Abstract
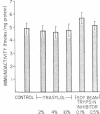
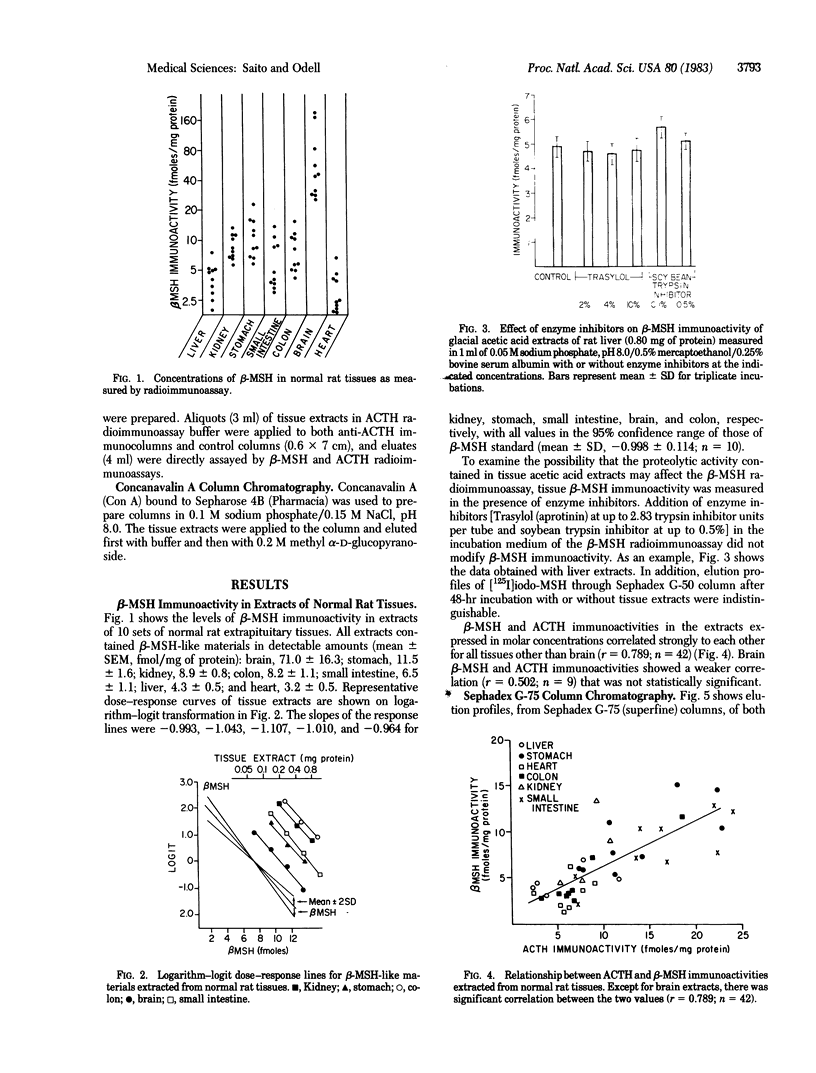
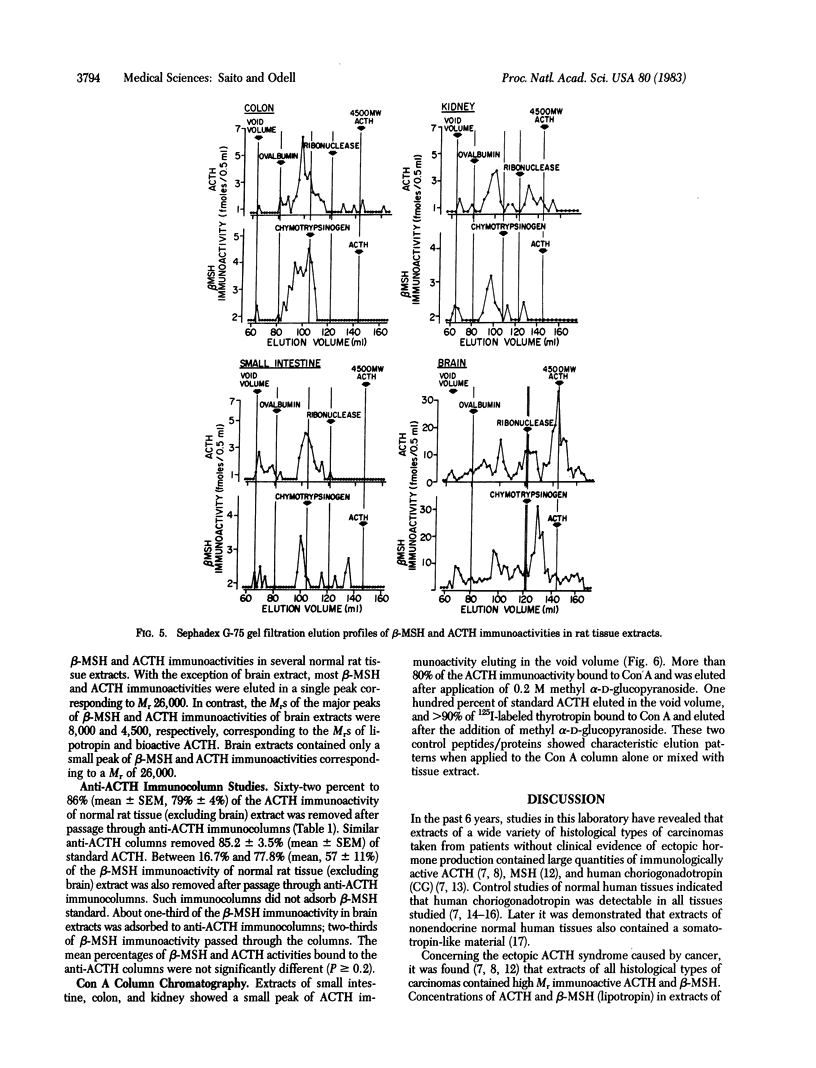
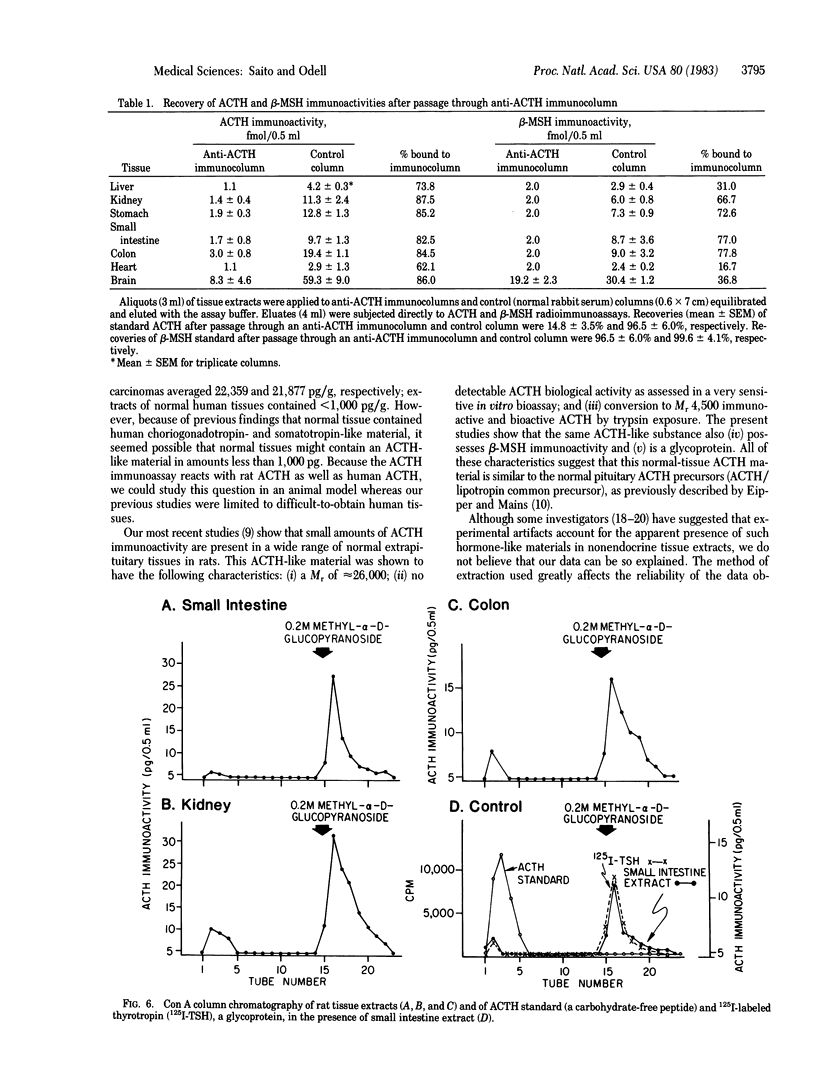
A Mr 26,000 corticotropin (ACTH)-like material is present in glacial acetic acid extracts of all normal rat extrapituitary tissues. In the present study, beta-melanotropin (beta-MSH) immunoactivity was detected in glacial acetic acid extracts of normal rat extrapituitary tissues. beta-MSH immunoactivity was also present in all extracts (mean +/- SEM, fmol/mg of protein): brain, 71.0 +/- 16.3; stomach, 11.5 +/- 1.6; kidney, 8.9 +/- 0.8; colon, 8.2 +/- 1.1; small intestine, 6.5 +/- 1.1; liver, 4.3 +/- 0.5; and heart, 3.2 +/- 0.5. Except in brain extracts, beta-MSH and ACTH immunoactivities of tissue extracts were strongly correlated to each other (r = 0.79; n = 42). When tissue extracts (except brain) were passed through a Sephadex G-75 (superfine) column, ACTH and beta-MSH immunoactivities were eluted in a single peak corresponding to Mr 26,000. In contrast, for brain extracts, the MrS of major peaks of ACTH and beta-MSH immunoactivities were 4,500 and 8,000, respectively; a smaller peak of Mr 26,000 ACTH/beta-MSH-like material was also eluted. Specific anti-ACTH immunocolumns, which did not bind purified synthetic beta-MSH, adsorbed both ACTH and beta-MSH immunoactivities of all tissue extracts except those of brain. One-third of the beta-MSH immunoactivity in brain extracts adsorbed to the anti-ACTH immunocolumn, but two-thirds of beta-MSH immunoactivity passed through the column. We conclude that ACTH and beta-MSH immunoactivities are present in all normal rat extrapituitary tissues and exist in most tissues on the same molecule. This Mr 26,000 molecule is closely related to the pituitary ACTH/beta-lipotropin common precursor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo H. F., Slifkin M., Pouchet G. R., Pardo M. Immunohistochemical localization of a choriogonadotropin-like protein in bacteria isolated from cancer patients. Cancer. 1978 Apr;41(4):1217–1229. doi: 10.1002/1097-0142(197804)41:4<1217::aid-cncr2820410401>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bachelot I., Wolfsen A. R., Odell W. D. Pituitary and plasma lipotropins: demonstration of the artificial nature of betaMSH. J Clin Endocrinol Metab. 1977 May;44(5):939–946. doi: 10.1210/jcem-44-5-939. [DOI] [PubMed] [Google Scholar]
