Abstract
Corin is a serine protease originally isolated from the heart. Functional studies show that corin is the long-sought enzyme responsible for activating cardiac natriuretic peptides. In mice, lack of corin prevents the natriuretic peptide processing, causing salt-sensitive hypertension. In humans, corin variants and mutations that reduce corin activity have been identified in patients with hypertension and heart failure. Decreased plasma levels of corin antigen and activity have been reported in patients with heart failure and coronary artery disease. Low levels of urinary corin also have been found in patients with chronic kidney disease. Most recent studies show that corin also acts in the uterus to promote spiral artery remodeling and prevent pregnancy-induced hypertension. Here we review the role of corin in natriuretic peptide processing and cardiovascular diseases such as hypertension, heart disease, pre-eclampsia, and chronic kidney disease.
Keywords: African American, ANP, BNP, Cardiac hypertrophy, Chronic kidney disease, CNP, Corin, ENaC, Gene mutation, Gene variant, Heart failure, Hypertension, Natriuretic peptides, Pre-eclampsia, Salt-sensitive hypertension, Spiral artery remodeling, Trophoblast invasion
Introduction
Natriuretic peptides are important hormones conserved in all vertebrates [1]. In mammals, the natriuretic peptide family has three members, i.e. atrial natriuretic peptide (ANP), brain or B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) [2, 3]. ANP and BNP are produced in cardiomyocytes and function to regulate salt-water balance and blood pressure. Upon binding to their receptor, natriuretic peptide receptor-A (NPR-A), ANP and BNP promote renal sodium excretion and relax vascular smooth muscles. To date, ANP and NPR-A gene variants and mutations have been reported in patients with hypertension and cardiac hypertrophy [4, 5*, 6*, 7–9, 10*]. ANP and BNP also play a role in regulating energy metabolism by stimulating fat oxidation in skeletal muscles and lipolysis in adipocytes [11*, 12*, 13]. Under pathological conditions, such as heart failure, ANP and BNP expression is highly up-regulated, which serves as a compensatory mechanism to lower blood pressure and volume. Currently, ANP, BNP and their related peptides are used as biomarkers in assessing heart failure [14].
CNP differs from ANP and BNP in many aspects including tissue distribution and biological function. CNP is made mainly in vascular endothelial cells, smooth muscles and chondrocytes, where it acts through its receptor, natriuretic peptide receptor-B (NPR-B), to regulate cell growth, vascular remodeling and bone differentiation [15, 16]. Defects in CNP and NPR-B cause skeletal abnormalities [17–20]. CNP also functions in the reproductive system. In ovaries, for example, CNP has been shown to promote follicle development and regulate oocyte maturation [21, 22]. The expression of CNP in peripheral neurons also has been reported [23]. Recent studies show that CNP may function in the gastrointestinal track to stimulate intestinal motility [24, 25].
Peptide hormones commonly are synthesized in precursor forms, which are processed to mature forms by proteolytic enzymes either in specific intracellular compartments or extracellularly. This step is essential for activating the peptide hormones. Similarly, the natriuretic peptides are made as prepropeptides [19, 26]. Signal peptidase moves the prepeptide in the ER to generate pro-natriuretic peptides, which remain inactive. In recent years, corin, a serine protease identified in the heart [27], has been shown to play a critical role in processing natriuretic peptides. In this review, we will focus on recent findings of corin function and discuss the role of corin in hypertension and kidney disease.
Corin and natriuretic peptide processing
Corin is a trypsin-like protease highly expressed in cardiomyocytes [27–29]. Unlike trypsin, which is a secreted soluble protein, corin has an N-terminal transmembrane domain tethering corin on the cell surface [27]. The catalytic protease domain of corin is located at the C-terminus. Between these two domains, there are two frizzled-like domains, eight LDL receptor-like repeats and a scavenger receptor-like domain [27]. The overall corin domain structure and membrane topology are similar to those of type II transmembranse serine proteases [30]. Corin, however, is the only trypsin-like serine protease known to contain frizzled-like domains. The molecular biology and biochemical properties of corin have been described in previous reviews [26, 31].
