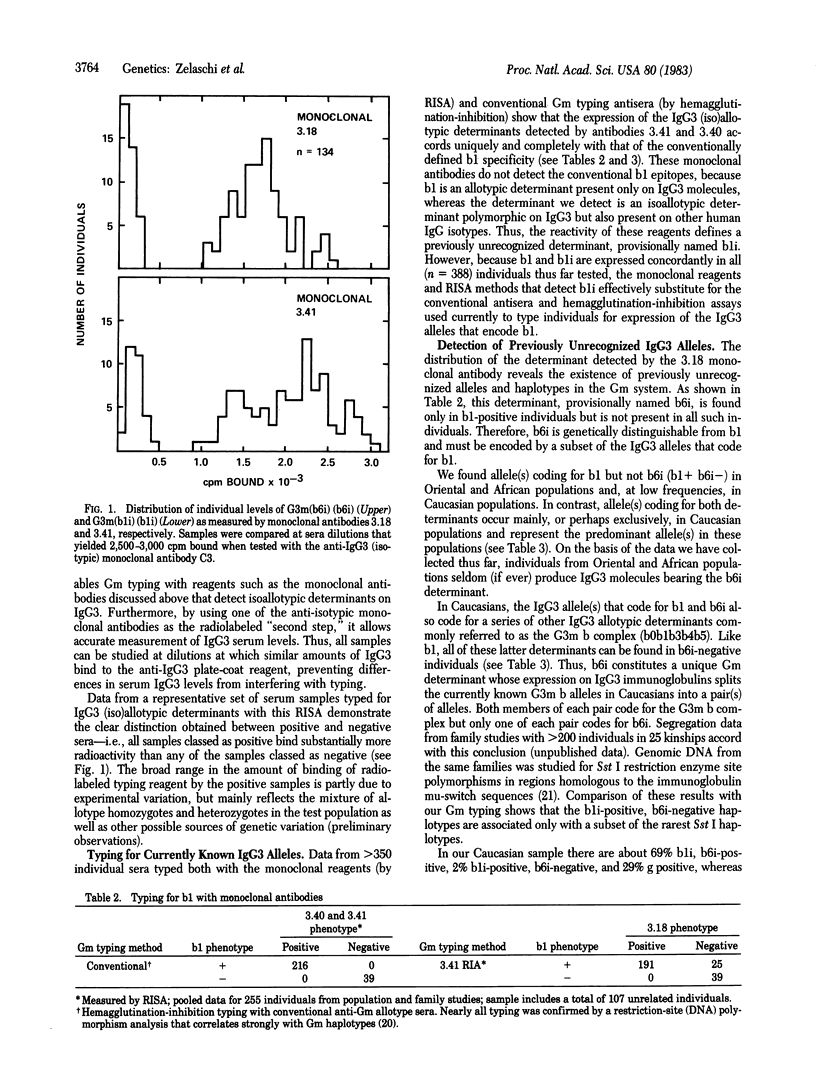
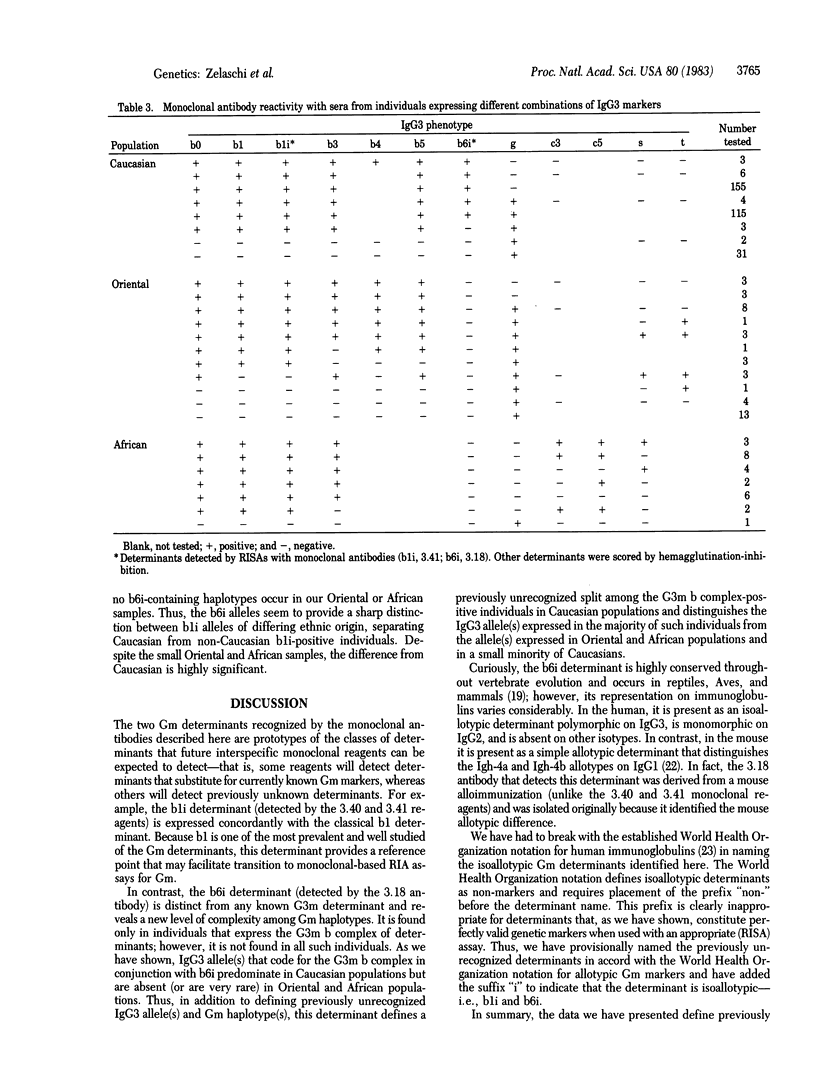
Abstract
The highly polymorphic system of serologically defined genetic markers on human IgG heavy chains (Gm allotypes) is second only to the HLA complex in terms of the large number of determinants, alleles, and haplotypes that can be used for analyses of disease associations and other genetic studies. However, present typing methods are based on the use of anti-Gm antisera that are derived mainly from fortuitously immunized human donors, often requiring processing before use, and must be used in a hemagglutination-inhibition assay that cannot be used in typing for isoallotypic determinants (currently termed "non-markers"). In studies presented here, we describe an allotyping system that utilizes monoclonal antibodies in a "sandwich" modification of the solid-phase radioimmunoassay, which is capable of reliable quantitative typing of allotypic, isoallotypic, and isotypic immunoglobulin determinants. We show that these highly reproducible, easily disseminated, and essentially inexhaustible reagents can be used for rapid, sensitive, and quantitative Gm typing. Using this system we define two previously unrecognized Gm determinants, one of which, found to date only in Caucasians, is different from all known Gm markers and thus defines previously unrecognized alleles and haplotypes. The other determinant co-segregates with the conventional G3m(b1) marker but is distinct from that marker on serological grounds. The successful preparation of mouse monoclonal antibodies that detect human Gm allotypic differences and the development of an assay system capable of typing isoallotypic as well as allotypic determinants opens the way to further dissection and application of this rich genetic system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farid N. R., Newton R. M., Noel E. P., Marshall W. H. Gm phenotypes in autoimmune thyroid disease. J Immunogenet. 1977 Dec;4(6):429–432. doi: 10.1111/j.1744-313x.1977.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Migone N., Feder J., Cann H., van West B., Hwang J., Takahashi N., Honjo T., Piazza A., Cavalli-Sforza L. L. Multiple DNA fragment polymorphisms associated with immunoglobulin mu chain switch-like regions in man. Proc Natl Acad Sci U S A. 1983 Jan;80(2):467–471. doi: 10.1073/pnas.80.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A., Käser H., Scherz R., Skvaril F. Uncommon Gm phenotypes in sera from neuroblastoma patients. J Immunol. 