Abstract
Barrett's esophagus (BE) is an acquired condition characterized by replacement of stratified squamous epithelium by a cancer predisposing metaplastic columnar epithelium. Endoscopy with systemic biopsy protocols plays a vital role in diagnosis. Technological advancements in dysplasia detection improves outcomes in surveillance and treatment of patients with BE and dysplasia. These advances in endoscopic technology radically changed the treatment for dysplastic BE and early cancer from being surgical to organ-sparing endoscopic therapy. A multimodal treatment approach combining endoscopic resection of visible and/or raised lesions with ablation techniques for flat BE mucosa, followed by long-term surveillance improves the outcomes of BE. Safe and effective endoscopic treatment can be either tissue acquiring as in endoscopic mucosal resection and endoscopic submucosal dissection or tissue ablative as with photodynamic therapy, radiofrequency ablation and cryotherapy. Debatable issues such as durability of response, recognition and management of sub-squamous BE and optimal management strategy in patients with low-grade dysplasia and non-dysplastic BE need to be studied further. Development of safer wide field resection techniques, which would effectively remove all BE and obviate the need for long-term surveillance, is another research goal. Shared decision making between the patient and physician is important while considering treatment for dysplasia in BE.
Keywords: Barrett’s esophagus, endoscopic mucosal resection, endoscopic submucosal dissection
INTRODUCTION
In response to injury associated with gastroesophageal reflux, the normal stratified squamous epithelium of the esophagus may be replaced by a metaplastic columnar intestinal-like epithelium—Barrett’s esophagus (BE)—which is predisposed to cancer development [1]. Three types of Barrett’s columnar epithelia have been described—a junctional (cardia) type-, a gastric fundic type- and intestinal-type metaplasia, the latter being specialized columnar epithelium, with prominent goblet cells [2]. Barrett’s epithelium appears to progress sequentially from intestinal metaplasia (IM) to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and finally to invasive adenocarcinoma. Although BE is associated with a low (0.5%) annual incidence of HGD or esophageal adenocarcinoma, a four-fold increase in incidence of esophageal cancer has been noted in certain patient populations [3, 4, 5]. Five-year survival with esophageal adenocarcinoma remains a dismal 13–16% [6, 7].
Since the description of BE 50 years ago, there have been tremendous advances in understanding the biology of BE, risk factors and progression towards cancer, and enhanced endoscopic imaging techniques for identification of dysplasia within BE. Despite the high mortality and morbidity associated with surgical resection, esophagectomy was once considered the therapeutic ‘gold standard’ for BE with HGD, due to a concern over a high risk of harboring occult invasive cancer [8–13].
The management of BE with dysplasia and early cancer has changed radically from morbid surgical resection to organ-sparing endoscopic therapy. With the advent of a multitude of safe and effective treatment options—such as endoscopic mucosal resection (EMR) and endoscopic sub-mucosal dissection (ESD) in combination with tissue ablative therapies, such as photodynamic therapy (PDT), radiofrequency ablation (RFA) and cryotherapy—endoscopic therapy has become the standard of care in expert centers throughout the world.
Endoscopic diagnosis of BE
BE is both an endoscopic and pathologic diagnosis. Endoscopic knowledge of the anatomy of the gastro-esophageal junction (GEJ) is key in the diagnosis of BE. During endoscopy, after gastric decompression, the endoscope should be withdrawn slowly to identify the diaphragmatic hiatus, the top of the gastric folds and the squamo-columnar junction (SCJ or Z-line) which coincide and are normally at the same distance from the incisors. BE is endoscopically suspected when the SCJ is proximal to the top of the gastric folds, with the presence of salmon-colored mucosa within this distance. Endoscopic biopsies should be taken from within this area to confirm the diagnosis of BE [14]. BE is classified as (i) short-segment BE (SSBE) when the distance between the top of the gastric folds and the SCJ is less than 3 cm and (ii) long-segment BE (LSBE), when the distance is greater than 3 cm. The Prague C and M criteria represent the standard classification system and categorize BE more precisely, based on the circumferential extent (C) and the maximum extent (M) of Barrett’s metaplasia [15]. Documenting the length of the BE has prognostic implications and may influence the method of ablation in the event of HGD or IMC being found.
Endoscopic screening of BE
American Gastroenterological Association (AGA) recommendations for screening for BE are shown in Table 1 [1]. The current practice of screening for BE with EGD in the general population with gastroesophageal reflux disease (GERD) is controversial and should be considered on a case-by-case basis. Traditionally, endoscopic screening for BE has been reserved for male Caucasians with long-term GERD. However, BE is known to be present in patients without GERD and up to 57% of patients with esophageal adenocarcinoma never report symptoms of typical GERD, limiting this approach and missing a significant portion of patients at risk for esophageal adenocarcinoma [16–19]. Similarly, the population prevalence of BE is 2–7% and the risk of HGD or IMC is only about 0.5% per year, making population screening a strategy that is not very cost-effective [3, 20, 21]. With the advent of newer and cheaper approaches for diagnosis of BE—such as unsedated examinations and non-endoscopic options (capsule esophagoscopy and cytosponge)—the cost-effectiveness of screening may improve [22–24].