The primary function of corin in the heart is to activate pro-ANP [32–34]. When pro-ANP is released from the dense granules of cardiomyocytes, corin cleaves pro-ANP at residue Arg-98, producing a 26-amino-acid C-terminal active ANP. Corin also participates in the conversion of pro-BNP to BNP [33, 35–38]. This activity, however, is not corin-specific. The proprotein convertase furin also activates pro-BNP [37–40]. Remarkably, O-glycans in the pro-BNP propeptide inhibit corin- and furin-mediated pro-BNP processing [37, 41]. It appears that O-glycans, which are absent in pro-ANP and pro-CNP [42], may have a specific role in regulating BNP production. To date, there is no evidence to indicate a role of corin in pro-CNP processing. Furin appears to be the primary enzyme responsible for pro-CNP processing [43]. Thus, the enzymes responsible for processing pro-ANP, pro-BNP and pro-CNP differ considerably, even though the genes encoding these peptides were derived from a common origin. Probably, the divergence reflects specific regulatory requirements, as the peptides evolve to perform separate functions in different cell types.
Mouse models of corin deficiency
The importance of corin in controlling natriuretic peptide production and blood pressure has been studied in knockout (ko) mice [44]. Despite corin mRNA is expressed in embryonic hearts as early as day E7.5 [27], corin ko mice had no apparent developmental defects [44]. In this regard, corin appears similar to several other type II transmembrane serine proteases, such as hepsin [45] and HAT [46], which are dispensable for embryonic development and postnatal survival.
In biochemical analysis, heart tissues from corin ko mice were found to contain high levels of unprocessed pro-ANP but no detectable amounts of mature ANP, reflecting a defect in pro-ANP processing [44]. Intravenous injection of a soluble active corin into the ko mice restored pro-ANP processing and elevated plasma cGMP levels [44]. The results show that corin is essential for pro-ANP processing and that its activity cannot be compensated by other proteases in vivo.
Natriuretic peptides are critical for normal blood pressure. In mice, lacking ANP or NPR-A leads to hypertension [47, 48]. A hypertensive phenotype also was found in corin ko mice [44, 49]. When the ko mice were challenged with high-salt diets, their blood pressure elevated further, indicating that the hypertension in corin ko mice was salt-sensitive [49]. Thus, the hypertensive phenotypes in corin, ANP and NPR-A ko mice were similar, supporting a critical role of corin in activating the ANP pathway in vivo.
In addition to the ko mice, corin deficiency has been reported in a naturally-occurring mutant mouse strain, C57BL/6-KitW-sh (Wsh), known for mast cell-deficiency due to an abnormal Kit locus [50]. Genomic sequencing revealed a genetic inversion disrupting the transcriptional regulatory region upstream of the c-kit gene, which accounts for the hematopoietic defects in these mice [51]. Interestingly, the generic inversion also disrupted the corin gene between exons 5 and 6. As a result, Wsh mice lacked corin mRNA expression and had high levels of unprocessed pro-ANP in the heart [51, 52]. The mice developed cardiac hypertrophy and had poor cardiac function [51, 52]. Similar pathological findings also were reported in corin ko mice [44, 53].
Unexpectedly, corin expression was detected in the dermal papilla of hair follicles in mice and humans [54, 55]. The biological significance of corin in the skin is not completely understood. In mice, corin appears involved in coat color regulation, as corin ko mice had a lighter yellowish color than that of wild-type mice [54]. This skin phenotype, however, occurred only when mice had a functional agouti allele [54], suggesting that corin may interact with a molecule in the agouti pathway to regulate hair pigmentation. To date, the specific corin substrate in the mouse skin has not been defined. It remains to be determined if corin has a similar role in human skin biology.
Human corin variants and mutations
The human corin gene is on chromosome 4p12–13, consisting of 22 exons [56]. To date, human corin gene variants have been identified. Dries et al. reported a corin variant allele (T555I/Q568P) in African Americans with hypertension and heart disease [57]. Individuals with this variant allele had severe cardiac hypertrophy and high levels of unprocessed natriuretic peptides in blood [58, 59], suggesting that corin protein encoded by this variant allele may be defective. This hypothesis was supported by biochemical studies, in which recombinant corin variant T555I/Q568P was shown to process pro-ANP and pro-BNP poorly [60]. Apparently, the amino acid changes caused by the gene variant in the propeptide region altered corin protein conformation and inhibited corin zymogen activation [60].
