Abstract
Background: Prepubertal varicocele has the most devastating effects on the testes. Oxidative stress is the major cause leading to infertility in varicocele. The antioxidant properties of Flaxseed (FS) treatment in some oxidative diseases have been reported.
Objective: This study aimed to evaluate the antioxidant effect of FS in prepubertal rats with experimental varicocele.
Materials and Methods: Forty two male prepubertal rats were divided into 6 groups: the varicocele group were either fed with 10% FS, or with regular diet, or with Vit E, the group with sham operation fed with 10% FS, or had regular diet, and control rats who had not been operated but received regular diet. Varicocele was created by Koksal method. After 6 weeks sperm superoxide anion and H2O2 were evaluated by flowcytometery. Semen total antioxidant capacity (TAC) by Koracevic method and testes malondialdehyde (MDA) by thiobarbituric acid with spectrophotometry was measured.
Results: While superoxide anion and H2O2 were significantly higher in varicocele grop with regular diet (p=0.0001), FS significantly decreased the previously-mentioned parameters (p=0.0001). There were no significant differences for seminal TAC between 6 groups (p=0.07). Left testicular MDA concentration were lower in varicocele or group that were fed with 10% FS compared with other groups (p=0.001).
Conclusion: Reactive oxygen species (ROS) may cause sperm oxidative damage. FS as a fat soluble antioxidant can scavenge intracellular ROS production in varicocele.
Key Words: Varicocele, Flax, Oxidative stress, Antioxidants
Introduction
The incidence of varicocele in Prepubertal age is up to 20%, which is similar to the incidence of varicoceles in adults. Varicocele in adolescence has the most devastating effects on the testes. The exact mechanism is not clear but oxidative stress is considered as a major cause that leads to infertility (1-8).
The optimum age for treating a child with varicocele is still controversial (9). On the other hand just some cases of pediatric varicocele are infertile in the future and it remains difficult to identify which varicocele subjects should be treated (10-11). Surgical correction is not enough effective too (2, 9). So, most authors suggest a ‘‘wait and watch’’ approach to the evolution of pediatric varicocele (12).
Therefore, it is still a worthwhile subject to investigate pharmacological treatment for varicocele related infertility. Antioxidant therapy is effective in reducing the oxidative stress in men or animals; although it is not clear which antioxidant and which dose is more effective in male infertility (13). Flaxseed (FS) is a nutritional whole grain, containing numerous chemical constituents and lignans that possess antioxidant activity (14). Lignan of matairesinol and secoisolariciresinol diglycoside (SDG) can be converted into mammalian lignans by colonic bacteria such as enterodiol and entrolactone that have antioxidant properties (14-15). In some studies the oxygen radical scavenging properties of FS lignans by inhibition of lipid peroxidation or direct hydroxyl radical scavenging activity are demonstrated (16).
The efficiency of oral FS treatment in animal models of diabetes, endotoxic shock, lung injury and cancer has been noticed. One possible mechanism for FS is scavenging of radical species (17-19). In the proposed scheme, a radical abstracts a hydrogen atom from the lignan phenol hydroxyl, yielding a resonance-stabilized phenoxyl radical. The lignan radical could then react with another lignan radical, to form a stable product (20). Considering the evidence present in the current and older literature we have evaluated the antioxidant efficacy of FS in rats with prepubertal varicocele. Since FS is lipophilic antioxidant so it can easily pass the blood testis barrier and can induce direct antioxidant activity (14). It can also be orally assumed. Vitamin E is the most lipophilic antioxidant that is sometimes used in enhancing of sperm quality.
Hence in our experiment FS antioxidant’s properties was only compared with vitamin E. This work aims to extend our original findings on the protective role of FS in scavenging ROS in prepubertal induced varicocele.
Materials and methods
The study design was experimental intervention. Forty two male prepubertal Sprague-Dawley rats (25 days, 30-60 gr) were randomly divided in to 6 groups of seven rats. The first group was the control group and was fed with ordinary pellets. The second group underwent a sham operation. The third group (sham+FS) was sham operation that were fed base diet which was supplemented with 10% FS (wt/wt).
