Abstract
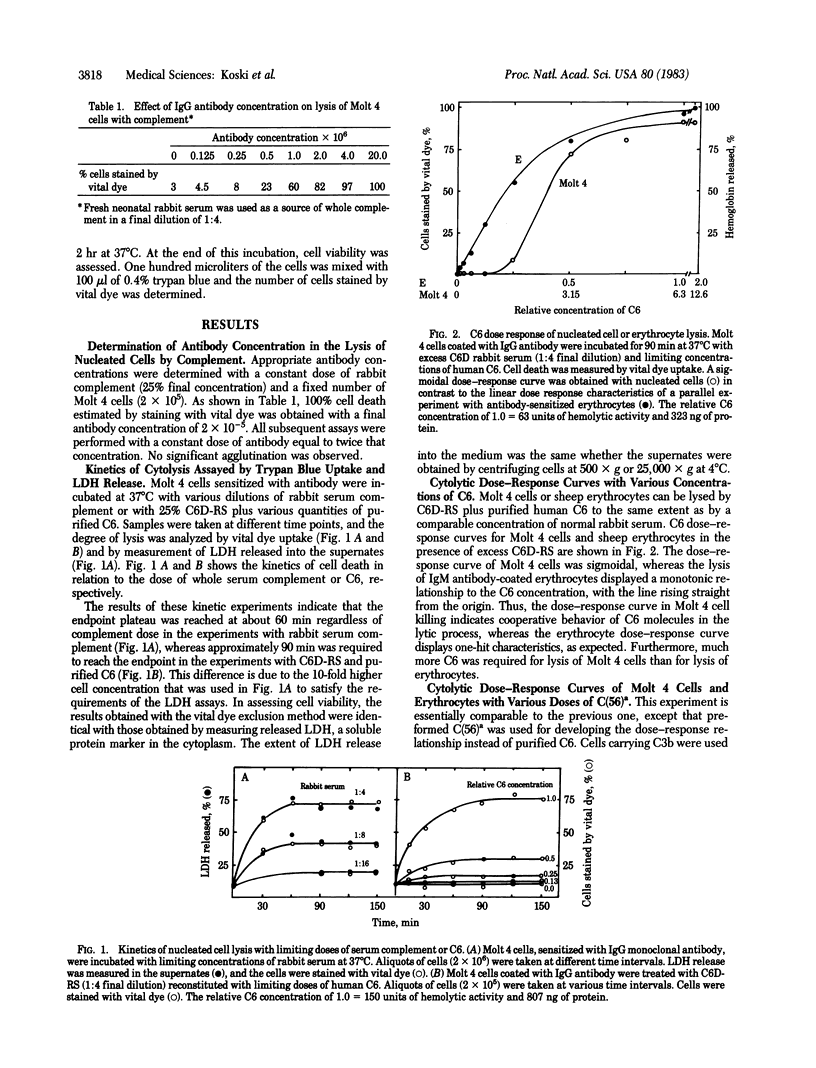
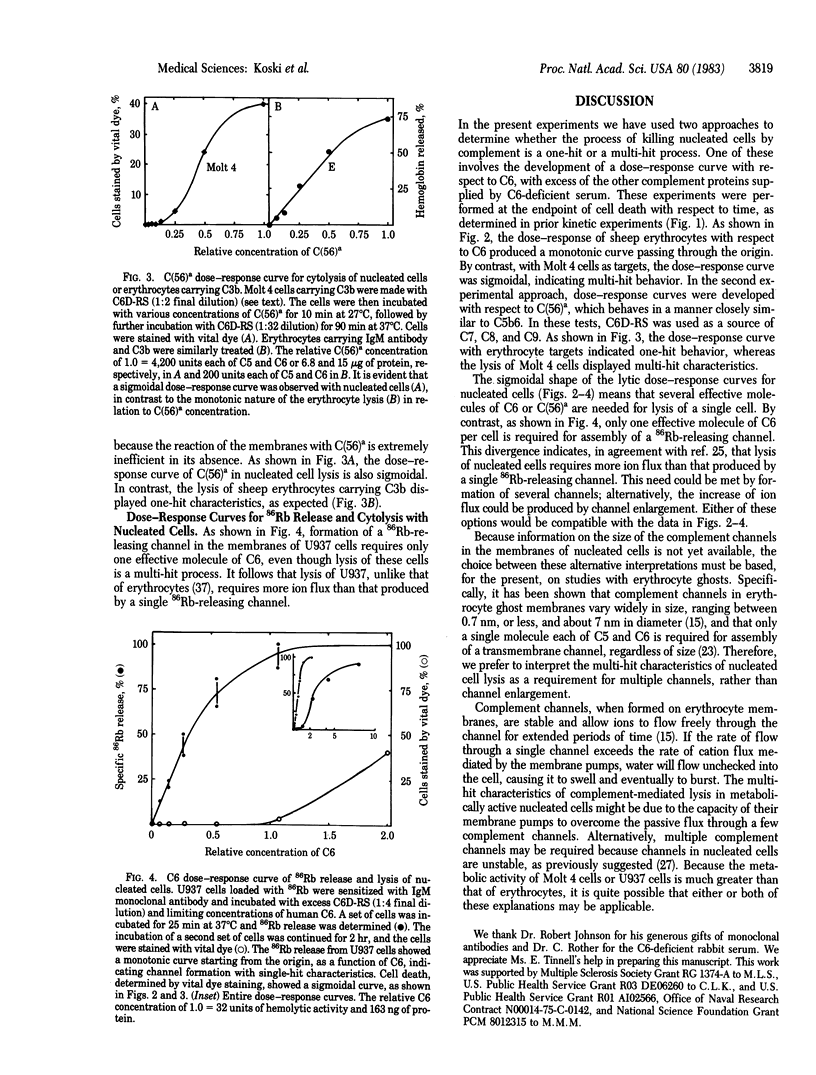
Lysis of nucleated cells by complement was studied to determine whether the lytic process by C5b-9 conforms to a one-hit mechanism as in the case of erythrocytes. Two nucleated cell lines, Molt 4 and U937, derived from human T lymphocytes and histiocytes, respectively, were employed as targets. The antibody-sensitized cells were used to develop the titration curves, measuring cell death as a function of limiting quantities of human C6 or C5,6 complex in the presence of an excess of other complement components. The cytolysis curves generated in both experiments were sigmoidal, in sharp contrast to the monotonic curves observed in lysis of erythrocytes treated similarly. The sigmoidal curves of cytolysis indicate a cooperative action of several molecules of C6 or acid-activated C5,6 complex, C(56)a. In contrast to the multi-hit characteristics of cytolysis, dose-response measurements of the release of 86Rb indicated that only one effective molecule of C6 per cell is required for assembly of a 86Rb-releasing channel. This divergence indicates that lysis requires formation of several channels or, alternatively, assembly of large channels that are formed by several molecules of C6. Because prior studies with erythrocyte ghosts have shown that only a single effective molecule of C6 is required for assembly of a transmembrane channel, regardless of size, we prefer to interpret the multi-hit characteristics of nucleated cell lysis as an indication of a multi-channel requirement, rather than channel enlargement.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Bjerrum O. J., Bhakdi-Lehnen B., Tranum-Jensen J. Complement lysis: evidence for an amphiphilic nature of the terminal membrane C5b-9 complex of human complement. J Immunol. 1978 Dec;121(6):2526–2532. [PubMed] [Google Scholar]
- Bhakdi S., Bjerrum O. J., Rother U., Knüfermann H., Wallach D. F. Immunochemical analyses of membrane-bound complement. Detection of the terminal complement complex and its similarity to "intrinsic" erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Sep 16;406(1):21–35. doi: 10.1016/0005-2736(75)90039-5. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Borsos T. Studies on the terminal stages of immune hemolysis. VI. Osmotic blockers of differing Stokes' radii detect complement-induced transmembrane channels of differing size. J Immunol. 1979 Jul;123(1):77–82. [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Lysis of tumor cells by antibody and complement. VII. Complement-dependent 86Rb release--a nonlethal event? J Immunol. 1976 Oct;117(4):1346–1350. [PubMed] [Google Scholar]