Wang et al. further examined the effect of this corin variant on blood pressure by generating a transgenic mouse model that expressed T555I/Q568P variant in a corin null background [53*]. In these mice, corin activity was significantly reduced, resulting in high levels of pro-ANP in the heart. The results confirmed that the corin variant is defective in vivo. More importantly, the transgenic mice developed hypertension and cardiac hypertrophy, which were exacerbated upon high salt-diet challenge [53*]. The overall hypertensive phenotype of the transgenic mice mimics the clinical features in the African Americans carrying the variant allele [57, 58]. These data suggest that the corin variant allele, which is present in ~10–12% of African Americans [57], may contribute to hypertension and heart disease in this high-risk population.
The corin T555I/Q568P variant appears to originate in Africa, as this allele is present mostly in African Americans but not in other ethnic groups [57]. Recently, Dong et al. reported a C-to-T mutation in exon 12 of the corin gene in a Chinese patient family of hypertension [61]. The mutation resulted in an Arg-to-Cys change at residue 539 in corin frizzled-2 domain. Within this family, individuals carrying the mutantion had high systolic and/or diastolic blood pressure. In functional studies, the R539C mutant had reduced pro-ANP processing activity and exhibited a dominant-negative effect on wild-type corin. It appeared that the mutant Cys formed an alternative disulfide bond [61], altering the conformation of corin frizzled-2 domain, which is required for interacting with pro-ANP [62]. The R539C mutation also caused corin self-cleavage, producing an alternative inactive fragment [61]. These results indicate that genetic mutations reducing corin activity may represent a molecular mechanism underlying hypertension. As more genetic studies are conducted, additional corin mutations are expected to be identified in hypertensive patients.
Corin in patients with heart disease
Hypertension is a major risk factor for heart disease. In patients with heart failure, plasma levels of unprocessed natriuretic peptides are highly elevated [63–65], suggesting that corin activity could not be compensated adequately to meet the demand under the pathological condition [66–68]. Presently, how corin expression and activity are regulated in the heart is not well understood. Studies indicate that a number of regulatory mechanisms may be involved, including transcriptional control [56], cell surface targeting [69], N-glycosylation [70, 71], and zymogen activation [60, 72].
In cell-based studies, active corin molecules on the cell surface were shown to undergo proteolytic shedding [73], a process that has been found in many membrane-bound proteases [30, 74]. Understandably, excessive proteolytic activities in tissues could be detrimental. It is possible that the shedding process may serve as a mechanism to control corin activity in the heart. In principle, shed corin fragments could be degraded in the heart, which is known containing many proteolytic enzymes [75]. It is also possible that some of the corin fragments may enter the circulation.
Indeed, corin antigen and activity have been detected in human blood [36, 76, 77]. Dong et al. showed that plasma corin levels decreased progressively in patients with late stages of heart failure [78]. Similar findings of reduced corin antigen and activity levels in heart failure patients were reported in another study [79]. Most recently, low serum corin levels were found to be an independent predictor for poor clinical outcomes in patients with coronary disease [80*]. These results are consistent with the elevated levels of unprocessed natriuretic peptides in patients with heart disease, suggesting that corin deficiency may be a contributing factor in failing hearts [81]. In supporting this hypothesis, a recent study showed that corin overexpression improved cardiac function, reduced pulmonary edema, and increased survival in a mouse model of dilated cardiomyopathy [82*]. The data also support a possible therapeutic strategy to enhance corin activity to treat patients with heart disease.
Corin in pre-eclampsia
Pre-eclampsia is a serious complication in pregnancy, afflicting millions of women worldwide [83]. It has been long suspected that a defective uteroplacental interface is a primary factor underlying the disease [84, 85]. Among pathologic findings in pre-eclamptic patients, delayed trophoblast invasion and poorly remodeled spiral arteries in the uterus are common [84, 85]. Possibly, the maternal hypertension reflects a compensatory response attempting to increase blood flow to the ischemic placenta caused by narrow uterine spiral arteries. To date, the mechanism underlying such uteroplacental defects in pre-eclamptic patients remains poorly understood.