Table 1.
AGA recommendations for screening for Barrett’s esophagus
| *Screen patients with multiple risk factors associated with esophageal adenocarcinoma: |
|
| *Recommend against screening the general population with GERD |
Endoscopic surveillance of BE
AGA guidelines on endoscopic surveillance of BE are as shown in Table 2 [1]. Although the special image-enhanced endoscopic technique is not usually required, a high-resolution endoscope (>850 000 pixels) should be used to evaluate patients with BE and standard-resolution endoscopes are not recommended [25]. Currently, endoscopic surveillance is suggested for patients without BE-related dysplasia and in patients with LGD in BE not opting for ablation. In contrast, surveillance without therapy for HGD is highly controversial and no longer practiced by most clinicians. Even after ablation for dysplastic BE, surveillance is performed based on the highest degree of dysplasia prior to ablation. In contrast to endoscopic surveillance practice in North America, the British societies and other groups do not require IM and survey all columnar epithelium in the esophagus. AGA endoscopic surveillance recommendations include detailed endoscopic evaluation using white light endoscopy, followed by biopsy specimens of any mucosal irregularities and four-quadrant biopsy specimens obtained at least every 2 cm. If dysplasia is suspected, then the four-quadrant biopsy specimens should be obtained every 1 cm [1].
Table 2.
Professional society guidelines for surveillance intervals
| Organization | Surveillance interval |
||
|---|---|---|---|
| No dysplasia | LGD | HGD | |
| American College of Gastroenterology (ACG) |
|
|
|
| American Gastroenterological Association (AGA) |
|
|
|
| American Society for Gastrointestinal Endoscopy (ASGE) |
|
|
|
| British Society of Gastroenterology (BSGE) |
|
|
|
The interpretation of dysplasia can be a matter of contention. At least two experienced gastrointestinal pathologists should evaluate all Barrett’s biopsies when a diagnosis of dysplasia is considered [25]. The use of large-capacity or ‘jumbo’ forceps may improve tissue acquisition and dysplasia detection [26]. Rigorous surveillance with a systematic biopsy protocol improves detection of dysplasia and early cancers [27]. In addition, patients with BE in a surveillance program may have cancers that are detected at an earlier stage, with improved survival [28, 29]. Narrow-band imaging (NBI), chromo-endoscopy, optical coherence tomography, confocal-microendoscopy, spectroscopic probe and endoscopic image enhancement technology (such as ‘i-scan’) may be helpful for targeting biopsies during surveillance of BE for dysplasia but, in large part, these novel imaging technologies remain experimental [30–38].
Endoscopic treatment of BE
The key rationale of endoscopic treatment is to resect and/or ablate the dysplastic mucosa, followed by acid suppression to permit re-epithelialization with neosquamous mucosa. Patients with HGD are at high risk for recurrence and it is thus important to ablate the residual metaplastic epithelium after the dysplastic epithelium has been addressed [39–44]. By eradicating dysplasia and intestinal metaplasia (IM), the cancer rate may decrease, leading to improved survival [39–44].
Accurate pre-treatment staging is essential to ensure an appropriate choice of therapy and optimal long-term outcomes. An accepted multimodal endoscopic treatment approach is targeted EMR of visible lesions, in combination with one or more ablative therapies after a confirmed BE pathology report. Endoscopic treatment can be tissue-acquiring, as in endoscopic mucosal resection (EMR), and endoscopic sub-mucosal dissection (ESD) or tissue ablative, as with photodynamic therapy (PDT), radiofrequency ablation (RFA) and cryotherapy. Treatment is then tailored after detailed discussion of the available endoscopic treatment options including risks, benefits and surveillance as an alternative.
HGD has a higher risk of concomitant cancer and a 6% per year rate of progression to cancer [45–47]. A greater emphasis on accurate diagnosis of BE with HGD, as well as better prediction of risk for progression to esophageal adenocarcinoma (EAC), has been advocated [45–47, 50]. Hence treatment of dysplastic BE is now widely acknowledged and preferred over surveillance [45–50]. However, recent studies confirmed a much smaller risk of occult cancer with HGD and <1% incidence of lymph node metastasis with intra-mucosal cancer (IMC) [11–13, 49]. Endoscopic therapy for BE with HGD is highly effective, safe, with a long-term survival rate similar to esophagectomy [42–44]. In patients with multifocal HGD, the risk of occult cancer is higher and selected patients may be considered for surgery [45–51].
