Abstract
Objective
To determine whether Libman-Sacks endocarditis is a pathogenic factor for cerebrovascular disease (CVD) in systemic lupus erythematosus (SLE).
Background
A cardioembolic pathogenesis of SLE CVD manifested as 1) neuropsychiatric SLE (NPSLE) including stroke and transient ischemic attacks (TIA), 2) neurocognitive dysfunction, and 3) MRI focal brain lesions has not been established.
Methods
A 6-year study of 30 patients with acute NPSLE (27 women, age 38±12 years), 46 age-and-sex matched SLE controls without NPSLE (42 women, age 36±12 years), and 26 age-and-sex matched healthy controls (22 women, age 34±11 years) who underwent clinical and laboratory evaluations, TEE, carotid duplex, transcranial Doppler, neurocognitive testing, and brain MRI/MRA. NPSLE patients were re-evaluated after 4.5 months of therapy. All patients were followed clinically for a median of 52 months.
Results
Libman-Sacks vegetations (87%), cerebromicroembolism (27% with 2.5 times more events per hour), neurocognitive dysfunction (60%), and cerebral infarcts (47%) were more common in NPSLE than in SLE (28%, 20%, 33%, and 0%) and healthy controls (8%, 0%, 4%, and 0%, respectively) (all p≤0.009). Patients with vegetations had 3 times more cerebromicroemboli per hour, lower cerebral blood flow, more stroke/TIA and overall NPSLE events, neurocognitive dysfunction, cerebral infarcts, and brain lesion load than those without (all p≤0.01). Libman-Sacks vegetations were independent risk factors of NPSLE (OR=13.4, p<0.001), neurocognitive dysfunction (OR=8.0, p=0.01), brain lesions (OR=5.6, p=0.004), and all 3 outcomes combined (OR=7.5, p<0.001). Follow-up re-evaluations in 18 (78%) of 23 surviving NPSLE patients demonstrated improvement of vegetations, microembolism, brain perfusion, neurocognitive dysfunction, and lesion load (all p≤0.04). Finally, patients with vegetations had reduced event free survival time to stroke/TIA, cognitive disability, or death (p=0.007).
Conclusion
The presence of Libman-Sacks endocarditis in patients with SLE is associated with higher risk for embolic CVD. This suggests that Libman-Sacks endocarditis may be a source of cerebral emboli.
Keywords: Libman-Sacks endocarditis, cerebrovascular disease, stroke, microembolism, transesophageal echocardiography
BACKGROUND
Cerebrovascular disease (CVD) in systemic lupus erythematosus (SLE) is common, associated with increased morbidity and mortality, and manifested as 1) major neuropsychiatric (NPSLE) syndromes of stroke, transient ischemic attacks (TIA), confusional state, or seizures; 2) acute or cumulative neurocognitive dysfunction; and 3) focal brain lesions on MRI (1–5). CVD in SLE is usually attributed to cerebritis, vasculitis, hypercoagulability, atherosclerosis, or antineuronal antibodies (5–9). However, CVD in SLE often occurs independently of these conditions (10–12). Libman-Sacks endocarditis characterized by non-infective inflammatory and/or thrombotic vegetations is also common in SLE and associated with increased morbidity and mortality (13). The relationship of Libman-Sacks endocarditis with CVD in SLE has not been established due to small, retrospective, or non-controlled studies using transthoracic echocardiography (TTE), a method with lower sensitivity and specificity than TEE for detection of Libman-Sacks endocarditis (14,15); incomplete clinical or imaging data not timed to clinical events; and inclusion of patients with confounding age-related heart and brain disease. Thus, this 6-year, fully integrated, controlled, cross-sectional and longitudinal study was designed to establish the relationship between Libman-Sacks endocarditis detected by TEE and cerebroembolism, NPSLE, neurocognitive dysfunction, and focal brain injury.
METHODS
Study Populations
This study design and protocol was approved by the National Institutes of Health and our Institutional Review Board, and participants provided informed consent. From December 2006 to December 2012, 76 (29%) of 266 patients with SLE actively followed at the rheumatology clinics of the University of New Mexico were consecutively recruited and classified at enrollment into 2 study groups:
Acute NPSLE Group: 30 patients (27 women, age 38±12 years) manifesting as acute stroke/TIA (n=23), cognitive dysfunction (n=11), confusional state (n=7), or seizures (n=4). The occurrence of NPSLE in 30 of 266 screened patients over a 6-year period constitute cumulative and annual event rates of 11.3% and 1.9%, respectively, similar to those reported in inception studies (1).
