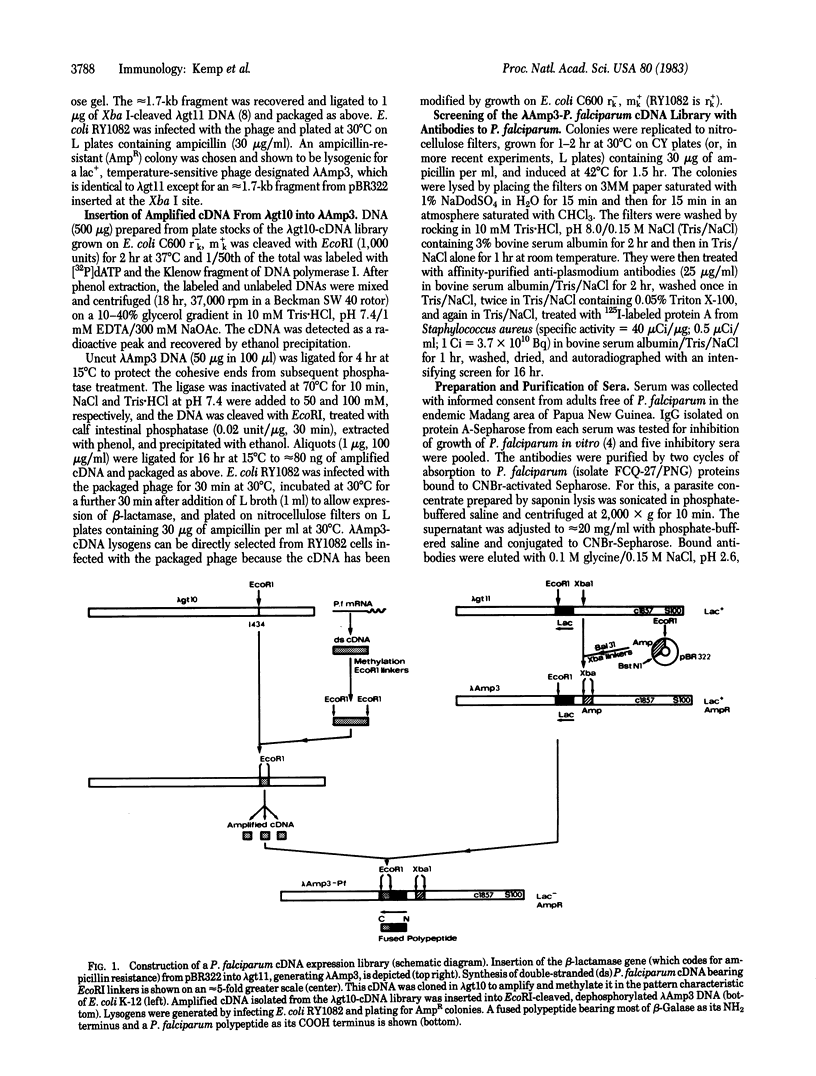
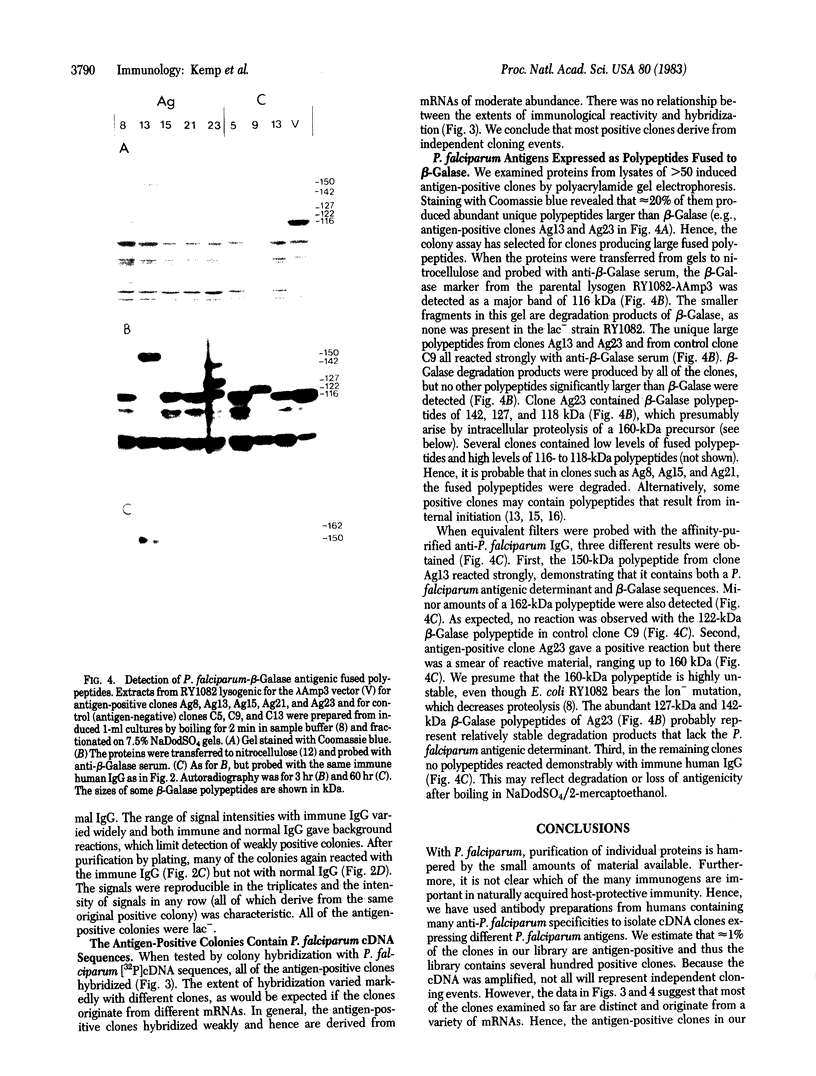
Abstract
Many proteins produced by blood stages of the malaria parasite Plasmodium falciparum are natural immunogens in man. As an approach to determining which of these are relevant to protective immunity we have constructed an expression library of P. falciparum cDNA sequences, cloned in Escherichia coli. The cDNA sequences were inserted into the beta-galactosidase gene of an ampicillin-resistant derivative of the temperature-sensitive lysogenic bacteriophage lambda gt11. About 5% of the resulting clones expressed P. falciparum sequences as polypeptides fused to beta-galactosidase. We have identified many clones that express P. falciparum antigens by immunological screening in situ with antibodies from immune human sera that inhibit P. falciparum growth in vitro. The antigen-positive clones contain P. falciparum cDNA sequences, as determined by hybridization. Some express polypeptides that are larger than beta-galactosidase and react both with antibodies to beta-galactosidase and with antibodies from humans immune to P. falciparum. The cloned P. falciparum antigens should facilitate new approaches to the identification of potential vaccine molecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Mitchell G. F., Heywood P. F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature. 