Similarly to HGD, the long-term survival rate of patients with BE and intra-mucosal cancer (IMC) undergoing endoscopic therapy is equal to patients undergoing surgery [42–44]. Extensive EMR for removal of BE with early neoplasia is thought to be safe, with no procedure-related perforations or mortality, but strictures have been reported in 27% and major bleeding in 2% [48]. Outcomes for complete BE eradication are modest at 49.4% and eradication of high-grade dysplasia at 81%. Barrett's length of less than 5 cm is the only significant predictor of complete response [48]. Dunbar et al. reported a 1–2% risk of unexpected lymph-node metastases in patients with BE and IMC [49]. EMR and less so endoscopic ultrasound (EUS) in non-nodular BE helps with diagnosis of sub-mucosal invasion, which is associated with a higher nodal metastasis risk and requires surgery or systemic therapy [13, 49–51]
Management of low-grade dysplasia (LGD) is somewhat controversial. High inter-observer variability among the pathologists in diagnosis LGD seems to affect the natural history of LGD and its rate of progression to HGD and cancer [52]. High rates of eradication of intestinal metaplasia (IM) and LGD, using RFA as reported, is enticing [54]. However, the survival benefits and cost-effectiveness of ablation over surveillance are not clear as estimated from a modeling study [55]. This study estimated the risk of progression rate of 0.7% per year and concluded that although patients with LGD can be managed optimally with ablation, long-term post-ablation surveillance may not be cost-effective [55]. At this time, offering ablation to patients with LGD is made on a case-by-case base and the decision is a shared one between the physician and the patient. Young age at diagnosis, presence of multifocal LGD and LGD on several biopsy sessions may pose a higher risk of progression and, hence, are candidates for ablation [55].
Even though RFA can eradicate 92% of non-dysplastic Barrett’s esophagus (NDBE) with relatively low complication rate and a durable response, the absolute rate of progression to cancer in these patients is low and routine ablation of NDBE is not currently recommended. Histological changes in the gastric cardia, with development of nodules, dysplasia and adenocarcinoma after ablation of BE, have been reported and this calls for caution while considering ablation of BE with LGD or NDBE [56–58].
Mucosal resection
The goal of endoscopic treatment is resection of the mucosa and sub-mucosa of the targeted area to the lamina propria. Endoscopic treatment is not only curative but also allows for histological assessment of the resected specimen, which helps to accurately stage the lesion by assessing the depth of the tumor, involvement of lateral and deep margins, lymphatic and vascular invasion [59–69]. EMR and ESD are two organ-sparing endoscopic treatment techniques developed for removing tumors limited to the mucosa—and occasionally sub-mucosa—in the esophagus and elsewhere in the GI tract.
Inoue et al. were the first to describe the use of EMR for early gastrointestinal cancers, including esophageal cancer [59] (Fig. 1). EMR can be injection-, cap- or ligation-assisted. EMR can be performed en bloc for smaller lesions (<2 cm) or piecemeal [59–67]. Most endoscopists are familiar with band ligation and this technique has gained in popularity. The two techniques appear similar in terms of the depth of resection, efficacy and safety [59–67]. Although, in some situations, the cap technique may yield slightly larger pieces, the band ligation assisted method saves cost and time [59–67].
Figure 1.
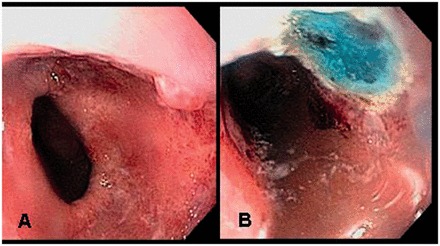
EMR of Barrett’s HGD nodular lesion.
A: Nodular lesion within the Barrett’s mucosa.
B: Post-EMR image.
EMR leads to complete remission rates of 97–100% with 5-year survival rates of 84–98% and 21.5% rate of recurrence with metachronous lesions [59–67]. Ablative therapy after ER could decrease this risk [68–70]. Complications of EMR include bleeding, stricture formation and stenosis. Mucosal defects involving over three-fourths the circumference of the esophagus and mucosal defects longer than 30 mm are associated with greater severity of stenosis [59–72]. Complete Barrett’s eradication EMR (CBE-EMR) with a reported 97.5% efficacy is a recently introduced concept, wherein the entire length of BE is eradicated in multiple sessions [72]. CBE-EMR also provides for the most accurate staging of BE with neoplasia, at a cost of a high rate of esophageal stenosis (49.7%) [72]. In a European, multicenter, randomized study of 43 patients, the efficacy of CBE-EMR was similar to RFA for eradication of all IM (96 vs 95%), but was associated with much higher rates of bleeding (23 vs 5%) and stricture formation (86 vs 14%) [72].
Endoscopic sub-mucosal dissection (ESD) has been developed for en bloc resection and removal of larger than 2 cm flat GI tract lesions [73–78] (Fig. 2). Feasibility of ESD for early esophageal cancers has been demonstrated in small case series from Asia and Europe [74–78]. Even though ESD may have a better rate of tumor-free margins for resection, it is technically challenging, associated with complications such as perforation and stricture formation [74–78]. At the present time, there is no evidence to suggest that ESD is superior to EMR with ablation to achieve complete remission and improve survival in patients with BE and early cancer (73).
Figure 2.