The varicocele group (fourth group) underwent partial ligation of the left renal vein. The fifth group (varicocele+FS) consisted of varicocele rats that were fed base diet which was supplemented with 10% FS. In order to compare the antioxidant effects of FS10% with a standard fat soluble antioxidant we chose vitamin E (α tocopherol acetate, Sigma-Aldrich, Steinheim, Germany). Therefore, the sixth group (varicocele+vit E) consisted of the varicocele group by injection of vitamin E (IM-100 mg kg-1/3 days in a week) (21). FS diet was the basal diet by adding 10% (wt/wt) freshly whole ground FS. The diets were stored at 4oC to avoid oxidative degradation and fresh diet was provided every 3 days (17-19, 22-24). To induce the varicocele to the animals, the rats were firstly anaesthetized with intra-peritoneal injection xylazine (15 mg/kg-1) and ketamine (75 mg/kg-1).
As a method described by Koksal et al, the upper left abdominal quadrant was approached through a midline laparotomy incision (25). The abdominal contents were gently pushed to the right. A tunnel was made under the left renal vein proximal to the venacava with tearing. By using the 24-gauge angiocatheter the left renal vein was ligated up to 50% with silk 4/0. An angiocatheter was interposed at the point medial to the insertion of the adrenal and spermatic vein into the left renal vein. The ligature was placed around the renal vein over the parallel angiocatheter. After placing the ligature, the angiocatheter was removed and abdominal wall was repaired (25).
Six weeks later, the left spermatic vein was checked for significant dilation and renal atrophy. Dilation of the internal spermatic veins was identified during this procedure as an internal control. All rats in experimental group which failed dilation or presence renal atrophy were excluded from the study (n=5). Sham operated prepubertal rats underwent a similar procedure except for the ligation. Samplings were conducted after 6 weeks when all rats were adults. After laparatomy the left and right caudal epididymis of the rats was used in sperm collection.
The caudal epididymis was placed into a Petri dish containing 1 mL of normal saline at 37oC for 5 minutes. For flow cytometric assays, sperm cells were separated by centrifugation (500g, 3 minutes, 37oC) to remove pellet serum. The cells were then resuspended in phosphate-buffered saline (PBS; pH=7.4, 37oC) to a final concentration of 1×106 cells/mL-1. To detect intracellular superoxide anion production, parallel samples were stained with Dihydroethidium (DHE) using 2 protocols, first DHE procedure, aliquots of 1×106 cells were incubated in PBS containing 100 μmol DHE (Sigma-Aldrich, Steinheim, Germany) for 15 minutes at 37oC in a dark place; then the samples were washed with PBS and immediately analyzed.
In the second DHE procedure, cells were stained with DHE, as described above. After being washed with PBS, the samples were loaded with menadione (1 mmol; Axxora Deutschland, Germany), incubated for 1 hour, and then analyzed immediately. Menadione can induce superoxide anion production and was used for the positive control group (26). To detect H2O2 in sperms, aliquots of 1×106 cells were incubated in PBS containing 10 μmol 2′, 7′-Dichlorofluorescin diacetate (DCF) (Sigma- Aldrich, Steinheim, Germany) for 30 minutes at room temperature in a dark place; in the second DCF procedure, cells were stained with DCF, as described above.
After being washed with PBS, the samples were loaded with 5μl H2O2 as positive control, incubated for 20 min. The samples were then analyzed immediately (27). Before analysis, all aliquots were counterstained with propidium iodide (PI) (Sigma- Aldrich, Steinheim, Germany) which assesses cell viability. This fluorochrome can enter dead cells through their damaged plasma membrane. The samples stained with DCF and DHE were then incubated in PI with 23.9 μmol for 5 minutes at 37oC in the dark and immediately analyzed (26).
All samples were analyzed with a Partec PAS FacScan Flow Cytometer (DAKO Cytomation, Denmark) with a 488-nm excitation laser and Flowmax software. A forward and side scatter gate was used to select single sperms out of clumps and debris. Approximately 10,000 gated events were analyzed per sample. Fluorescence from the DHE- stained sperm was collected in a fluorescence detector FL2 with a 585-nm and and DCF in a FL1 530 nm bandpass filter. To limit the evaluation of DHE fluorescence to viable spermatozoa, only the subpopulation of PI-negative sperm was included in the evaluation.