1982 Jun 17;297(5867):591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Stace J. D., Alpers M. P., Mitchell G. F. Immunoprecipitation of biosynthetically-labelled proteins from different Papua New Guinea Plasmodium falciparum isolates by sera from individuals in the endemic area. Parasite Immunol. 1981 Winter;3(4):283–298. doi: 10.1111/j.1365-3024.1981.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. Progress in malaria vaccine development. Br Med Bull. 1982 May;38(2):161–165. doi: 10.1093/oxfordjournals.bmb.a071753. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Tacon W. C., Catlin G. H., Jenkins B., Porter A. G., Carey N. H. Influenza antigenic determinants are expressed from haemagglutinin genes cloned in Escherichia coli. Nature. 1980 Jan 10;283(5743):171–174. doi: 10.1038/283171a0. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R., Hofer-Warbinek R. Reaction of immune sera with components of the human malarial parasite, Plasmodium falciparum. Am J Trop Med Hyg. 1981 Nov;30(6):1168–1178. doi: 10.4269/ajtmh.1981.30.1168. [DOI] [PubMed] [Google Scholar]
- Sanderson A., Walliker D., Molez J. F. Enzyme typing of Plasmodium falciparum from African and some other Old World countries. Trans R Soc Trop Med Hyg. 1981;75(2):263–267. doi: 10.1016/0035-9203(81)90331-x. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]