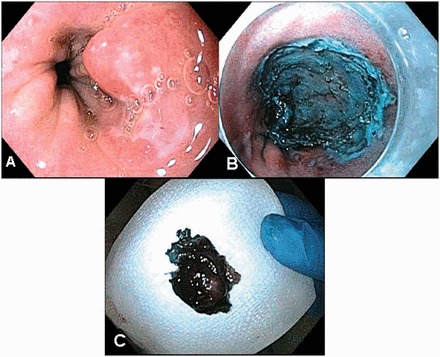
ESD of Barrett’s HGD nodular lesion.
A: Nodular lesion within the Barrett’s mucosa.
B: Post-ESD image.
C: Gross specimen.
Mucosal ablation
Mucosal ablation using endoscopic laser therapy, such as multi-polar electro coagulation (MPEC) and argon plasma coagulation (APC), was demonstrated almost two decades ago [79–82]. Photodynamic therapy (PDT) was a relatively new therapy. However, low response rates and high rates of adverse reactions, such as strictures and risk of buried Barrett’s glands, led to replacement of these above techniques with radiofrequency ablation (RFA) and cryotherapy.
Photodynamic therapy (PDT) utilizes the photochemical energy of a photosensitizer [porphimer sodium (Ps), 5-aminolevulinic acid (5-ALA) or m-tetrahydroxyphenylchlorin (mTHPC)], which is concentrated in neoplastic tissue, followed by activation with endoscopically delivered laser light (balloon based or bare cylinder) of an appropriate power and wavelength. The activated drug reacts with oxygen, generating free radicals, and induces cell membrane damage and apoptosis [83]. The greatest body of data pertaining to efficacy and long-term outcomes in the treatment of BE with dysplasia or IMC is related to porphimer sodium [84]. PDT (using Ps) with PPI was more effective than PPI alone in eradicating BE with HGD (77% vs 39%), along with a lower rate of progression to cancer (13% vs 28%) and a significantly longer time to progression [84]. Only 48% of patients with PDT remained in complete remission, compared to 4% of those on PPI alone [84].
Photosensitivity (69%), esophageal strictures (36%), chest pain (20%), fever (20%) and dysphagia (19%) are the common side-effects of PDT [84–91]. Older age, smoking and presence of residual non-dysplastic BE may result in recurrence and/or presence of buried Barrett’s glands [84–91]. Adenocarcinoma can arise from buried Barrett’s glands and limit the effectiveness of PDT therapy [84–91].
Radiofrequency ablation (RFA) uses an alternating electrical current to induce an electromagnetic field [92–95]. The electromagnetic field causes charged ions to rapidly oscillate, collide with one another and create molecular friction and a rapid, exothermic release of thermal energy, resulting in controlled thermal injury [92–95]. The coagulated mucosal tissue acts as an insulator, limiting the ablation depth in a superficial, controlled and consistent manner (Fig. 3). There are two commercially available devices to perform RFA in the esophagus: the HALO360 and HALO90 (BARRX Medical, Inc, Sunnyvale, CA, USA).
Figure 3.
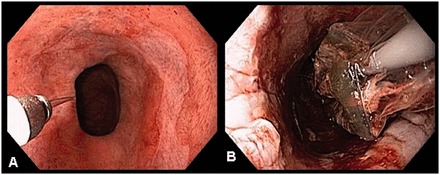
RFA of Barrett’s HGD flat mucosa.
A: Barrett’s mucosa pre-RFA.
B: Barrett’s mucosa with tissue coagulum post-RFA.
In a prospective, multicenter study of dysplastic BE patients, complete remission of IM (CRIM) was seen in 77% and complete remission of dysplasia (CRD) was seen in 86% [92–95]. In patients with HGD, CRIM was seen in 74% and CRD in 81%. There was less disease progression (3.6%) and fewer cancers noted (1.2%) in patients from the ablation group and the response sustained for 2–3 years [92–95]. However, more long-term studies are needed to demonstrate continued durability.
Non-cardiac chest pain (8.9%), nausea (7.5%), bleeding (1.6%) and minor discomfort requiring pain medications (44%) are the common complications of RFA [92–95]. Serious complications seen with RFA, such as strictures (6.4%), buried Barrett’s and dysplasia (0.5–1%), are much less than the complications observed with PDT [92–95]. Given the superficial nature of thermal injury and requirement of adequate tissue apposition, RFA may not be appropriate in patients with nodular BE. However, RFA can be successfully performed after focal EMR of nodular or visible lesion. Akiyama et al. retrospectively studied RFA outcomes in Barrett’s patients with acid suppression [96]. Despite treatment with proton-pump inhibitors (PPI), about 29% of the 45 patients treated with RFA for BE still exhibited moderate-to-severe esophageal acid exposure (EAE) [96]. Increased reduction of BE surface area and complete eradication of BE were noted in patients with normal–mild-, compared to moderate–severe, EAE (99 vs 95%) [96]. RFA is currently the best available ablation technique for treatment of flat HGD and for eradication of residual BE mucosa after focal EMR [25].