- Gaither T. A., Hammer C. H., Frank M. M. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of beta 1H and the C3b inactivator (C3bINA). J Immunol. 1979 Sep;123(3):1195–1204. [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Hammer C. H., Nicholson A., Mayer M. M. On the mechanism of cytolysis by complement: evidence on insertion of C5b and C7 subunits of the C5b,6,7 complex into phospholipid bilayers of erythrocyte membranes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5076–5080. doi: 10.1073/pnas.72.12.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Shin M. L., Abramovitz A. S., Mayer M. M. On the mechanism of cell membrane damage by complement: evidence on insertion of polypeptide chains from C8 and C9 into the lipid bilayer of erythrocytes. J Immunol. 1977 Jul;119(1):1–8. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hingson D. J., Massengill R. K., Mayer M. M. The kinetics of release of 86rubidium and hemoglobin from erythrocytes damaged by antibody and complement. Immunochemistry. 1969 Mar;6(2):295–307. doi: 10.1016/0019-2791(69)90166-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann L. G. Statistical evaluation of reaction mechanisms in immune hemolysis. II. The kinetics of release of 86rubidium and hemoglobin from erythrocytes damaged by antibody and complement. Immunochemistry. 1969 Mar;6(2):309–325. doi: 10.1016/0019-2791(69)90167-0. [DOI] [PubMed] [Google Scholar]
- Hu V. W., Esser A. F., Podack E. R., Wisnieski B. J. The membrane attack mechanism of complement: photolabeling reveals insertion of terminal proteins into target membrane. J Immunol. 1981 Jul;127(1):380–386. [PubMed] [Google Scholar]
- Hänsch G. M., Hammer C. H., Mayer M. M., Shin M. L. Activation of the fifth and sixth component of the complement system: similarities between C5b6 and C(56)a with respect to lytic enhancement by cell-bound C3b or A2C, and species preferences of target cell. J Immunol. 1981 Sep;127(3):999–1002. [PubMed] [Google Scholar]
- Inoue K., Kinoshita T., Okada M., Akiyama Y. Release of phospholipids from complement-mediated lesions on the surface structure of Escherichia coli. J Immunol. 1977 Jul;119(1):65–72. [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schlager S. I. Humoral immune killing of nucleated cells: mechanisms of complement-mediated attack and target cell defense. Crit Rev Immunol. 1981 Jan;1(3):165–209. [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [PubMed] [Google Scholar]