Fluorescent measurements were compensated to minimize spillover fluorescence between red and green spectrum. Data were reported as fluorescence intensity. Seminal plasma total antioxidant activity (Total Antioxidant Capacity, TAC) was measured using the Koracevic method. The assay measured the capacity of seminal plasma to inhibit the production of thiobarbituric acid reactive substance (TBARS) from sodium benzoate under the influence of ROS generated through the Fenton reaction. Uric acid solution was used as the standard (28). Malondialdehyde (MDA) is a stable product of lipid peroxidation. MDA assay was performed with thiobarbituric acid test in the supernatant, according to the method suggested by Cheeseman and Esterbaue (29).
The testes MDA results are expressed as nmol gr tissue-1. MDA reacts with thiobarbituric acid to give a red compound absorbing at 532 nm in a spectrophotometer. After laparatomy both testes were delivered into the abdomen. Testis tissue was excised from fat and adhering tissues and washed in cold saline, then transferred to a -70oC freezer. Tissues were homogenized in phosphate buffer (pH=7.4) and the supernatant was used for MDA experiments. The protocol of this study was approved by the Ethics Committee of Hormozgan University of Medical Sciences )HEC-91-4-6(.
Statistical analysis
The statistical analysis was performed with SPSS for windows (SPSS, Chicago, II). Numerical data were expressed as mean±SE. Statistical analysis was performed using one-way analysis of variance among groups and t test for comparing right and left data. The Kruskal-Wallis test was used when the distribution of data was not normal. P-value ≤0.05 was considered as statistically significant.
Results
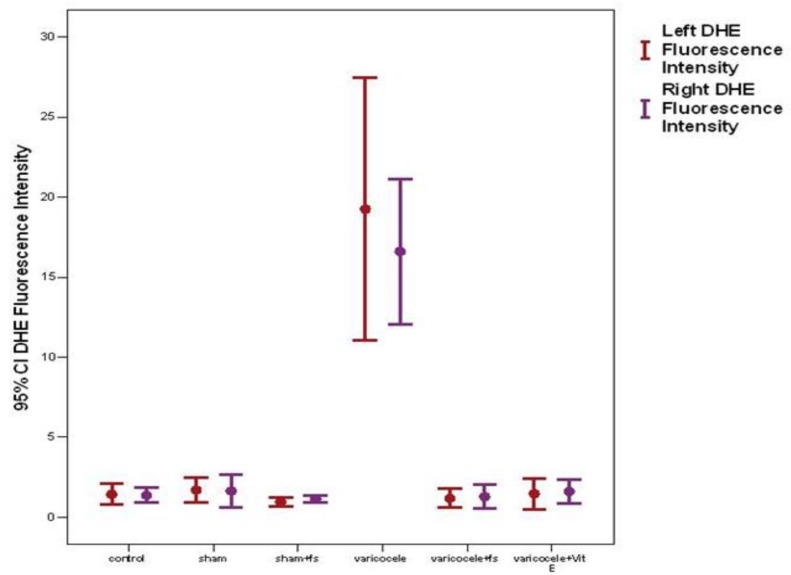
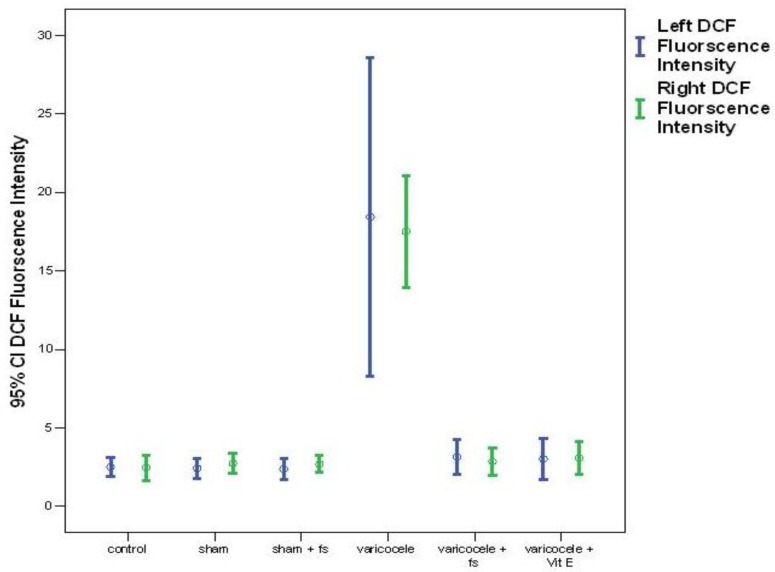
Dietary FS supplementation ameliorates sperm injury after varicocele. Intracellular superoxide anion production, as evaluated by using the fluorescence intensity of DHE-positive sperm cells, was significantly higher in varicocele groups compared with the control and sham groups both on the left and right sides (p=0.0001). But in varicocele+FS and varicocele+Vit E group it decreased significantly (p=0.0001). There was no difference between varicocele+FS and varicocele+Vit E groups (p=0.08, Figure 1). H2O2 production, as evaluated using the fluorescence intensity of DCF-positive sperm cells, was significantly higher in varicocele group compared with the control and sham groups both on the left and right sides (p=0.0001). But it decreased significantly in varicocele+FS and varicocele+Vit E groups. (p=0.0001). There was no difference between varicocele+FS and varicocele+Vit E groups (p=0.08, Figure 2).