Pasricha et al. described the use of endoscopic cryotherapy, wherein application of a cryogen (liquid CO2 or liquid N2) to the BE, with repeated cycles of rapid freezing and slow thawing, causes direct cell injury, vascular stasis and cellular apoptosis [97–104]. The efficacy depends on the tissue temperature, duration of freezing, cooling rate, thaw rate, number of freeze–thaw cycles and interval between the cycles [97–104] (Fig. 4).
Figure 4.
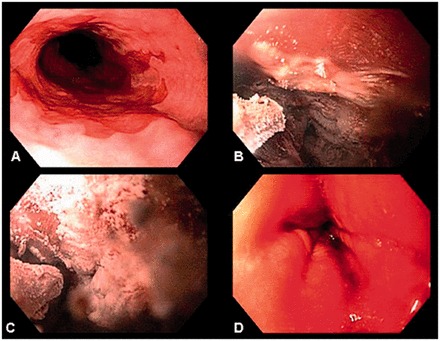
Cryotherapy of Barrett’s HGD flat mucosa.
A: Barrett’s mucosa pre-cryotherapy.
B & C: Cryotherapy catheter application with cycles of rapid freezing.
D: Thawing cycle slow redness indicating direct cell injury.
EMR for resection of visible lesions, followed by cryotherapy, was prospectively studied in non-surgical BE patients with HGD. The treatment resulted in 90% improved histology and 30–40% complete resolution of dysplasia [97–104]. Similarly 97% HGD eradication and 57% IM eradication was noted in a multicenter, retrospective cohort study of 98 patients with BE and HGD at a 10.4 month follow-up following a mean total of four treatments [97–104]. Management of local disease in unresectable cancer is another application of endoscopic cryotherapy. In a retrospective study of 79 subjects with T1–T4 cancer and a mean tumor burden of 4 cm, complete response of intraluminal disease was seen in 61% (75% with mucosal cancer), with 13% developing benign strictures [102].
Common side-effects include strictures (3%) and chest pain (2%). Buried Barrett’s was seen in 3% [97–104]. Contra-indications to esophageal cryotherapy ablation include mucosal breaks, coagulopathy and retained food in the stomach [97–104]. Altered surgical anatomy, eosinophilic esophagitis and presence of large hiatal hernia pose significant risk of perforation, due to restricted volume or distensibility of the gastrointestinal tract [97–104]. Similarly to RFA, cryotherapy appears promising, with good efficacy and safety profile. However larger studies and long-term durability data of treatment are necessary.
CONCLUSION
Endoscopic therapy in an appropriately selected patient population appears to be safe and effective for management of BE with dysplasia and IMC. BE eradication is recommended for treatment and prevention of metachronous and synchronous lesions. Further studies are needed to assess the long-term durability of endoscopic therapy, to recognize and manage buried Barrett’s and to identify optimal management strategy in patients with LGD and non-dysplastic BE. Management of BE is a dynamic process and will continue to evolve as we make advances in our understanding of the development of dysplasia and cancer in BE, genetics of BE, identify molecular markers or less-expensive methods of screening and surveillance for cancer and dysplasia and develop safer wide-field resection techniques, which would effectively remove all Barrett’s and obviate the need for long-term surveillance.
BE with dysplasia and cancer often entails complex decision-making. Its management requires a multidisciplinary approach, in collaboration with expert endoscopists, surgeons, oncologists and pathologists. A clear understanding of the biology of BE—risk of neoplastic progression, appropriate screening and surveillance, patient selection, availability of various endoscopic ablation techniques, their benefits, risk profile and applicability to the patient to be treated—will help successful endoscopic and/or surgical management of BE.
Conflict of interest: none declared.