1977 Mar;118(3):1083–1085. [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Mizuno N., Arima N., Wakisaka A., Okimoto K., Akazawa Y., Tsuji K., Fujita T. IgG heavy-chain (Gm) allotypes and immune response to insulin in insulin-requiring diabetes mellitus. N Engl J Med. 1981 Feb 12;304(7):407–409. doi: 10.1056/NEJM198102123040706. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Nishitani H., Ota K., Fujita T., Tsuji K. Gm allotypes in myasthenia gravis. Lancet. 1980 Mar 29;1(8170):677–680. [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Nishitani H., Takatsuki K., Kasukawa R., Nakayama S., Izumi S., Fujita T., Tsuji K. IgG heavy chain allotypes (Gm) in autoimmune diseases. Clin Exp Immunol. 1980 Oct;42(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Watanabe S., Mukojima T., Kawashima R., Fujita T., Tsuji K. Immunoglobulin G heavy-chain allotypes as possible genetic markers for human cancer. J Natl Cancer Inst. 1981 Jul;67(1):47–50. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M., Herzenberg L. A. A monoclonal mouse antiallotype antibody reacts with certain human and other vertebrate immunoglobulins: genetic and phylogenetic findings. Immunogenetics. 1981;12(3-4):207–219. doi: 10.1007/BF01561665. [DOI] [PubMed] [Google Scholar]
- Salier J. P., Goust J. M., Pandey J. P., Fudenberg H. H. Preferential synthesis of the G1m(1) allotype of IgG1 in the central nervous system of multiple sclerosis patients. Science. 1981 Sep 18;213(4514):1400–1402. doi: 10.1126/science.6973823. [DOI] [PubMed] [Google Scholar]
- Salier J. P., Rivat L., Daveau M., Lefranc G., Breton P., de Menibus C. H., Henocq A., Fudenberg H. H. Quantitative studies of Gm allotypes. V. Simultaneous presence of latent Gm allotypes and deficient Gm genes in a family with hypogammaglobulinaemic probands. J Immunogenet. 1980 Apr;7(2):123–135. doi: 10.1111/j.1744-313x.1980.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Uno H., Sasazuki T., Tamai H., Matsumoto H. Two major genes, linked to HLA and Gm, control susceptibility to Graves' disease. Nature. 1981 Aug 20;292(5825):768–770. doi: 10.1038/292768a0. [DOI] [PubMed] [Google Scholar]
- Whittingham S., Mathews J. D., Schanfield M. S., Tait B. D., Mackay I. R. Interaction of HLA and Gm in autoimmune chronic active hepatitis. Clin Exp Immunol. 1981 Jan;43(1):80–86. [PMC free article] [PubMed] [Google Scholar]