Figure 1.
The effect of FS 10% on superoxide anion production in sperm cells on the left and right side
Figure 2.
The effect of FS 10% on H2O2 production on the sperm cells in left and right sides
The effect of flaxseed on total antioxidant capacity in the cudal epididymis in varicocele is shown in table I. There was no significant difference in the seminal plasma total antioxidant capacity among all groups (p=0.07). Testicular MDA concentration of varicocele+FS rats was lower on the left side (10.42±1.63) compared with varicocele groups (13.93±1.04), p=0.001).
Table I.
Effect of FS 10% on testicular MDA and cudal epididim TAC on the left and right side
|
MDA (nmol gr tissue
-1
)
|
TAC (mmol Lit
-1
)
|
|||||
|---|---|---|---|---|---|---|
| Left side | Right side | Left side | Right side | |||
| Control | 8.96 ± 1.1 | 9.26 ± 0.81 | 0.29± 0.02 | 0.27 ± 0.02 | ||
| Sham | 8.86 ±0.9 | 7.88 ± 0.76 | 0.25 ± 0.02 | 0.23 ± 0.01 | ||
| Sham+ FS | 9.1 ± 0.96 | 8.07 ± 0.7 | 0.28 ± 0.01 | 0.26 ± 0.02 | ||
| Varicocele | 13.93 ± 0.39* | 10.21 ± 0.49 | 0.23 ± 0.01 | 0.2 ± 0.03 | ||
| Varicocele+ FS | 10.42 ± 0.61≠ | 8.88 ± 0.58 | 0.22 ± 0.02 | 0.16 ± 0.02 | ||
| Varicocele+ vitamin E | 9.9 ± 0.9≠ | 7.6 ± 0.59 | 0.16 ± 0.01 | 0.21 ± 0.01 | ||
MDA= Malondialdehyde.
TAC= Total Antioxidant Capacity.
Values are mean±SEM
Significant difference compared to control and sham groups (p<0.05).
Significant difference compared to varicocele group (p≤0.05).
Consuming FS as Vitamin E (9.90±2.40, p=0.001) decreased testicular MDA. No significant difference was seen between varicocele+FS and varicocele+vitamin E (p=0.06, Table I).
Discussion
Our results showed a significant increase in the production of superoxide anion and H2O2 inside the sperms in both sides in rat with varicocele compared with the control and sham groups. However, rats supplemented with FS had a lower production level of superoxide anion and H2O2. So it seems that dietary FS are protective against oxidative stress in rats with varicocele. There was no similar study to ours but the oxygen hydroxyl radical scavenging activity of FS has been shown in previous studies (18, 24).
FS showed antioxidant activities in diabetes, in carbon tetrachloride-induced oxidative stress, radiotrapy, cancer and also in invitro models (17-19, 23, 30, 31). Some antioxidants like fat soluble vitamin E neutralize radical species by forming stable non- radical products. It is possible that FS and vitamin E have similar mechanisms. A possible mechanism for lignans of FS is described by Hosseinian et al. In this proposed mechanism a radical can abstract a hydrogen atom from the lignan phenol hydroxyl and produce a stable radical so that the lignan radical reacts with a second radical and forms a stable product (20).