Interestingly, corin expression was found in the pregnant uterus in mice and humans [27, 86, 87], suggesting a possible corin function in pregnancy. Studies have shown that ANP and NPR-A are expressed in the uterus, where ANP antagonized uteroplacental vessel contraction and stimulated myometrial relaxation [88]. ANP also is known to promote vessel wall remodeling in angiogenic processes [89, 90]. In a matrigel-based invasion study, ANP was shown to enhance human trophoblast transmigration [86**]. These data suggest that corin and ANP may participate in tissue and vascular remodeling in the pregnant uterus, which is necessary for developing a healthy maternal-fetal interface.
Consistent with this hypothesis, pregnant ko mice lacking either corin or ANP had delayed trophoblast invasion and poorly remodeled spiral arteries in the uterus [86**]. The mice also developed late gestational hypertension and proteinuria, which was alleviated once pups were delivered [86**]. The overall phenotype of the pregnant corin and ANP ko mice resembled pathological features in pre-eclamptic patients [86, 91]. Importantly, low levels of uterine corin expression were found in pre-eclamptic patients [86**]. Moreover, missense mutations in the corin gene were identified in patients with pre-eclampsia. In functional studies, the corin mutants were shown to have markedly reduced activity in processing pro-ANP [86**]. Paradoxically, plasma corin levels were increased in patients with pre-eclampsia [86, 92]. The results suggest that the observed role of corin in the uterus was likely mediated by locally produced, but not heart-derived, corin and ANP. Together, these findings suggest that defects in the corin and ANP pathway may be a novel mechanism underlying pre-eclampsia.
Corin in kidney disease
In mice, corin mRNA expression was detected in the medulla of developing kidneys [27]. In rat and human kidneys, corin protein was found in the proximal tubule, thick ascending limb, connecting tubule, and collecting duct [36, 93]. In rat models of proteinuric kidney disease that were associated with sodium retention, renal corin mRNA and protein expression was found to be markedly reduced and the reduction was associated with increased renal -ENaC expression [93]. These results are intriguing because ENaC is known to play a critical role in renal sodium reabsorption. The findings from these rat model studies suggest that corin may function in the kidney to promote sodium excretion in an ENaC-dependent manner and that impaired corin expression and/or function may contribute to salt and water retention in kidney disease [94].
Consistent with these findings, corin ko mice were shown to have an impaired response to promote sodium excretion when dietary salt contents increased, resulting in sodium and water retention and exacerbated hypertension [49]. When corin ko mice on high-salt diet were treated with an ENaC inhibitor, amiloride, renal sodium excretion was significantly improved, leading to lowered blood pressure and reduced body weight [49]. The results point to an important role of corin and ANP in regulating renal sodium excretion and body fluid balance in response to fluctuating dietary salt levels [49, 95]. In migratory fish species such as eel and salmon that live in both salty and fresh water environments, natriuretic peptides are essential for maintaining electrolyte balance [1]. Apparently, such a physiological mechanism is well preserved in mammals, even though their living environments are drastically different from that of fish.
At this time, it is unknown if renal and cardiac corin expression is controlled under similar transcriptional mechanism. Interestingly, corin protein expressed on renal epithelial cells also undergoes a shedding process. Fang et al. reported soluble corin in human urine samples [96]. In patients with chronic kidney disease (CKD), urinary corin levels were significantly lower than that in normal controls [96]. The low levels of urinary corin were associated with hypertension and poor renal function in these patients. By immunostaining, reduced corin protein levels also were found in kidney biopsies in CKD patients [96]. The results suggest that reduced renal corin protein expression and/or activity may be a contributing factor in the pathogenesis of CKD. These findings are consistent with previous reports of fibrosis, tubular dilation and enhanced local inflammation in the kidney of NPR-A ko mice [97].
Conclusions
Hypertension is a major cardiovascular disease. As proposed by Irvine H. Page in his "mosaic theory of hypertension" more than half a century ago [98], a steady state exists in the circulation in which the important regulatory factors are in equilibrium to maintain blood pressure. To date, it is well accepted that environmental, behavioral and genetic factors disrupting such equilibrium can cause hypertension.