- Podack E. R., Stoffel W., Esser A. F., Müller-Eberhard H. J. Membrane attack complex of complement: distribution of subunits between the hydrocarbon phase of target membranes and water. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4544–4548. doi: 10.1073/pnas.78.7.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Mayer M. M. Life-span and size of the trans-membrane channel formed by large doses of complement. J Immunol. 1980 May;124(5):2281–2287. [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Size distribution and stability of the trans-membrane channels formed by complement complex C5b-9. Mol Immunol. 1983 Feb;20(2):155–160. doi: 10.1016/0161-5890(83)90126-8. [DOI] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Transmembrane channel formation by complement: functional analysis of the number of C5b6, C7, C8, and C9 molecules required for a single channel. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4751–4755. doi: 10.1073/pnas.79.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske-Kunz A. B., Scheid M. P., Abbott J., Metakis L. J., Polley M. J., Boyse E. A. Action of complement in the lysis of mouse sarcoma cells sensitized with H-2 alloantibody. Transplantation. 1979 Aug;28(2):149–153. doi: 10.1097/00007890-197908000-00016. [DOI] [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H., Borsos T. Correlation between the ability of tumor cells to resist humoral immune attack and their ability to synthesize lipid. J Immunol. 1978 Feb;120(2):463–471. [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H., Borsos T. Stimulation of the synthesis and release of lipids in tumor cells under attack by antibody and C. J Immunol. 1978 Mar;120(3):895–901. [PubMed] [Google Scholar]
- Shin H. S., Pickering R. J., Mayer M. M. The fifth component of the guinea pig complement system. II. Mechanism of SAC1,4,2,3,5b formation and C5 consumption by EAC1,4,2,3. J Immunol. 1971 Feb;106(2):473–479. [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Abramovitz A. S., Mayer M. M. On the mechanism of membrane damage by C: exposure of hydrophobic sites on activated C proteins. J Immunol. 1977 Oct;119(4):1358–1364. [PubMed] [Google Scholar]
- Stephens C. L., Henkart P. A. Electrical measurements of complement-mediated membrane damage in cultured nerve and muscle cells. J Immunol. 1979 Feb;122(2):455–458. [PubMed] [Google Scholar]
- Yamamoto K. I., Gewurz G. The complex of C5b and C6: isolation, characterization, and identification of a modified form of C5b consisting of three polypeptide chains. J Immunol. 1978 Jun;120(6):2008–2015. [PubMed] [Google Scholar]