REFERENCES
- 1.American Gastroenterology Association. AGA Medical Position Statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett's esophagus. N Engl J Med. 1976;295:476–80. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol and Hepatol. 2006;4:566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage and age. J Natl Cancer Inst. 2008;100:1184–87. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spechler SJ, Fitzgerald RC, Prasad GA, et al. History, molecular mechanisms and endoscopic treatment of Barrett's esophagus. Gastroenterology. 2010;138:854–69. doi: 10.1053/j.gastro.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98–100. doi: 10.1002/ijc.11029. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson MK, Naunheim KS. Resection for Barrett's mucosa with high-grade dysplasia: implications for prophylactic photodynamic therapy. J Thorac Cardiovasc Surg. 1997;114:824–29. doi: 10.1016/S0022-5223(97)70087-4. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini CA, Pohl D. High-Grade dysplasia in Barrett’s esophagus: surveillance or operation? J Gastrointest Surg. 2000;4:131–34. doi: 10.1016/s1091-255x(00)80048-7. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 11.Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol. 2008;6:159–64. doi: 10.1016/j.cgh.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Feith M, Stein HJ, Siewert JR. Pattern of lymphatic spread of Barrett's cancer. World J Surg. 2003;27:1052–57. doi: 10.1007/s00268-003-7060-2. [DOI] [PubMed] [Google Scholar]
- 13.Rice TW, Zuccaro G, Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787–92. doi: 10.1016/s0003-4975(97)01387-8. [DOI] [PubMed] [Google Scholar]
- 14.Levine DS, Reid BJ. Endoscopic biopsy technique for acquiring larger mucosal samples. Gastrointest Endosc. 1991;37:332–37. doi: 10.1016/s0016-5107(91)70726-8. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–99. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Lagergren J, Bergström R, Lindgren A, et al. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340–6. [PubMed] [Google Scholar]
- 17.Chak A, Faulx A, Eng C, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006;107:2160–66. doi: 10.1002/cncr.22245. [DOI] [PubMed] [Google Scholar]
- 18.Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 19.Reavis KM, Morris CD, Gopal DV, et al. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Surg. 2004;239:849–56. doi: 10.1097/01.sla.0000128303.05898.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn 1. Gastroenterology. 2003;125:1670–77. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson M, Chak A. Unsedated small-caliber endoscopy—a new screening and surveillance tool for Barrett's esophagus? Nat Clin Pract Gastroenterol Hepatol. 2007;4:426–27. doi: 10.1038/ncpgasthep0880. [DOI] [PubMed] [Google Scholar]
- 23.Galmiche JP, Sacher-Huvelin S, Coron E, et al. Screening for esophagitis and Barrett's esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538–45. doi: 10.1111/j.1572-0241.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- 24.Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komanduri S, Swanson G, Keefer L, et al. Use of a new jumbo forceps improves tissue acquisition of Barrett's esophagus surveillance biopsies. Gastrointest Endosc. 2009;70:1072–78. doi: 10.1016/j.gie.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Abela JE, Going JJ, Mackenzie JF, et al. Systematic four-quadrant biopsy detects Barrett's dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol. 2008;103:850–85. doi: 10.1111/j.1572-0241.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 27.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald RC, Saeed IT, Khoo D, et al. Rigorous surveillance protocol increases detection of curable cancers associated with Barrett's esophagus. Dig Dis Sci. 2001;46:1892–98. doi: 10.1023/a:1010678913481. [DOI] [PubMed] [Google Scholar]
- 29.Mannath J, Subramanian V, Hawkey C, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Endoscopy. 2010;42:351–59. doi: 10.1055/s-0029-1243949. [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett's esophagus. Gastrointest Endosc. 2006;64:167–75. doi: 10.1016/j.gie.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Marcon N, Wani S, et al. Non-biopsy detection of intestinal metaplasia and dysplasia in Barrett's esophagus: a prospective multicenter study. Endoscopy. 2006;38:1206–12. doi: 10.1055/s-2006-944974. [DOI] [PubMed] [Google Scholar]
- 32.Canto MIF, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett's esophagus. Gastrointest Endosc. 2000;51:560–68. doi: 10.1016/s0016-5107(00)70290-2. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Izatt JA, Kulkarni MD, et al. High-resolution cross-sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc. 1998;47:515–23. doi: 10.1016/s0016-5107(98)70254-8. [DOI] [PubMed] [Google Scholar]
- 34.Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett's esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19–24. doi: 10.1016/j.gie.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2001;120:1620–29. doi: 10.1053/gast.2001.24842. [DOI] [PubMed] [Google Scholar]
- 36.Kendall C, Stone N, Shepherd N, et al. Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett's oesophagus. J Pathol. 2003;200:602–9. doi: 10.1002/path.1376. [DOI] [PubMed] [Google Scholar]
- 37.Hancock S, Bowman E, Prabhakaran J, et al. Use of i-scan endoscopic image enhancement technology in clinical practice to assist in diagnostic and therapeutic endoscopy: a case series and review of literature. Diagn Ther Endosc. doi: 10.1155/2012/193570. doi:10.1155/2012/193570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang KK, Sampliner R. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 39.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Watson A, Heading RC, Shepherd NA. Guidelines for the diagnosis and management of Barrett's columnar-lined oesophagus. British Society of Gastroenterology 2005. [Google Scholar]