- Bertagna X. Y., Nicholson W. E., Sorenson G. D., Pettengill O. S., Mount C. D., Orth D. N. Corticotropin, lipotropin, and beta-endorphin production by a human nonpituitary tumor in culture: evidence for a common precursor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5160–5164. doi: 10.1073/pnas.75.10.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTY N. P. Adrenocorticotrophic activity in the plasma of patients with Cushing's syndrome associated with pulmonary neoplasms. Lancet. 1961 Jan 14;1(7168):85–86. doi: 10.1016/s0140-6736(61)92125-0. [DOI] [PubMed] [Google Scholar]
- Clark D., Thody A. J., Shuster S., Bowers H. Immunoreactive alpha-MSH in human plasma in pregnancy. Nature. 1978 May 11;273(5658):163–164. doi: 10.1038/273163a0. [DOI] [PubMed] [Google Scholar]
- Cohen H., Strampp A. Bacterial synthesis of substance similar to human chorionic gonadotrophin. Proc Soc Exp Biol Med. 1976 Jul;152(3):408–410. doi: 10.3181/00379727-152-39407. [DOI] [PubMed] [Google Scholar]
- Dubé D., Lissitzky J. C., Leclerc R., Pelletier G. Localization of alpha-melanocyte-stimulating hormone in rat brain and pituitary. Endocrinology. 1978 Apr;102(4):1283–1291. doi: 10.1210/endo-102-4-1283. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Eskay R. L., Brownstein M. J., Long R. T. alpha-Melanocyte-stimulating hormone: reduction in adult rat brain after monosodium glutamate treatment of neonates. Science. 1979 Aug 24;205(4408):827–829. doi: 10.1126/science.462194. [DOI] [PubMed] [Google Scholar]
- Gewirtz G., Yalow R. S. Ectopic ACTH production in carcinoma of the lung. J Clin Invest. 1974 Apr;53(4):1022–1032. doi: 10.1172/JCI107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllt V., Przewłocki R., Herz A. beta-Endorphin-like immunoreactivity in plasma, pituitaries and hypothalamus of rats following treatment with opiates. Life Sci. 1978 Sep 11;23(10):1057–1065. doi: 10.1016/0024-3205(78)90667-7. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A. S., Brownstein M. J., Zimmerman E. A. ACTH, beta-lipotropin, and related peptides in brain, pituitary, and blood. Recent Prog Horm Res. 1980;36:277–344. doi: 10.1016/b978-0-12-571136-4.50015-2. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A. S., Hauser H., Brownstein M. J. Effect of stress, adrenocorticotropin or corticosteroid treatment, adrenalectomy, or hypophysectomy on hypothalamic immunoreactive adrenocorticotropin concentrations. Endocrinology. 1979 Sep;105(3):737–742. doi: 10.1210/endo-105-3-737. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A. S., Nicholsen G., Kizer J. S. Brain ACTH and endorphin reduced in rats with monosodium glutamate-induced arcuate nuclear lesions. Nature. 1979 Apr 5;278(5704):562–563. doi: 10.1038/278562a0. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A., Brownstein M. J. Presence of corticotropin in limbic system of normal and hypophysectomized rats. Brain Res. 1977 Jun 17;128(3):575–579. doi: 10.1016/0006-8993(77)90185-8. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A., Suda T., Palkovits M., Brownstein M. J. Presence of immunoassayable beta-lipotropin in bovine brain and spinal cord: lack of concordance with ACTH concentrations. Biochem Biophys Res Commun. 1977 Jun 6;76(3):930–936. doi: 10.1016/0006-291x(77)91591-1. [DOI] [PubMed] [Google Scholar]
- Kyle C. V., Evans M. C., Odell W. D. Growth hormone-like material in normal human tissues. J Clin Endocrinol Metab. 1981 Dec;53(6):1138–1144. doi: 10.1210/jcem-53-6-1138. [DOI] [PubMed] [Google Scholar]
- LaBella F., Queen G., Senyshyn J., Lis M., Chretien M. Lipotropin: localization by radioimmunoassay of endorphin precursors in pituitary and brain. Biochem Biophys Res Commun. 1977 Mar 21;75(2):350–357. doi: 10.1016/0006-291x(77)91049-x. [DOI] [PubMed] [Google Scholar]
- Larsson L-I Corticotropin-like peptides in central nerves and in endocrine cells of gut and pancreas. Lancet. 1977 Dec 24;2(8052-8053):1321–1323. doi: 10.1016/s0140-6736(77)90368-3. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Distribution of ACTH-like immunoreactivity in rat brain and gastrointestinal tract. Histochemistry. 1978 Apr 4;55(3):225–233. doi: 10.1007/BF00495761. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Immunocytochemical characterization of ACTH-like immunoreactivity in cerebral nerves and in endocrine cells of the pituitary and gastrointestinal tract by using region-specific antisera. J Histochem Cytochem. 1980 Feb;28(2):133–141. doi: 10.1177/28.2.6243680. [DOI] [PubMed] [Google Scholar]
- LeRoith D., Lesniak M. A., Roth J. Insulin in insects and annelids. Diabetes. 1981 Jan;30(1):70–76. doi: 10.2337/diab.30.1.70. [DOI] [PubMed] [Google Scholar]
- LeRoith D., Shiloach J., Roth J., Liotta A. S., Krieger D. T., Lewis M., Pert C. B. Evolutionary origins of vertebrate hormones: material very similar to adrenocorticotropic hormone, beta-endorphin, and dynorphin in protozoa. Trans Assoc Am Physicians. 1981;94:52–60. [PubMed] [Google Scholar]
- Liddle G. W., Nicholson W. E., Island D. P., Orth D. N., Abe K., Lowder S. C. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283–314. doi: 10.1016/b978-0-12-571125-8.50009-0. [DOI] [PubMed] [Google Scholar]
- Liotta A., Osathanondh R., Ryan K. J., Krieger D. T. Presence of corticotropin in human placenta: demonstration of in vitro synthesis. Endocrinology. 1977 Nov;101(5):1552–1558. doi: 10.1210/endo-101-5-1552. [DOI] [PubMed] [Google Scholar]
- Livingston V. W., Livingston A. M. Some cultural, immunological, and biochemical properties of Progenitor cryptocides. Trans N Y Acad Sci. 1974 Jun;36(6):569–582. doi: 10.1111/j.2164-0947.1974.tb01602.x. [DOI] [PubMed] [Google Scholar]
- MEADOR C. K., LIDDLE G. W., ISLAND D. P., NICHOLSON W. E., LUCAS C. P., NUCKTON J. G., LUETSCHER J. A. Cause of Cushing's syndrome in patients with tumors arising from "nonendocrine" tissue. J Clin Endocrinol Metab. 1962 Jul;22:693–703. doi: 10.1210/jcem-22-7-693. [DOI] [PubMed] [Google Scholar]
- Maruo T., Segal S. J., Koide S. S. Studies on the apparent human chorionic gonadotropin-like factor in the crab Ovalipes ocellatus. Endocrinology. 1979 Apr;104(4):932–939. doi: 10.1210/endo-104-4-932. [DOI] [PubMed] [Google Scholar]
- Matsukura S., Yoshimi H., Sueoka S., Kataoka K., Ono T., Ohgushi N. The regional distribution of immunoreactive beta-endorphin in the monkey brain. Brain Res. 1978 Dec 22;159(1):228–233. doi: 10.1016/0006-8993(78)90125-7. [DOI] [PubMed] [Google Scholar]