The natriuretic peptides act as an important endocrine system, connecting the heart and kidney in maintaining salt-water balance and blood pressure. Most recent studies show that ANP may also participate in a gut-heart cross-talk in regulating blood pressure and energy metabolism [99, 100**]. Corin was discovered as a novel cardiac protease [27]. Identification of corin as the long-sought pro-ANP convertase provides important insights into the biochemical mechanism underlying natriuretic peptide processing [26] (Fig. 1). As more studies are being conducted, we now know that corin acts not only in the heart, but also in many other tissues including the kidney, uterus and skin. Recent animal models and human genetic studies have shown that corin plays a critical role in major cardiovascular diseases, including hypertension, heart disease, pre-eclampsia and kidney disease (Fig. 1). These findings are expected to stimulate more studies to understand the biology of corin and its role in cardiovascular disease. Such studies may also help to translate basic discoveries in corin and natriuretic peptide research into novel therapies to treat hypertension and heart disease in patients.
Fig. 1.
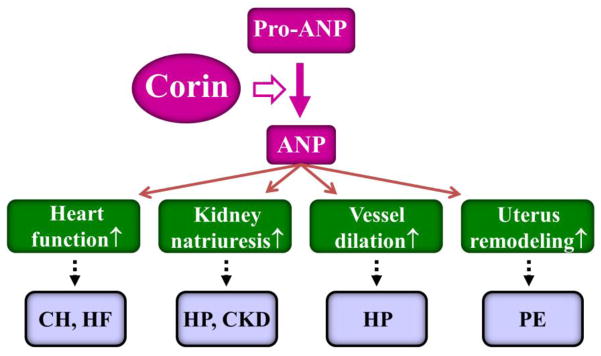
Corin and ANP in cardiovascular biology and disease. Corin converts pro-ANP to ANP, which in return enhances cardiac function, renal sodium excretion, vasodilation, and uterine spiral artery remodeling. Defects in the corin and ANP pathway may lead to major diseases, such as cardiac hypertrophy (CH), heart failure (HF), hypertension (HP), chronic kidney disease (CKD), and pre-eclampsia (PE).
Acknowledgments
We would like to thank our co-workers, past and present, who contributed to corin studies. This work was supported in part by NIH grants R01 HL089298 and HD064634, and grants from the National Natural Science Foundation of China (31070716, 81170247 and 31161130356) and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Yiqing Zhou and Qingyu Wu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Takei Y, Kawakoshi A, Tsukada T, Yuge S, Ogoshi M, Inoue K, et al. Contribution of comparative fish studies to general endocrinology: structure and function of some osmoregulatory hormones. J Exp Zool A Comp Exp Biol. 2006;305:787–98. doi: 10.1002/jez.a.309. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto I, Tokudome T, Nakao K, Kangawa K. Natriuretic peptide system: an overview of studies using genetically engineered animal models. Febs J. 2011;278:1830–41. doi: 10.1111/j.1742-4658.2011.08116.x. [DOI] [PubMed] [Google Scholar]
- 3.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–77. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Arora P, Wu C, Khan AM, Bloch DB, Davis-Dusenbery BN, Ghorbani A, et al. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. 2013;123:3378–82. doi: 10.1172/JCI67383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Barbato E, Bartunek J, Mangiacapra F, Sciarretta S, Stanzione R, Delrue L, et al. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J Am Coll Cardiol. 2012;59:1763–70. doi: 10.1016/j.jacc.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 6*.Cannone V, Huntley BK, Olson TM, Heublein DM, Scott CG, Bailey KR, et al. Atrial natriuretic peptide genetic variant rs5065 and risk for cardiovascular disease in the general community: a 9-year follow-up study. Hypertension. 2013;62:860–5. doi: 10.1161/HYPERTENSIONAHA.113.01344. References 5 and 6 describe the association of a minor ANP gene allele, encoding an ANP variant with two extra C-terminal Arg residues, with an increased risk of cardiovascular disease in European and American populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–65. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker-Foster C, et al. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. Jama. 2008;299:296–307. doi: 10.1001/jama.299.3.296. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K. Functional deletion mutation of the 5'-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res. 2000;86:841–5. doi: 10.1161/01.res.86.8.841. [DOI] [PubMed] [Google Scholar]
- 10*.Sciarretta S, Marchitti S, Bianchi F, Moyes A, Barbato E, Di Castro S, et al. C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res. 2013;112:1355–64. doi: 10.1161/CIRCRESAHA.113.301325. This study shows that the ANP variant with two C-terminal Arg residues impaired endothelial cell survival and function through abnormal activation of natriuretic peptide receptor-C. [DOI] [PubMed] [Google Scholar]