SLE Control Group: 46 age-and-sex frequency matched SLE patients (42 women, age 36±12 years) without clinically manifested acute or past NPSLE.
Patients were excluded due to age <18 or >60 years, pregnancy, heart or brain disease unrelated to SLE, atrial fibrillation or flutter, cardiomyopathy, intracardiac thrombi, drug abuse, renal dysfunction, difficult venous access, self-withdrawal or non-compliance with study protocol, or contraindications to TEE or MRI.
To validate blinded interpretation and diagnostic accuracy of tests and provide a normality reference, 26 apparently healthy volunteers age-and-sex frequency matched to patients were studied. Controls were recruited by the study coordinator from available listings of volunteer subjects in the Office of Research of the Health Sciences Center, employees of the University of New Mexico, and from patient’s relatives or acquaintances. Candidate subjects were then screened with a standard general health questionnaire.
All 102 participants underwent a standardized protocol of clinical and laboratory evaluations, TEE, carotid duplex, transcranial Doppler, complete neurocognitive testing, and brain MRI/MRA within 1 week of enrollment. All studies were coded, de-identified, and interpreted by experienced observers blinded to subjects’ clinical and imaging data.
Clinical and laboratory evaluations
Patients were characterized with regard to disease duration, activity, injury, therapy, and standard autoantibodies (11,16,17) (Supplemental Table 1). All 102 participants were further characterized for demographics, atherogenic risk factors, and specific parameters of inflammation, platelet activity, coagulation, and fibrinolysis (Supplemental Table 2).
Transesophageal echocardiography
Participants underwent complete TEE with IE-33 Philips systems with images digitally acquired for off-line interpretation. Heart valves were imaged in multiple planes at a depth of 4–8 cm with a narrow sector scan to improve image resolution. Criteria for Interpretation: Libman-Sacks vegetations were defined as abnormal localized echodensities with well-defined borders either as part of or adjacent to valve leaflets or subvalvular apparatus (13). Size of vegetations was determined by planimetry. Valve thickening was determined using M-mode imaging and considered present when thickness >3 mm (mitral valve) or >2 mm (aortic valve) was observed in ≥2 leaflets, or in one leaflet if associated with vegetation, ≥mild regurgitation, or both (13,18). Mitral or aortic regurgitation assessed by standard color-Doppler criteria was present if >mild, or if mild and associated with a vegetation or thickening of the respective valve (13,19). In 30 randomly selected TEE studies (22 patients, 8 controls), inter-observer agreement for detection of valve vegetations, thickening, and regurgitation were 93%, 83%, and 90%, respectively (Kappa 0.87, 0.67, and 0.73, respectively).
The left atrium and ventricle were assessed for spontaneous echocardiographic contrast or thrombus; the atrial septum was interrogated by two-dimensional, color-Doppler, and saline contrast images for detection of aneurysms, patent foramen ovale, or atrial septal defects. The ascending aorta, arch, and descending thoracic aorta were assessed by two-dimensional and M-mode images for intima-media thickening (≥2 SD above the mean of healthy controls) and plaques (focal thickening of intima-media exceeding 50% of the surrounding wall) (20).
Carotid duplex
From longitudinal B-mode images of both common carotid arteries, 6 measurements of intima-media thickness along the far and near walls were performed at end-diastole (21). Carotid intima-media thickening and plaques were determined with criteria described for the aorta.
Transcranial Doppler
Both middle cerebral arteries were interrogated for 90 minutes for detection of microembolism using a 2.0 MHz DWL Doppler Box with power Doppler M-Mode, 32 gate spatial imaging, and dual channel emboli detection software. Microemboli were defined as audible, high intensity (>13db), and <100ms unidirectional signals within both Doppler blood flow velocity and vessel lumen (22). Intraobserver agreement for detection of microembolism was 96% (Kappa 0.83).
Neurocognitive evaluation
Participants underwent complete neurocognitive testing for premorbid intelligence, attention, memory, language, processing speed, executive function, motor function, and global neurocognitive function (23).
