASN.2004110999. [DOI] [PubMed] [Google Scholar]