- 11*.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–36. doi: 10.1172/JCI59701. This study shows that ANP and BNP enhance the thermogenic program in mouse and human brown adipose tissues, revealing a novel mechanism of the cardiac natriuretic peptides in regulating energy metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–9. doi: 10.1172/JCI64526. This study shows that ANP and BNP promote oxidative metabolism in human skeletal muscles, which may contribute to improved skeletal muscle fat oxidation through exercise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–7. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Del Ry S. C-type natriuretic peptide: a new cardiac mediator. Peptides. 2013;40:93–8. doi: 10.1016/j.peptides.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira CC, Agoston H, Beier F. Nitric oxide, C-type natriuretic peptide and cGMP as regulators of endochondral ossification. Dev Biol. 2008;319:171–8. doi: 10.1016/j.ydbio.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–21. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 20.Yasoda A, Nakao K. Translational research of C-type natriuretic peptide (CNP) into skeletal dysplasias. Endocr J. 2010;57:659–66. doi: 10.1507/endocrj.k10e-164. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Cheng Y, Kawamura K, Takae S, Hsueh AJ. C-type natriuretic Peptide stimulates ovarian follicle development. Mol Endocrinol. 2012;26:1158–66. doi: 10.1210/me.2012-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–9. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishimoto I, Tokudome T, Horio T, Soeki T, Chusho H, Nakao K, et al. C-type natriuretic peptide is a Schwann cell-derived factor for development and function of sensory neurones. J Neuroendocrinol. 2008;20:1213–23. doi: 10.1111/j.1365-2826.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 24.Sabbatini ME. Natriuretic peptides as regulatory mediators of secretory activity in the digestive system. Regul Pept. 2009;154:5–15. doi: 10.1016/j.regpep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Sogawa C, Wakizaka H, Aung W, Jin ZH, Tsuji AB, Furukawa T, et al. C-type natriuretic peptide specifically acts on the pylorus and large intestine in mouse gastrointestinal tract. Am J Pathol. 2013;182:172–9. doi: 10.1016/j.ajpath.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–6. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 28.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–7. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 29.Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1625–31. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 30.Antalis TM, Bugge TH, Wu Q. Membrane-anchored serine proteases in health and disease. Prog Mol Biol Transl Sci. 2011;99:1–50. doi: 10.1016/B978-0-12-385504-6.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–90. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 32.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–5. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 33.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Jiang J, Cui Y, Wu Q. Corin, atrial natriuretic peptide and hypertension. Nephrol Dial Transplant. 2009;24:1071–3. doi: 10.1093/ndt/gfn727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichiki T, Huntley BK, Burnett JC., Jr BNP molecular forms and processing by the cardiac serine protease corin. Adv Clin Chem. 2013;61:1–31. doi: 10.1016/b978-0-12-407680-8.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, et al. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–7. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 37.Peng J, Jiang J, Wang W, Qi X, Sun XL, Wu Q. Glycosylation and processing of pro-B-type natriuretic peptide in cardiomyocytes. Biochem Biophys Res Commun. 2011;411:593–8. doi: 10.1016/j.bbrc.2011.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem. 2010;56:1166–76. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 39.Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, et al. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem. 1997;272:20545–54. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- 40.Semenov AG, Seferian KR. Biochemistry of the human B-type natriuretic peptide precursor and molecular aspects of its processing. Clin Chim Acta. 2011;412:850–60. doi: 10.1016/j.cca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Semenov AG, Postnikov AB, Tamm NN, Seferian KR, Karpova NS, Bloshchitsyna MN, et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem. 2009;55:489–98. doi: 10.1373/clinchem.2008.113373. [DOI] [PubMed] [Google Scholar]
- 42.Jiang J, Pristera N, Wang W, Zhang X, Wu Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin Chem. 2010;56:959–66. doi: 10.1373/clinchem.2009.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of pro-C-type natriuretic peptide. J Biol Chem. 2003;278:25847–52. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 44.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–90. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Q, Yu D, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest. 1998;101:321–6. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sales KU, Hobson JP, Wagenaar-Miller R, Szabo R, Rasmussen AL, Bey A, et al. Expression and genetic loss of function analysis of the HAT/DESC cluster proteases TMPRSS11A and HAT. PLoS One. 2011;6:e23261. doi: 10.1371/journal.pone.0023261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–81. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 48.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–8. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, et al. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckley CL, Stokes AJ. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul Pept. 2011;172:44–50. doi: 10.1016/j.regpep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Wang W, Cui Y, Shen J, Jiang J, Chen S, Peng J, et al. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension. 2012;60:1352–8. doi: 10.1161/HYPERTENSIONAHA.112.201244. This study shows that the corin variant T555I/Q568P was defective in vivo and contibuted to salt-sensitive hypertension and cardiac hypertrophy, which resembled the phenotype in the African Americans carrying the corin variant allele. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–25. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shim JH, Lee TR, Shin DW. Enrichment and characterization of human dermal stem/progenitor cells by intracellular granularity. Stem Cells Dev. 2013;22:1264–74. doi: 10.1089/scd.2012.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–8. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 57.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 58.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–64. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 59.Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, et al. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail. 2009;2:541–8. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–8. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong N, Fang C, Jiang Y, Zhou T, Liu M, Zhou J, et al. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J Biol Chem. 2013;288:7867–74. doi: 10.1074/jbc.M112.411512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–71. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 63.Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol. 2007;49:1089–91. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–8. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 65.Xu-Cai YO, Wu Q. Molecular forms of natriuretic peptides in heart failure and their implications. Heart. 2010;96:419–24. doi: 10.1136/hrt.2008.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S, Sen S, Young D, Wang W, Moravec CS, Wu Q. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–92. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dries DL. Process matters: Emerging concepts underlying impaired natriuretic peptide system function in heart failure. Circ Heart Fail. 2011;4:107–10. doi: 10.1161/CIRCHEARTFAILURE.111.960948. [DOI] [PubMed] [Google Scholar]
- 68.Ichiki T, Boerrigter G, Huntley BK, Sangaralingham SJ, McKie PM, Harty GJ, et al. Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R102–9. doi: 10.1152/ajpregu.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi X, Jiang J, Zhu M, Wu Q. Human corin isoforms with different cytoplasmic tails that alter cell surface targeting. J Biol Chem. 2011;286:20963–9. doi: 10.1074/jbc.M110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gladysheva IP, King SM, Houng AK. N-glycosylation modulates the cell-surface expression and catalytic activity of corin. Biochem Biophys Res Commun. 2008;373:130–5. doi: 10.1016/j.bbrc.2008.05.181. [DOI] [PubMed] [Google Scholar]
- 71.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–35. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 72.Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–42. doi: 10.1016/j.yjmcc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–72. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larsen BR, Steffensen SD, Nielsen NV, Friis S, Godiksen S, Bornholdt J, et al. Hepatocyte growth factor activator inhibitor-2 prevents shedding of matriptase. Exp Cell Res. 2013;319:918–29. doi: 10.1016/j.yexcr.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q, Kuo HC, Deng GG. Serine proteases and cardiac function. Biochim Biophys Acta. 2005;1751:82–94. doi: 10.1016/j.bbapap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Dong N, Dong J, Liu P, Xu L, Shi S, Wu Q. Effects of anticoagulants on human plasma soluble corin levels measured by ELISA. Clin Chim Acta. 2010;411:1998–2003. doi: 10.1016/j.cca.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peleg A, Jaffe AS, Hasin Y. Enzyme-linked immunoabsorbent assay for detection of human serine protease corin in blood. Clin Chim Acta. 2009;409:85–9. doi: 10.1016/j.cca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Dong N, Chen S, Yang J, He L, Liu P, Zhen D, et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–11. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;2011:114–20. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Peleg A, Ghanim D, Vered S, Hasin Y. Serum corin is reduced and predicts adverse outcome in non-ST-elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2013;2:159–65. doi: 10.1177/2048872613483588. This study shows that reduced serum soluble corin levels were associated with major adverse cardiovascular events in patients with acute coronary syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong N, Chen S, Wang W, Zhou Y, Wu Q. Corin in clinical laboratory diagnostics. Clin Chim Acta. 2012;413:378–83. doi: 10.1016/j.cca.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Gladysheva IP, Wang D, McNamee RA, Houng AK, Mohamad AA, Fan TM, et al. Corin overexpression improves cardiac function, heart failure, and survival in mice with dilated cardiomyopathy. Hypertension. 2013;61:327–32. doi: 10.1161/HYPERTENSIONAHA.112.193631. This study shows in a genetic mouse model of heart failure that overexpression of corin improved cardiac function, reduced pulmonary edema and increased survival, supporting a therapeutic strategy to enhance corin activity to treat heart failure patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–6. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 84.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 85.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–54. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86**.Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–50. doi: 10.1038/nature10897. This study identifies a novel corin function in the uterus to promote trophoblast invasion and uterine spiral artery remodeling, which are important for preventing pregnancy-induced hypertension. This study also reports corin gene mutations that reduced corin activity in patients with pre-eclampsia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaitu'u-Lino TJ, Ye L, Tuohey L, Dimitriadis E, Bulmer J, Rogers P, et al. Corin, an enzyme with a putative role in spiral artery remodeling, is up-regulated in late secretory endometrium and first trimester decidua. Hum Reprod. 2013;28:1172–80. doi: 10.1093/humrep/det028. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Y, Wu Q. Role of corin and atrial natriuretic peptide in preeclampsia. Placenta. 2013;34:89–94. doi: 10.1016/j.placenta.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhn M, Volker K, Schwarz K, Carbajo-Lozoya J, Flogel U, Jacoby C, et al. The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–30. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tokudome T, Kishimoto I, Yamahara K, Osaki T, Minamino N, Horio T, et al. Impaired recovery of blood flow after hind-limb ischemia in mice lacking guanylyl cyclase-A, a receptor for atrial and brain natriuretic peptides. Arterioscler Thromb Vasc Biol. 2009;29:1516–21. doi: 10.1161/ATVBAHA.109.187526. [DOI] [PubMed] [Google Scholar]
- 91.Armstrong DW, Tse MY, O'Tierney-Ginn PF, Wong PG, Ventura NM, Janzen-Pang JJ, et al. Gestational hypertension in atrial natriuretic peptide knockout mice and the developmental origins of salt-sensitivity and cardiac hypertrophy. Regul Pept. 2013;186:108–15. doi: 10.1016/j.regpep.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Zaki MA, El-Banawy SE-DS, El-Gammal HH. Plasma soluble corin and N-terminal pro-atrial natriurectic peptide levels in pregnancy induced hypertensioin. Pregnancy Hypertens. 2012;2:48–52. doi: 10.1016/j.preghy.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Polzin D, Kaminski HJ, Kastner C, Wang W, Kramer S, Gambaryan S, et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–9. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klein JD. Corin: an ANP protease that may regulate sodium reabsorption in nephrotic syndrome. Kidney Int. 2010;78:635–7. doi: 10.1038/ki.2010.223. [DOI] [PubMed] [Google Scholar]
- 95.Bouley R. Corin: a key protein of an adaptive renal mechanism to respond to salt variation? Kidney Int. 2012;82:7–8. doi: 10.1038/ki.2012.123. [DOI] [PubMed] [Google Scholar]
- 96.Fang C, Shen L, Dong L, Liu M, Shi S, Dong N, et al. Reduced urinary corin levels in patients with chronic kidney disease. Clin Sci (Lond) 2013;124:709–17. doi: 10.1042/CS20120517. [DOI] [PubMed] [Google Scholar]
- 97.Das S, Au E, Krazit ST, Pandey KN. Targeted disruption of guanylyl cyclase-A/natriuretic peptide receptor-A gene provokes renal fibrosis and remodeling in null mutant mice: role of proinflammatory cytokines. Endocrinology. 2010;151:5841–50. doi: 10.1210/en.2010-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Page IH. Arterial hypertension in retrospect. Circ Res. 1974;34:133–42. doi: 10.1161/01.res.34.2.133. [DOI] [PubMed] [Google Scholar]
- 99.Buglioni A, Burnett JC., Jr A gut-heart connection in cardiometabolic regulation. Nat Med. 2013;19:534–6. doi: 10.1038/nm.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100**.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–75. doi: 10.1038/nm.3128. This study identifies a novel gut-heart link, in which gut-derived glucagon-like peptide-1 acts on glucagon-like peptide-1 receptor in the heart to promote ANP secretion, thereby regulating blood pressure and energy metabolism. [DOI] [PubMed] [Google Scholar]