- Moldow R. L., Yalow R. S. Artifacts in the radioimmunoassay of ACTH in tissue extracts and plasma. Horm Metab Res. 1980 Mar;12(3):105–110. doi: 10.1055/s-2007-996215. [DOI] [PubMed] [Google Scholar]
- Moldow R., Yalow R. S. Extrahypophysial distribution of corticotropin as a function of brain size. Proc Natl Acad Sci U S A. 1978 Feb;75(2):994–998. doi: 10.1073/pnas.75.2.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y., Nakao K., Oli S., Imura H. Presence of immunoreactive beta-lipotropin and beta-endorphin in human placenta. Life Sci. 1978 Nov 13;23(20):2013–2018. doi: 10.1016/0024-3205(78)90233-3. [DOI] [PubMed] [Google Scholar]
- Odagiri E., Sherrell B. J., Mount C. D., Nicholson W. E., Orth D. N. Human placental immunoreactive corticotropin, lipotropin, and beta-endorphin: evidence for a common precursor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2027–2031. doi: 10.1073/pnas.76.4.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell W. D., Wolfsen A. R., Bachelot I., Hirose F. M. Ectopic production of lipotropin by cancer. Am J Med. 1979 Apr;66(4):631–638. doi: 10.1016/0002-9343(79)91174-4. [DOI] [PubMed] [Google Scholar]
- Odell W., Wolfsen A., Yoshimoto Y., Weitzman R., Fisher D., Hirose F. Ectopic peptide synthesis: a universal concomitant of neoplasia. Trans Assoc Am Physicians. 1977;90:204–227. [PubMed] [Google Scholar]
- Oliver C., Porter J. C. Distribution and characterization of alpha-melanocyte-stimulating hormone in the rat brain. Endocrinology. 1978 Mar;102(3):697–705. doi: 10.1210/endo-102-3-697. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Guillemin R., Ling N., Nicholson W. E. Immunoreactive endorphins, lipotropins and corticotropins in a human nonpituitary tumor: evidence for a common precursor. J Clin Endocrinol Metab. 1978 May;46(5):849–852. doi: 10.1210/jcem-46-5-849. [DOI] [PubMed] [Google Scholar]
- Orwoll E. S., Kendall J. W. Beta-endorphin and adrenocorticotropin in extrapituitary sites: gastrointestinal tract. Endocrinology. 1980 Aug;107(2):438–442. doi: 10.1210/endo-107-2-438. [DOI] [PubMed] [Google Scholar]
