Abstract
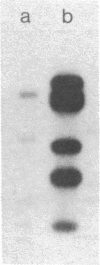
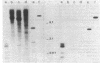
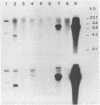
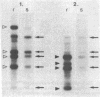
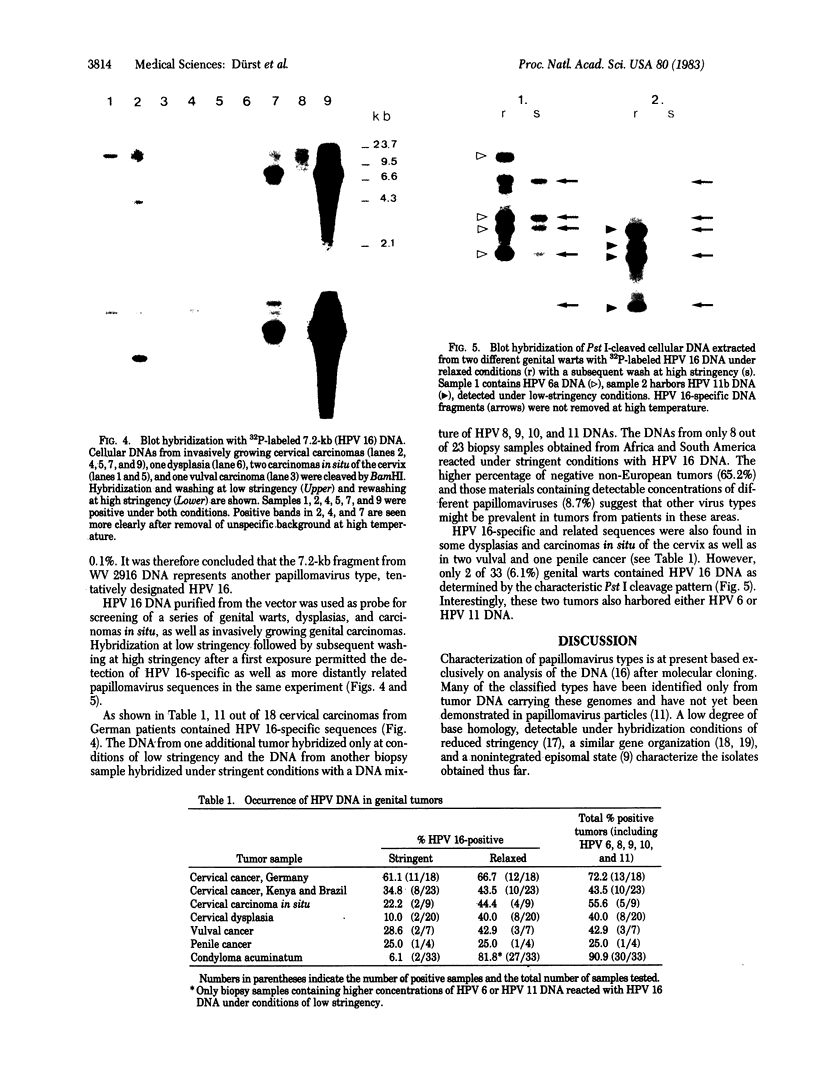
DNA from one biopsy sample of invasive cancer of the cervix contained sequences hybridizing with human papillomavirus (HPV) type 11 DNA only under nonstringent conditions. This DNA was molecularly cloned in lambda phage. Under stringent conditions of hybridization it cross-hybridized to a minor extent (less than 0.1%) with HPV types 10, 14, and 15 and showed no homology with DNA of other human HPV types. We therefore propose to designate it tentatively as HPV 16. HPV 16 DNA was used as a probe to test additional cancer biopsy samples from cervical, vulval, and penile cancer, as well as benign genital warts (condylomata acuminata) and cervical dysplasias for the presence of homologous sequences. In 61.1% (11/18) of cervical cancer samples from German patients sequences were found hybridizing with HPV 16 DNA under conditions of high stringency. In contrast, only 34.8% (8/23) of cancer biopsy samples from Kenya and Brazil revealed this DNA. Vulval and penile cancer biopsy samples hybridized to 28.6% (2/7) or 25% (1/4), respectively. Only 2 out of 33 condylomata acuminata contained HPV 16 DNA. Both positive tumors harbored in addition HPV 6 or HPV 11 DNA. The data thus indicate that HPV 16 DNA prevails in malignant tumors, rendering an accidental contamination with papillomavirus DNA from adjacent papillomas rather unlikely. The rare presence in benign genital papillomas in addition to common genital papillomaviruses suggests a dependence of HPV 16 replication on helper virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Diehl V., Schultz-Coulon H. J., zur Hausen H. Molecular cloning and characterization of human papilloma virus DNA derived from a laryngeal papilloma. J Virol. 1982 Oct;44(1):393–400. doi: 10.1128/jvi.44.1.393-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., deVilliers E. M., zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982 Feb 15;29(2):143–146. doi: 10.1002/ijc.2910290205. [DOI] [PubMed] [Google Scholar]