Salama et al reported that polymorph nuclear leukocytes accumulate in testicular tissue of rats with varicocele (32). There are some reports about the ability of FS in inhibition of oxygen free radicals by neutrophils, so the other possible benefit of FS in varicocele is that FS lignans can inhibit the production of ROS by neutrophils (14, 24).
Although, the TAC level of seminal plasma among the groups was not statistically significant, as reported by Elsayed et al, the possible clarification is mobilization of antioxidant from other organs, for example the liver response to oxidative stress (33). Jafari et al also reported no change in TAC level in adult varicocele induction rats (26). In some clinical studies there was also no difference between TAC in seminal plasma of the control group and patients with varicocele (34).
In our finding, FS has no effect on TAC. Although in Naqshbandi et al research dietary supplementation of flaxseed oil in Cisplatin treated rats increased the activities of catalase, superoxide dismutase and glutathione peroxidase (35). MDA level measurements are widely used as an indicator of lipid peroxidation. In some adolescent patients with varicocele, the MDA level increases (36).
In our study, left testes tissue levels of MDA increased in rats with varicocele compared with the control and sham groups. Rats fed with FS 10% or those that received vitamin E had a significantly lower level of MDA on the left side. There was no similar study to compare but other studies confirm the effect of FS in decreasing MDA levels in other tissues.
Lee et al showed that mice that were fed FS 10% and then underwent pulmonary ischemia reperfusion had a lower level of MDA in the pulmonary tissue (16). In Kinniry et al study, the same effect was also seen in the lung tissue. In rats with diabetes mellitus, SDG (the lignan of FS) treatment lowered serum and pancreatic MDA, and diabetes did not develop. FS lignan like SDG has antioxidant activity and they can scavenge of reactive oxygen specious (ROS) production (17). Solomidou et al reported that, mice fed with a 10%FS diet (whether given preventively or therapeutically) maintained lower MDA level at 4 months post- X-ray radiation therapy (23).
In a randomized crossover research in obese and diabetic patients’ consumption of FS in form of ground grain or bread for 12 weeks decreased TBARS. TBARS is an indicator for lipid peroxidation, and it is measured in MDA equivalents. FS decreases lipid peroxidation by scavenging hydroxyl radical (23). Increased production of ROS and/or the decrease of local antioxidant capacity, result in oxidative stress impairment of sperm parameters in varicocele. Several studies have reported the effects of increased oxidative stress in the serum, semen, and testicular tissues of patients with varicocele.
However, the etiology of oxidative stress elevation in association with varicoceles is unclear (30). A metaanalysis of four studies published in 2006 showed patients with varicocele had significantly greater ROS and lower TAC levels. Similar to the findings in the spermatic vein, the finding of increased MDA and NO levels in the semen of patients with varicocele is also suggestive of oxidative stress (36). Several naturally occurring antioxidants have been studied in stress oxidative related disease, but some may produce undesirable side effects, thus limiting their benefit under which it can be used safely (24). Flaxseed is a safe, economically affordable nutritional agent, which is currently used in a number of clinical trials and can thus prove to be a useful alternative (30, 37).
Dietary flaxseed has high contents of omega-3 fatty acid and lignans with antioxidant properties (16). Some study show that plasma levels of FS lignans in human subjects that fed 25 g of raw FS for 8 days is comparable to rodent. If the bioactive components of FS can be isolated and formulated, it would be effective in stress oxidative related disease (24). One of the limitations of our study is that pregnancy rate was not measured. Pregnancy rate is the most effective outcome in varicocele treatment. Unfortunately we did not measure DNA fragmentation. FS could reduce DNA fragmentation too. We conclude that dietary FS can reduce sperm and testis oxidative damage in prepubertal varicocele model and this protective effect may in part be mediated by scavenging of ROS. However there is a need for prospective randomized clinical trials including pregnancy rates on the basis of this experimental observation to reach definitive conclusion.
Conflict of interest
The authors declare that there is no conflict of interest in this article.