Brain MRI-MRA
Standard T1-weighted, fluid attenuated inversion recovery (FLAIR), and diffusion-weighted images were obtained. Dynamic susceptibility contrast MRI was performed in 89 (92%) participants (64 patients, 25 controls) to assess brain perfusion (24). Brain lesions were classified as old or recent cerebral infarcts and small focal periventricular or deep white abnormalities using standard criteria (3,4,10). Counts of brain lesions and hemispheric and whole brain lesion load in cm3 were determined using semi-automated methods (25). Cerebral atherosclerosis, thrombosis, vasculitis, or aneurysms were determined using MRA (26). Inter-observer agreement for detection of brain lesions in 68 studies was 94% (Kappa 0.88).
Follow-up
To further assess the relationship of Libman-Sacks vegetations with cerebroembolism and CVD, 18 (78%) of 23 surviving NPSLE patients underwent reevaluations after 4.5 months (interquartile range, 2.1–8.4) of clinically indicated antimalarial (87%), corticosteroid (50%), immunosuppressive (58%), antiplatelet (71%), or anticoagulant (35%) therapy. Five patients had no follow-up studies because were too ill to undergo TEE or MRI. All 76 patients underwent clinical follow-up for a median of 52 months (interquartile range, 24–64 months) for development of new or recurrent stroke/TIA, cognitive disability (defined as formal physician recommendation for cognitive disability and a global neurocognitive score ≥1.5SD below pre-morbid intelligence score), or death.
Statistical analysis
Descriptive statistics were mean±SD, or median and interquartile ranges in asymmetrically distributed variables, or frequencies (%). Comparisons between the 3 study groups (Table 1) were performed by analysis of variance (ANOVA) for continuous measures and verified by Kruskal-Wallis tests. Two-tailed Fisher’s exact tests were used for binary measures. Pair-wise comparisons among 3 groups for each variable were done by Fisher’s least significant difference method. Association of vegetations with cerebromicroembolism and neurologic outcomes are reported in Table 2. The rate ratio (95% confidence interval) of microembolism in NPSLE and SLE patients and in those with and without vegetations was estimated by Poisson regression with individual observation time as the offset. Neurocognitive z-scores were computed using controls as reference. Differences in cerebral blood flow in gray and white matter of 4 cerebral lobes and 2 hemispheres due to vegetations and microembolism were analyzed by repeated measures (RM) ANOVA. Using clinical, laboratory, and cardiovascular imaging measures listed in Table 1 and Supplemental Tables 1–2, significant risk factors in univariate logistic regression for NPSLE, neurocognitive dysfunction, brain lesions, and all 3 outcomes combined were considered as candidate risk factors in multivariate logistic regression analyses (Table 3). The list of candidate risk factors is detailed in Supplemental Table 3. Effects are reported as adjusted odds ratios and 95% confidence intervals with Firth’s bias correction in near-separation conditions. The effect of therapy in follow up is assessed by Wilcoxon’s signed rank test as a robust, non-parametric paired comparison (Table 4). Kaplan-Meier event free survival curves for stroke/TIA, cognitive disability, or death related to vegetations were compared by log rank tests. Cox proportional hazard model was used to select predictors of this combined event. Two tailed p-values ≤0.05 were considered significant. All statistical analyses were performed in SAS 9.3.
Table 1.