- Pacold S. T., Kirsteins L., Hojvat S., Lawrence A. M. Biologically active pituitary hormones in the rat brain amygdaloid nucleus. Science. 1978 Feb 17;199(4330):804–806. doi: 10.1126/science.203034. [DOI] [PubMed] [Google Scholar]
- Ratcliffe J. G., Knight R. A., Besser G. M., Landon J., Stansfeld A. G. Tumor and plasma ACTH concentrations in patients with and without the ectopic ACTH syndrome. Clin Endocrinol (Oxf) 1972 Jan;1(1):27–44. doi: 10.1111/j.1365-2265.1972.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Burke C. W., Chard T., Evans S. W., Letchworth A. T. Possible placental origin of ACTH in normal human pregnancy. Nature. 1975 Apr 17;254(5501):620–622. doi: 10.1038/254620b0. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Nakai Y., Nakao K., Oki S., Masaki N., Ohtsuki H., Imura H. Presence of immunoreactive gamma-melanocyte-stimulating hormone, adrenocorticotropin, and beta-endorphin in human gastric antral mucosa. J Clin Endocrinol Metab. 1982 Feb;54(2):392–396. doi: 10.1210/jcem-54-2-392. [DOI] [PubMed] [Google Scholar]
- Vaudry H., Tonon M. C., Delarue C., Vaillant R., Kraicer J. Biological and radioimmunological evidence for melanocyte stimulating hormones (MSH) of extrapituitary origin in the rat brain. Neuroendocrinology. 1978;27(1-2):9–24. doi: 10.1159/000122796. [DOI] [PubMed] [Google Scholar]
- Wolfsen A. R., Odell W. D. ProACTH: use for early detection of lung cancer. Am J Med. 1979 May;66(5):765–772. doi: 10.1016/0002-9343(79)91114-8. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y., Wolfsen A. R., Hirose F., Odell W. D. Human chorionic gonadotropin--like material: presence in normal human tissues. Am J Obstet Gynecol. 1979 Aug 1;134(7):729–733. doi: 10.1016/0002-9378(79)90937-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y., Wolfsen A. R., Odell W. D. Glycosylation, a variable in the production of hCG by cancers. Am J Med. 1979 Sep;67(3):414–420. doi: 10.1016/0002-9343(79)90787-3. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y., Wolfsen A. R., Odell W. D. Human chorionic gonadotropin-like substance in nonendocrine tissues of normal subjects. Science. 1977 Aug 5;197(4303):575–577. doi: 10.1126/science.195341. [DOI] [PubMed] [Google Scholar]