- 41.Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340–45. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 42.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2007;132:1226–33. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology. 2009;137:815–23. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 45.Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108:200–7. doi: 10.1038/ajg.2012.387. [DOI] [PubMed] [Google Scholar]
- 46.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–98. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Thomas T, Ayaru L, Lee EY, et al. Length of Barrett's segment predicts success of extensive endomucosal resection for eradication of Barrett's esophagus with early neoplasia. Surg Endosc. 2011;25:3627–35. doi: 10.1007/s00464-011-1769-z. [DOI] [PubMed] [Google Scholar]
- 48.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol. 2012;107:850–62. doi: 10.1038/ajg.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tharavej C, Hagen JA, Peters JH, et al. Predictive factors of co-existing cancer in Barrett’s high-grade dysplasia. Surg Endosc. 2006;20:439–43. doi: 10.1007/s00464-005-0255-x. [DOI] [PubMed] [Google Scholar]
- 50.Van Sandick JW, Van Lanschot JJ, Ten Kate FJ, et al. Pathology of early invasive adenocarcinoma of the esophagus or esophagogastric junction. Cancer. 2000;88:2429–37. doi: 10.1002/1097-0142(20000601)88:11<2429::aid-cncr1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 51.Curvers WL, Ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: over diagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–30. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 52.Ajumobi A, Bahjri K, Jackson C, et al. Surveillance in barrett’s esophagus: an audit of practice. Dig Dis Sci. 2010;55:1615–21. doi: 10.1007/s10620-009-0917-y. [DOI] [PubMed] [Google Scholar]
- 53.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 54.Inadomi JM, Somsouk M, Madanick RD, et al. A cost-utility analysis of ablative therapy for Barrett's esophagus. Gastroenterology. 2009;136:2101–14. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients (with video) Gastrointest Endosc. 2007;65:185–95. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 56.Weston AP, Sharma P, Banerjee S, et al. Visible endoscopic and histologic changes in the cardia, before and after complete Barrett's esophagus ablation. Gastrointest Endosc. 2005;61:515–21. doi: 10.1016/s0016-5107(05)00131-8. [DOI] [PubMed] [Google Scholar]
- 57.Sampliner R, Camargo E, Prasad A. Association of ablation of Barrett's esophagus with high grade dysplasia and adenocarcinoma of the gastric cardia. Dis Esophagus. 2006;19:277–79. doi: 10.1111/j.1442-2050.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 58.Inoue H, Endo M, Takeshita K, et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC) Surg Endosc. 1992;6:264–65. doi: 10.1007/BF02498820. [DOI] [PubMed] [Google Scholar]
- 59.ASGE Technology Committee, Kantsevoy SV, Adler DG, Conway JD, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11–18. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 60.Fleischer DE, Wang GQ, Dawsey S, et al. Tissue band ligation followed by snare resection (band and snare): a new technique for tissue acquisition in the esophagus. Gastrointest Endosc. 1996;44:68–72. doi: 10.1016/s0016-5107(96)70233-x. [DOI] [PubMed] [Google Scholar]
- 61.Abrams J, Fedi P, Vakiani E, et al. Depth of resection using two different endoscopic mucosal resection techniques. Endoscopy. 2008;40:395–99. doi: 10.1055/s-2007-995529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.May A, Gossner L, Behrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167–75. doi: 10.1067/mge.2003.339. [DOI] [PubMed] [Google Scholar]
- 63.Peters FP, Kara MA, Curvers WL, et al. Multiband mucosectomy for endoscopic resection of Barrett's esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol. 2007;19:311–15. doi: 10.1097/MEG.0b013e328080ca90. [DOI] [PubMed] [Google Scholar]
- 64.May A, Gossner L, Pech O, et al. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett's esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy. 2002;34:604–10. doi: 10.1055/s-2002-33236. [DOI] [PubMed] [Google Scholar]
- 65.Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett's esophagus with advanced neoplasia. Gastrointest Endosc. 2012;76:733–39. doi: 10.1016/j.gie.2012.04.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 67.Manner H. Rabenstein T, Braun K et al. What should we do with the remainder of the Barrett’s segment after endoscopic resection of early Barrett’s cancer? Intermediate results of the first prospective-randomized trial on the APC ablation of residual Barrett’s mucosa with concomitant esomeprazole therapy versus surveillance without ablation after ER of Early Barrett’s Cancer. Gastrointest Endosc. 2010;71: AB175. [Google Scholar]
- 68.Gondrie J, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359–69. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 69.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for Barrett's esophagus with early neoplasia. Clinl Gastroenterol Hepatol. 2010;8:23–29. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165–69. doi: 10.1067/mge.2003.73. [DOI] [PubMed] [Google Scholar]
- 71.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684–92. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 72.Conio M, Sorbi D, Batts K, et al. Endoscopic circumferential esophageal mucosectomy in a porcine model: an assessment of technical feasibility, safety and outcome. Endoscopy. 2001;33:791–94. doi: 10.1055/s-2001-16516. [DOI] [PubMed] [Google Scholar]