Findings on Cardiovascular and Brain Imaging and Neurocognitive Testing
| Abnormality | Acute NPSLE (n=30) |
SLE (n=46) |
Controls (n=26) |
P value |
|---|---|---|---|---|
|
Transesophageal Echocardiography mean ± SD or n (%) |
||||
| Valve vegetations | 26 (87%)*† | 13 (28%) | 2 (8%) | <0.001 |
| Mitral valve | 20 (67%)*† | 6 (13%) | 1 (4%) | <0.001 |
| Aortic valve | 14 (47%)*† | 11 (24%) | 2 (8%) | 0.004 |
| Valve thickening | 26 (87%)*† | 16 (35%)* | 2 (8%) | <0.001 |
| Mitral valve | 20 (67%)*† | 8 (17%)* | 0 | <0.001 |
| Aortic valve | 18 (60%)*† | 12 (26%) | 2 (8%) | 0.002 |
| Valve regurgitation | 15 (50%)*† | 5 (11%) | 0 | <0.001 |
| Mitral valve | 13 (43%)*† | 4 (9%) | 0/25 | <0.001 |
| Aortic valve | 2 (7%) | 3 (7%) | 0 | 0.60 |
| Any valve abnormality | 28 (93%)*† | 18 (39%)* | 3 (12%) | <0.001 |
| PFO or interatrial septal aneurysm |
1 (3%)† | 11 (24%) | 2 (8%) | 0.03 |
| LV ejection fraction <50% | 2 (7%) | 0 | 0 | 0.15 |
| Ao intima-media thickness | 0.88 ± 0.37* | 0.80 ± 0.23* | 0.66 ± 0.15 | 0.01 |
| Ao intima-media thickening | 7/29 (24%) | 10/45 (22%) | 1 (4%) | 0.07 |
| Ao plaque (any portion) | 9 (30%)* | 8 (17%)* | 0 | 0.007 |
| Ao intima-media thickening or plaque |
11 (37%)* | 14 (30%)* | 1 (4%) | 0.006 |
| Carotid Artery Duplex, mean ± SD or n (%) | ||||
| Intima-media thickness | 0.55 ± 0.11*† | 0.50 ± 0.07 | 0.48 ± 0.08 | 0.01 |
| Intima-media thickening | 4 (13%) | 1 (2.2%) | 1 (4%) | 0.11 |
| Plaque | 5 (17%) | 1 (2.2%) | 2 (8%) | 0.06 |
| Intima-media thickening or plaque |
8 (27%)† | 2 (4%) | 3 (12%) | 0.02 |
| Transcranial Doppler, n (%) | ||||
| Right or left MCA microemboli | 8 (27%)*† 17 events/43.1 hours*† |
9 (20%) 11 events/68.5 hours* |
0 | 0.009 HR 2.5 (p=0.02) |
| Clinical Domain | Neurocognitive Z-Scores, mean ± SD (range) | |||
| Pre-morbid intelligence | −0.67 ± 1.17 (27) | −0.46 ± 0.93 (45) | −0.06 ± 1.03 | 0.10 |
| Attention | −3.14 ± 3.60*† | −0.91 ± 0.92 (45) | −0.06 ± 0.82 | <0.001‡ |
| Memory | −1.54 ± 1.43(29)* | −1.03 ± 0.90 (45)* | −0.056 ± 0.81 | <0.001‡ |
| Language | −1.02 ± 1.04 (27)* | −0.99 ± 1.16 (45)* | −0.05 ± 0.89 | <0.001‡ |
| Processing speed | −2.19 ± 1.95 (28)*† | −0.87 ± 1.15 (45)* | −0.06 ± 0.93 | <0.001‡ |
| Executive function | −3.58 ± 3.58 (29)*† | −1.63 ± 2.20 (45)*† | −0.10 ± 0.79 | <0.001‡ |
| Motor function | −5.17 ± 8.67 (26)*† | −1.70 ± 1.66 (37) | −0.056 ± 0.63 | <0.00‡ |
| Global | −2.76 ± 2.54*† | −1.19 ± 0.91 (45)*† | −0.07 ± 0.52 | <0.001‡ |
| Global abnormal§ | 12 (60%)*† | 15 (33%)* | 1 (4%) | <0.001‡ |
| Brain Lesions on MRI, median (IQR) or n (%) | ||||
| Any focal brain lesion | 22 (73%)*† | 19/45 (42%)*∥ | 4 (15%)∥ | <0.001 |
| Focal brain lesions (n) | 12 (3, 47) * | 2 (0, 9) | 0 (0, 2) | <0.001¶ |
| Old or recent cerebral infarcts | 14 (47%)*† | 0 | 0 | <0.001 |
| Cerebral infarcts (n) | 0 (0, 2)*† | 0 | 0 | <0.001¶ |
| White matter abnormalities | 20 (67%)*† | 18/45 (40%)* | 4 (15%) | <0.001 |
| White matter abnormalities (n) | 9 (1, 46) *† | 2 (0, 9) | 0 (0, 2) | <0.001¶ |
|
White Matter Brain Lesion Load (cm3) Median (IQR) |
||||
| Left hemisphere | 0.96 (0.16, 2.21)*† | 0.13 (0.05, 0.23) | 0.04 (0.03, 0.15) | 0.003¶ |
| Right hemisphere | 1.23 (0.18, 2.84)* | 0.11 (0.05, 0.24) | 0.05 (0.03, 0.10) | 0.01¶ |
| Whole brain | 2.92 (3.18, 5.55)*† | 0.26 (0.13, 0.38) | 0.13 (0.07, 0.23) | 0.002¶ |
p<0.05 compared to controls by Fisher’s post hoc least significant difference method.
p<0.05 for acute NPSLE compared to SLE by Fisher’s post hoc least significant difference method.
all p≤0.009 after simultaneously adjusting for education, premorbid intelligence, and depression index.