References
- 1.Shiraishi K, Takihara H, Matsuyama H. Effects of grade 1 varicocele detected in the pediatric age-group on testicular development. J Pediatric Surg . 2009;44:1995–1998. doi: 10.1016/j.jpedsurg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Razi M, Sadrkhanloo RA, Malekinejad H, Sarrafzadeh-Rezaei F. Testicular biohistochemical alterations following experimental varicocele in rats. Iran J Reprod Med . 2012;10:209–218. [PMC free article] [PubMed] [Google Scholar]
- 3.Moein MR, Khalili MA, Davoudi A. The effect of oral administration of Pentoxifylline on sperm motility of asthenozoospermic ejaculates from men with or without testicular varicoceles. Iran J Reprod Med . 2005;3:25–29. [Google Scholar]
- 4.Moein MR, Soleimani M, Tabibnejad N. Reactive oxygen species (ROS) production in seminal fluid correlates with the severity of varicocele in infertile men. Iran J Reprod Med . 2008;6:65–69. [Google Scholar]
- 5.Amirzargar MA, Yavangi M, Basiri A, Hosseini Moghaddam SM, Babbolhavaeji H, Amirzargar N, et al. Comparison of recombinant human follicle stimulating hormone (rhFSH), human chorionic gonadotropin (HCG) and human menopausal gonadotropin (HMG) on semen parameters after varicocelectomy: a randomized clinical trial. Iran J Reprod Med . 2012;10:441–452. [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel Aziz MT, Mostafa T, Atta H, Kamal O, Kamel M, Hosni H, et al. Heme oxygenase enzyme activity in seminal plasma of oligoasthenoteratozoospermic males with varicocele. Andrologia. 2010;42:236–241. doi: 10.1111/j.1439-0272.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- 7.Cocuzza M, Athayde KS, Alvarenga C, Srougi M, Hallak J. Grade 3 varicocele in fertile men: a different entity. J Urol . 2012;187:1363–1368. doi: 10.1016/j.juro.2011.11.114. [DOI] [PubMed] [Google Scholar]
- 8.Zini A, Dohle G. Are varicocele associated with increased deoxyribonucleic acid fragmentation? Fertil Stril . 2011;96:1283–1286. doi: 10.1016/j.fertnstert.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Fideleff H, Boquete H, Suarez M, Ruibal G, Sobrado P, Azaretsky M, et al. Controversies in the evolution of paediatric-adolescent varicocele: clinical, biochemical and histological studies. Eur J Endocrinol . 2000;143:775–781. doi: 10.1530/eje.0.1430775. [DOI] [PubMed] [Google Scholar]
- 10.Giagulli VA, Carbone MD. Varicocele correction for infertility: which patients to treat? Int J Androl . 2010;34:236–341. doi: 10.1111/j.1365-2605.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- 11.Keene D JB, Sajad Y, Rakoczy G, Cervellione RM. Testicular volume and semen parameters in patients aged 12 to 17 years with idiopathic varicocele. J pediatr surg . 47:383–385. doi: 10.1016/j.jpedsurg.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Zampieri N, Pellegrino M, Ottolenghi A, Camoglio FS. Effects of bioflavonoids in the management of subclinical varicocele. Pediatr Surg Int . 2010;25:505–508. doi: 10.1007/s00383-010-2574-9. [DOI] [PubMed] [Google Scholar]
- 13.Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchtti A, Innominato PF, Pace G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26:97–102. doi: 10.1007/s00345-007-0218-z. [DOI] [PubMed] [Google Scholar]
- 14.Basch E, Steve Bent M, Collins J, Dacey C, Hammerness P, Harrison M, et al. Flax and Flaxseed Oil (Linum usitatissimum): A Review by the Natural Standard Research Collaboration. J Soc Integ Oncol . 2007;5:92–105. doi: 10.2310/7200.2007.005. [DOI] [PubMed] [Google Scholar]
- 15.Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr . 2010;103:929–938. doi: 10.1017/S0007114509992753. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, et al. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther . 2009;8:47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinniry P, Amrani Y, Vachani A, Solomides C, Arguiri E, Workman A, et al. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr . 2006;136:1545–1551. doi: 10.1093/jn/136.6.1545. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Bhora F, Sun J, Cheng G, Arguiri E, Solomides C, et al. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol . 2008;294:255–265. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Hui E, Ip T, Thompson LU. Dietary flaxseed enhances the inhibitory effect of tamoxifen on the growth of estrogen-dependent human breast cancer (mcf-7) in nude mice. Clin Cancer Res . 2004;10:7703–7711. doi: 10.1158/1078-0432.CCR-04-1130. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinian FS, Muir AD, Westcott ND, Krol ES. AAPH-mediated antioxidant reactions of secoisolariciresinol and SDG. Organ Biomol Chem . 2007;5:644–654. doi: 10.1039/b617426d. [DOI] [PubMed] [Google Scholar]