- 73.Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy. 2012;44:1105–13. doi: 10.1055/s-0032-1310155. [DOI] [PubMed] [Google Scholar]
- 74.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169–77. doi: 10.1136/gut.2010.210229. [DOI] [PubMed] [Google Scholar]
- 75.Van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–73. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 76.Deprez PH, Piessevaux H, Aouattah T. ESD in Barrett’s esophagus high grade dysplasia and mucosal cancer: prospective comparison with CAP mucosectomy. Gastrointest Endosc. 2010;71:AB126. [Google Scholar]
- 77.Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202–9. doi: 10.1016/j.gie.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 78.Brandt LJ, Kauvar DR. Laser-induced transient regression of Barrett's epithelium. Gastrointest Endosc. 1992;38:619–22. doi: 10.1016/s0016-5107(92)70536-7. [DOI] [PubMed] [Google Scholar]
- 79.Sampliner RE, Fennerty B, Garewal HS. Reversal of Barrett's esophagus with acid suppression and multipolar electrocoagulation: preliminary results. Gastrointest Endosc. 1996;44:532–35. doi: 10.1016/s0016-5107(96)70004-4. [DOI] [PubMed] [Google Scholar]
- 80.Dulai GS, Jensen D M, Cortina G, et al. Randomized trial of argon plasma coagulation vs multipolar electrocoagulation for ablation of Barrett's esophagus. Gastrointest Endosc. 2005;61:232–40. doi: 10.1016/s0016-5107(04)02576-3. [DOI] [PubMed] [Google Scholar]
- 81.Nishioka NS. Drug, light and oxygen: a dynamic combination in the clinic. Gastroenterology. 1998;114:604–6. doi: 10.1016/s0016-5085(98)70546-3. [DOI] [PubMed] [Google Scholar]
- 82.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 83.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007;66:460–68. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 84.Pech O, Gossner L, May A, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett's cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc. 2005;62:24–30. doi: 10.1016/s0016-5107(05)00333-0. [DOI] [PubMed] [Google Scholar]
- 85.Badreddine RJ, Prasad GA, Wang KK, et al. Prevalence and predictors of recurrent neoplasia after ablation of Barrett's esophagus. Gastrointest Endosc. 2010;71:697–703. doi: 10.1016/j.gie.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hage M, Siersema PD, Van Dekken H, et al. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett’s oesophagus: a randomised trial. Gut. 2004;53:785–90. doi: 10.1136/gut.2003.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prasad GA, Wang KK, Buttar NS, et al. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett's esophagus. Gastrointest Endosc. 2007;65:60–66. doi: 10.1016/j.gie.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 88.Ban S, Mino M, Nishioka NS, et al. Histopathologic aspects of photodynamic therapy for dysplasia and early adenocarcinoma arising in Barrett's esophagus. Am J Surg Pathol. 2004;28:1466–73. doi: 10.1097/01.pas.0000141392.91677.7f. [DOI] [PubMed] [Google Scholar]
- 89.Mino-Kenudson M, Ban S, Ohana M, et al. Buried dysplasia and early adenocarcinoma arising in Barrett esophagus after porfimer-photodynamic therapy. Am J Surg Pathol. 2007;31:403–9. doi: 10.1097/01.pas.0000213407.03064.37. [DOI] [PubMed] [Google Scholar]
- 90.Bronner MP, Overholt BF, Taylor SL, et al. Squamous overgrowth is not a safety concern for photodynamic therapy for Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2009;136:56–64. doi: 10.1053/j.gastro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 91.Bulsiewicz WJ, Shaheen NJ. The role of radiofrequency ablation in the management of barrett's esophagus. Gastrointest Endosc Clin N Am. 2011;21:95–109. doi: 10.1016/j.giec.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Shaheen NJ, Fleischer D, Eisen G, et al. Durability of epithelial reversion after radiofrequency ablation: follow-up of the AIM Dysplasia Trial. Gastroenterology. 2010;138:S16–S17. [Google Scholar]
- 93.Vassiliou MC, Von Renteln D, Wiener DC, et al. Treatment of ultralong-segment Barrett’s using focal and balloon-based radiofrequency ablation. Surg Endosc. 2010;24:786–91. doi: 10.1007/s00464-009-0639-4. [DOI] [PubMed] [Google Scholar]
- 94.Pouw RE, Gondrie JJ, Rygiel AM, et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett's esophagus containing neoplasia. Am J Gastroenterol. 2009;104:1366–73. doi: 10.1038/ajg.2009.88. [DOI] [PubMed] [Google Scholar]
- 95.Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95:1187–91. doi: 10.1111/j.1464-410X.2005.05502.x. [DOI] [PubMed] [Google Scholar]
- 96.Pasricha PJ, Hill S, Wadwa KS, et al. Endoscopic cryotherapy: experimental results and first clinical use. Gastrointest Endosc. 1999;49:627–31. doi: 10.1016/s0016-5107(99)70393-7. [DOI] [PubMed] [Google Scholar]
- 97.Raju G, Ahmed I, Xiao SY, et al. Graded esophageal mucosal ablation with cryotherapy and the protective effects of submucosal saline. Endoscopy. 2005;37:523–26. doi: 10.1055/s-2005-861312. [DOI] [PubMed] [Google Scholar]
- 98.Johnston L, Johnston M. Cryospray ablation (CSA) in the esophagus: optimization of dosimetry. Am J Gastroenterol. 2006;101:S532. [Google Scholar]
- 99.Chen AM, Pasricha PJ. Cryotherapy for Barrett's esophagus: who, how and why? Gastrointest Endosc Clin N Am. 2001;21:111–18. doi: 10.1016/j.giec.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 100.Dumot JA, Vargo JJ, 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635–44. doi: 10.1016/j.gie.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680–85. doi: 10.1016/j.gie.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–693. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]