Global abnormal defined as ≥1.5 SD below the mean total of controls.
One SLE patient did not complete MRI studies due to claustrophobia; includes 2 controls with history of sports related head trauma.
by Kruskal-Wallis test.
NPSLE = neuropsychiatric systemic lupus erythmatosus, PFO = patent foramen ovale, Ao = aorta, LV = left ventricle, MCA = middle cerebral artery, HR = hazard ratio.
Table 2.
Association of Libman-Sacks Vegetations with Microembolism, Acute Stroke/TIA and Overall NPSLE, Neurocognitive Dysfunction, and Brain Lesions and Lesion Load
| Abnormality | Patients with Vegetations (n=39) |
Patients without Vegetations (n=37) |
P value |
|---|---|---|---|
| Microembolism | |||
| Right or left MCA microemboli |
12 (31%) 21 events/56.6 hours |
5 (14%) 7 events/55 hours |
adjusted HR* 3.0, p = 0.01 |
| NPSLE, n (%) | |||
| Acute stroke/TIA | 22 (56%) | 1(3%) | <0.001 |
| Acute Overall NPSLE | 26 (67%) | 4(11%) | <0.001 |
| Neurocognitive z-scores, mean ± SD | |||
| Attention | −2.36 ± 3.00 | −0.82 ± 0.91 | 0.02† |
| Memory | −1.75 ± 1.27 | −0.79 ± 1.00 | 0.001† |
| Processing speed | −1.90 ± 1.92 | −0.90 ± 1.15 | 0.04† |
| Executive function | −3.31 ± 3.60 | −1.68 ± 2.53 | 0.03† |
| Motor function | −4.38 ± 7.40 | −1.40 ± 1.54 | 0.005† |
| Global | −2.42 ± 2.32 | −1.17 ± 0.98 | 0.01‡ |
| Focal Brain Lesions, median (IQR) or n (%) | |||
| Focal brain lesions | 28 (72%) | 12/36 (34%)§ | <0.001 |
| Focal brain lesions (n) | 9 (3, 39) | 1 (0, 8) | 0.004† |
| Cerebral infarcts | 14 (36%) | 0/36 | <0.001 |
| Cerebral infarcts (n) | 0 (0, 2) | 0 | <0.001† |
| White matter lesions | 25/37 (68%) | 12/36 (34%) | 0.005 |
| White matter lesions (n) | 8 (1, 37) | 1 (0, 8) | 0.004† |
| Brain Lesion Load (cm3), median (IQR) | |||
| Left hemisphere | 0.05 (0, 1.23) | 0 (0, 0.09) | 0.016† |
| Right hemisphere | 0.14 (0, 1.15) | 0.01 (0, 0.06) | 0.002† |
| Whole brain | 0.18 (0, 2.34) | 0.026 (0, 0.16) | 0.008† |
Poisson regression with repeated measures adjusting for patent foramen ovale, interatrial septal aneurysm, carotid or aortic atherosclerosis, and antiphospholipid antibodies.
Wilcoxon test.
p=0.02 after simultaneously adjusting for age, depression index, pre-morbid intelligence, and education.
One of the patients without vegetations had no MRI due to claustrophobia.
TIA = transient ischemic attack, other abbreviations as in previous Table.
Table 3.
Independent Risk Factors of Acute NPSLE, Neurocognitive Dysfunction, Brain Lesions on MRI, and All 3 Outcomes Combined
| Variable | Odds Ratios (95% CI) | P value* |
|---|---|---|
| Acute NPSLE | ||
| Valve vegetations | 13.40 (3.31 – 54.35) | <0.001 |
| Valve regurgitation | 5.10 (1.19 – 21.93) | 0.03 |
| Triglyceride levels (per 20 mg/dL) | 1.27 (1.04 – 1.53) | 0.02 |
| Global Neurocognitive Dysfunction | ||
| Vegetations and microembolism | 8.01 (1.51 – 42.62) | 0.01 |
| Smoking (currently) | 3.79 (1.16 – 12.40) | 0.03 |
| Non-Neuro SLICC | 1.50 (1.06 – 2.13) | 0.02 |
| Age at diagnosis of SLE (per 10 years) | 2.08 (1.27 – 3.40) | 0.004 |
| Focal Brain Lesions | ||
| Valve vegetations | 5.57 (1.72–18.01) | 0.004 |
| P-selectin | 1.04 (1.00–1.07) | 0.02 |
| Complement C4 | 1.12 (1.03–1.22) | 0.009 |
| Acute NPSLE, Cognitive Dysfunction, or Brain Lesions | ||
| Valve vegetations | 7.49 (2.49–22.5) | <0.001† |
| Triglyceride levels (per 20 mg/dL) | 1.28 (1.03–1.60) | 0.03 |
| P-selectin | 1.05 (1.01–1.09) | 0.02 |
| Complement C4 | 1.17 (1.04–1.32) | 0.008 |
P-values for multivariate analysis adjusted for other variables in the “best” stepwise model.