- 21.Hajiani M, Golestani A, Shariftabrizi A, Rastegar R, Payabvash S, Hassanzadeh A, et al. Dose-dependent modulation of systemic lipid peroxidation and activity of anti-oxidant enzymes by vitamin E in the rat. Redox Report . 2008;13:60–66. doi: 10.1179/135100008X259114. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Tan KP, Ward WE, Thompson LU. Exposure to flaxseed or its purified lignan during suckling inhibits chemically induced rat mammary tumorigenesis. Exp Biol Med . 2003;228:951–958. doi: 10.1177/153537020322800811. [DOI] [PubMed] [Google Scholar]
- 23.Christofidou-Solomidou M, Tyagi S, Tan KS, Hagan S, Pietrofesa R, Dukes F, et al. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer. 2011;11:269–284. doi: 10.1186/1471-2407-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razi SS, Latif MJ, Li X, Afthinos JN, Ippagunta N, Schwartz G, et al. Dietary Flaxseed Protects Against Lung Ischemia Reperfusion Injury Via Inhibition of Apoptosis and Inflammation in a Murine Model. J Surg Res . 2011;171:113–121. doi: 10.1016/j.jss.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Koksal IT, Tefekli A, Usta M, Erol H, Abbasoqlu S, Kadioqlu A. The role of reactive oxygen species in testicular dysfunction associated with varicocele. BJU . 2000;86:261–265. doi: 10.1046/j.1464-410x.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- 26.Jafari A, Zahmatkesh M, Sadeghipour H, Kajbafzadeh A, Sarrafnejd A, Shahrestany T, et al. Flow Cytometric Evaluation of Sperm Superoxide Anion Production in Rats With Experimental Varicocele. Urology . 2010;75:217–222. doi: 10.1016/j.urology.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 27.Guthrie H, Welch G. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J Anim Sci . 2006;84:2089–2100. doi: 10.2527/jas.2005-766. [DOI] [PubMed] [Google Scholar]
- 28.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol . 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esterbauer H, Cheeseman K. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol . 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 30.Rhee Y, Brunt A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: a randomized crossover design. Nutr J. 2011;10:44–49. doi: 10.1186/1475-2891-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald-Wicks LK, Garg ML. Modulation of carbon tetrachloride-induced oxidative stress by dietary fat in rats. J Nutr Biochem . 2002;13:87–95. doi: 10.1016/s0955-2863(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 32.Salama N, Bergh A, Damber JE. The changes in testicular vascular permeability during progression of the experimental varicocele. Eur Urol. 2003;43:84–91. doi: 10.1016/s0302-2838(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 33.Elsayed NM, Mustafa MG, Mead JF. Increased vitamin E content in the lung after ozone exposure: A possible mobilization in response to oxidative stress. Arch Biochem Biophys . 1990;282:263–269. doi: 10.1016/0003-9861(90)90115-f. [DOI] [PubMed] [Google Scholar]
- 34.Smith R, Kaune H, Parodi D, Madariaga M, Rios R, Morales I, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod . 2006;21:986–993. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 35.Naqshbandi A, Khan W, Rizwan S, Khan F. Studies on the protective effect of flaxseed oil on cisplatin-induced hepatotoxicity. Hum Exp Toxicol . 2012;31:364–375. doi: 10.1177/0960327111432502. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Sharma R, Desai N, Prabakaran S, Tavares A, Sabaneqh E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology . 2009;73:461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 37.Nesbitt PD-Lam Y, Thompson LU. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr . 1999;69:549–555. doi: 10.1093/ajcn/69.3.549. [DOI] [PubMed] [Google Scholar]