- Gissmann L., zur Hausen H. Partial characterization of viral DNA from human genital warts (Condylomata acuminata). Int J Cancer. 1980 May 15;25(5):605–609. doi: 10.1002/ijc.2910250509. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Sanders P. R., Loewenstein P. M., Freel J. H., Eisinger M., Switlyk S. A. Isolation of a human papillomavirus from a patient with epidermodysplasia verruciformis: presence of related viral DNA genomes in human urogenital tumors. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4437–4441. doi: 10.1073/pnas.79.14.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Watts S. L., Anderson D. L., Faras A. J., Pass F. Anogenital warts contain several distinct species of human papillomavirus. J Virol. 1980 Oct;36(1):236–244. doi: 10.1128/jvi.36.1.236-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Jablonska S., Favre M., Croissant O., Obalek S., Jarzabek-Chorzelska M., Jibard N. Identification of papillomaviruses in butchers' warts. J Invest Dermatol. 1981 Feb;76(2):97–102. doi: 10.1111/1523-1747.ep12525394. [DOI] [PubMed] [Google Scholar]
- Ostrow R. S., Krzyzek R., Pass F., Faras A. J. Identification of a novel human papilloma virus in cutaneous warts of meathandlers. Virology. 1981 Jan 15;108(1):21–27. doi: 10.1016/0042-6822(81)90524-9. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Figueroa M. E., Melnick J. L. Herpesvirus type 2: association with carcinoma of the cervix. Science. 1968 Sep 20;161(3847):1255–1256. doi: 10.1126/science.161.3847.1255. [DOI] [PubMed] [Google Scholar]
- Rotkin I. D. A comparison review of key epidemiological studies in cervical cancer related to current searches for transmissible agents. Cancer Res. 1973 Jun;33(6):1353–1367. [PubMed] [Google Scholar]
- Schlehofer J. R., Hausen J. Z. Induction of mutations within the host cell genome by partially inactivated herpes simplex virus type 1. Virology. 1982 Oct 30;122(2):471–475. doi: 10.1016/0042-6822(82)90247-1. [DOI] [PubMed] [Google Scholar]
- Zachow K. R., Ostrow R. S., Bender M., Watts S., Okagaki T., Pass F., Faras A. J. Detection of human papillomavirus DNA in anogenital neoplasias. Nature. 1982 Dec 23;300(5894):771–773. doi: 10.1038/300771a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976 Feb;36(2 Pt 2):794–794. [PubMed] [Google Scholar]
- zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet. 1982 Dec 18;2(8312):1370–1372. doi: 10.1016/s0140-6736(82)91273-9. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977;78:1–30. doi: 10.1007/978-3-642-66800-5_1. [DOI] [PubMed] [Google Scholar]