OR and p value adjusted in “best” step wise model of predictors selected from all 3 components/outcomes as listed above.
Table 4.
Findings on Initial and Follow-up Cardiovascular and Brain Imaging and Neurocognitive Testing in 18 Patients with Treated NPSLE
| Finding | Initial Study | Follow-up Study | P Value* |
|---|---|---|---|
| TEE, mean ± SD | |||
| Vegetations (n) | 2.0 ± 1.41 | 1.33 ± 1.28 | 0.03 |
| Vegetations (area, cm2) | 0.38 ± 0.46 | 0.18 ± 0.19 | 0.09 |
| Transcranial Doppler, n (%) | |||
| Right or left MCA microemboli | 5 patients (28%) with 14 microemboli |
0 | 0.007† |
| Neurocognitive z-scores, mean ± SD | P value* | ||
| Attention | −3.55 ± 4.24 | −2.26 ± 3.20 | 0.002 |
| Memory | −1.62 ± 1.64 | −0.88 ± 1.61 | 0.001 |
| Motor function | −6.43 ± 10.46 | −2.32 ± 2.70 | 0.002 |
| Processing speed | −2.17 ± 2.01 | −1.73 ± 2.41 | 0.02 |
| Global cognitive dysfunction | −3.12 ± 3.08 | −1.86 ± 2.32 | <0.001 |
|
Brain Perfusion (ml/min/100 grams/tissue) (n=11), mean±SD |
Δ% / P value* | ||
| Overall gray matter | 28.10 ± 18.04 | 33.87 ± 15.02 | 34% / 0.02 |
| Overall white matter | 14.36 ± 9.73 | 17.47 ± 6.88 | 38% / 0.02 |
| Frontal lobe (gray matter) | 26.45 ± 17.58 | 32.19 ± 14.62 | 36% / 0.02 |
| Frontal lobe (white matter) | 12.76 ± 9.14 | 15.60 ± 6.53 | 40% / 0.01 |
| Parietal lobe (gray matter) | 29.25 ± 19.16 | 35.78 ± 15.20 | 38% / 0.02 |
| Parietal lobe (white matter) | 13.87 ± 9.02 | 17.21 ± 6.19 | 41% / 0.02 |
| Temporal lobe (gray matter) | 28.23 ± 16.97 | 33.78 ± 15.21 | 31% / 0.04 |
| Temporal lobe (white matter) | 14.70 ± 10.31 | 17.85 ± 7.68 | 38% / 0.02 |
| Occipital lobe (gray matter) | 28.46 ± 18.62 | 33.73 ± 15.39 | 32% / 0.03 |
| Occipital lobe (white matter) | 16.10 ± 10.56 | 19.21 ± 7.27 | 35% / 0.02 |
| Brain Lesion Load, median (IQR) | |||
| Whole brain lesion load (cm3) | 0.68 (0.17, 3.93) | 0.55 (0.07, 1.74) | 0.03 |
Wilcoxon Signed Rank test.
RESULTS
Cinical characteristics
NPSLE as compared to SLE patients were more often treated with statins and aspirin or warfarin, were more commonly SSA and β2-glycoprotein antibody positive, had higher triglyceride levels, had lower hemoglobin and serum albumin, and had higher levels of D-dimer and tissue plasminogen antigen (all p≤0.04) (Supplemental Tables 1,2). NPSLE and SLE patients differed from controls in multiple clinical and laboratory parameters.
Findings on cardiovascular imaging, neurocognitive testing, and brain MRI
NPSLE as compared to SLE patients and healthy controls had more Libman-Sacks vegetations, valve thickening, valve regurgitation, any valve abnormality, mild carotid atherosclerosis, cerebral microembolism (Poisson regression, 2.5 times more events per hour, 95% CI 1.2–5.3, p=0.02), neurocognitive dysfunction, and brain lesions and lesion load (all p≤0.02) (Table 1). Patent foramen ovale and interatrial septal aneurysms were more common in SLE than in NPSLE patients (p=0.03).
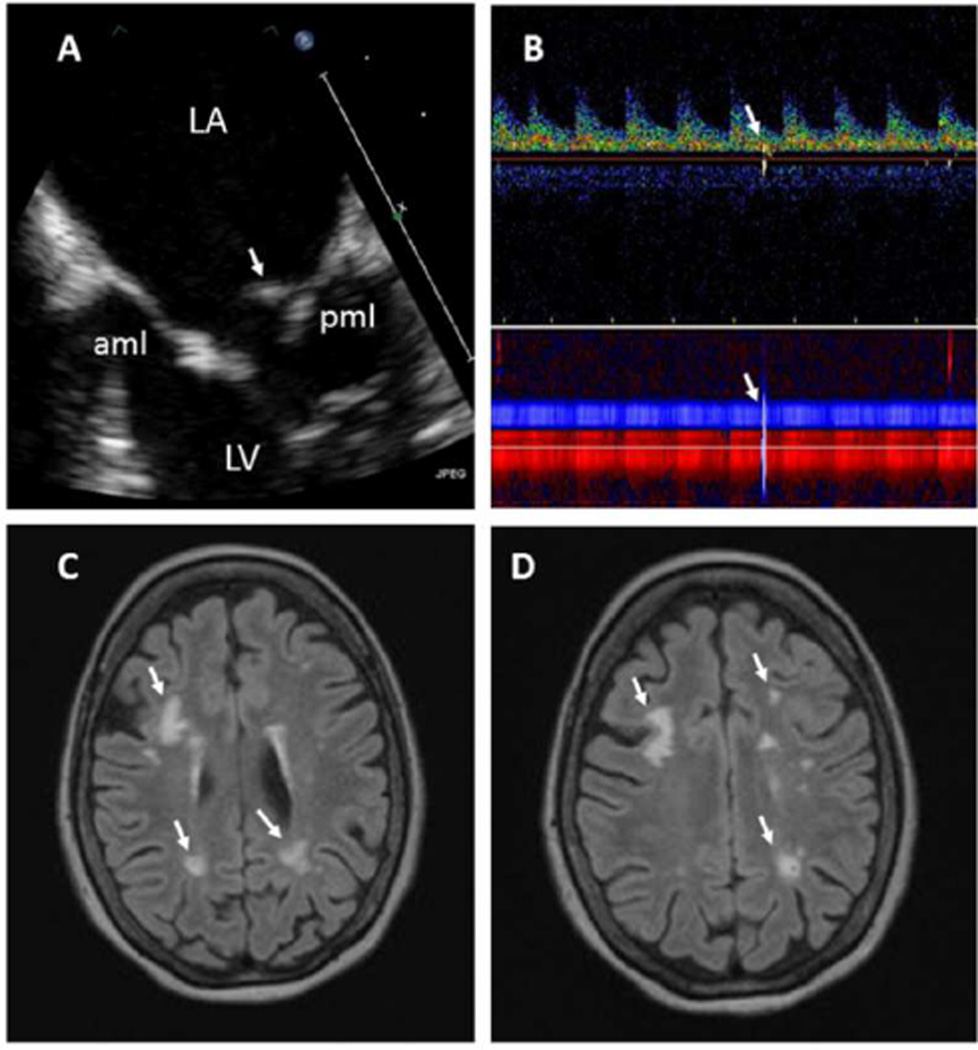
Vegetations were of oval shape, heterogeneous or soft tissue echoreflectance, sessile, and of variable maximal diameter (5.8±2.7 mm, range 2.8–14 mm) and area (0.37±0.36 cm2, range 0.04–1.51 cm2); were seen on the atrial side and tips of mitral leaflets and on the ventricular or aortic side of aortic cusps; and were highly associated with valve thickening (81%−92%, p<0.001) (Figure 1 & Supplemental Figures 1–5 with video clips).
Figure 1. Part 1. 55 year old female with SLE and acute transient ischemic attack.
A. This TEE view demonstrates a moderate size, elongated, sessile, and heterogeneously echoreflectant Libman-Sacks vegetation (arrow) on the atrial side of the posterior mitral leaflet (pml) (video clip Figure 1A). Moderate thickening and sclerosis with decreased mobility of the mid and distal portions of the anterior (aml) and posterior (pml) mitral leaflets is noted. B. Transcranial Doppler demonstrates a microembolic signal on the spectral Doppler (upper arrow) and vessels lumen (lower arrow) traveling through the left middle cerebral artery (red power M-mode) and anterior communicating artery (blue power M-mode). C,D. Brain MRI demonstrates multiple, bilateral, and variable size periventricular and deep white matter infarcts (arrows). This patient had 75 brain lesions and a lesion load of 6.04 cm3. Her global neurocognitive z-score was −4.28 indicative of severe neurocognitive dysfunction. Abbreviations: LA = left atrium, LV = left ventricle.
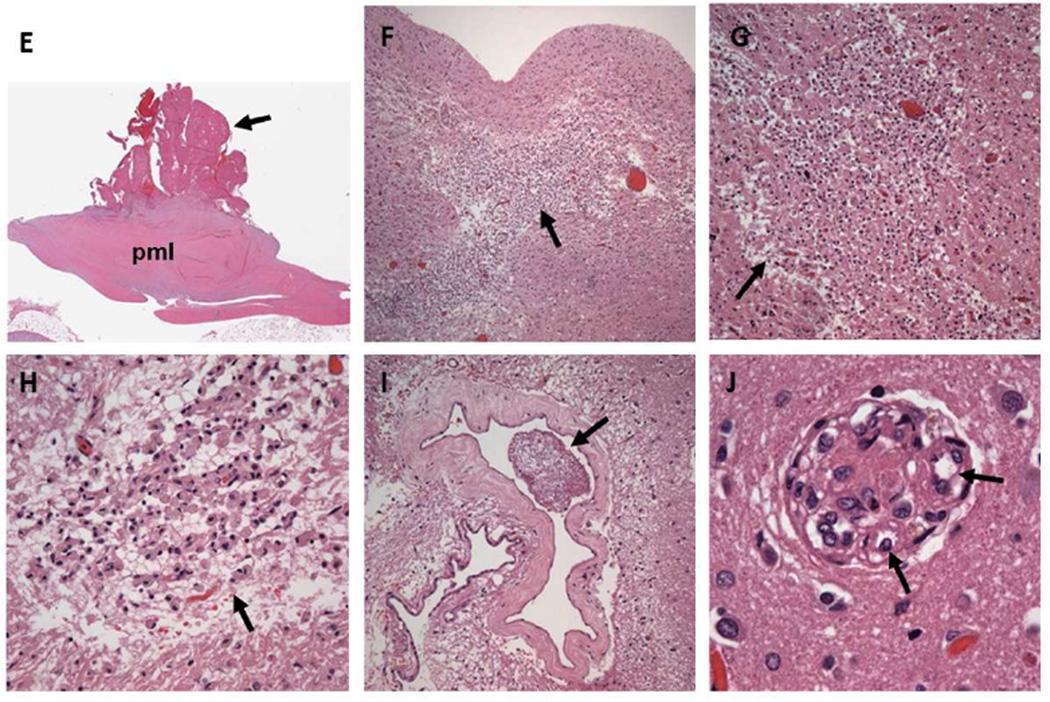
Part 2. 55 year old female with SLE and acute transient ischemic attack who during follow up died from a large intracerebral hemorrhage. Histopathology with H&E stains demonstrates: E (H&E 40X): Thickening and fibrosis of the posterior mitral leaflet (pml) with a well adhered, verrucoid, fibrinous vegetation (arrow). F (H&E 20X): Subacute cerebral infarct at the junction of the white and gray matter with necrotic debris and moderate cellular infiltration (arrow). G,H (H&E 40X, 100X): Old deeper (white matter) infarct with liquefactive necrosis, residual macrophages, and gliosis (arrows). I (H&E 100X): Large cerebral vessel with fibrin thrombi (arrow). J (H&E 100X): Small cerebral vessel with fibrin thrombi and neoangiogenesis (arrows). Multiple subacute and old microinfarcts and fibrin thrombosed microvasculature with neoangiogenesis characteristic of chronic cardiothromboembolic disease were demonstrated in both cerebral hemispheres.
Cerebromicroembolism was similarly frequent in the right and left middle cerebral arteries. Consequently, focal brain lesions always involved both hemispheres (Figure 1 & Supplemental Figures ,3–5).
Association of Libman-Sacks vegetations with cerebromicroembolism and CVD
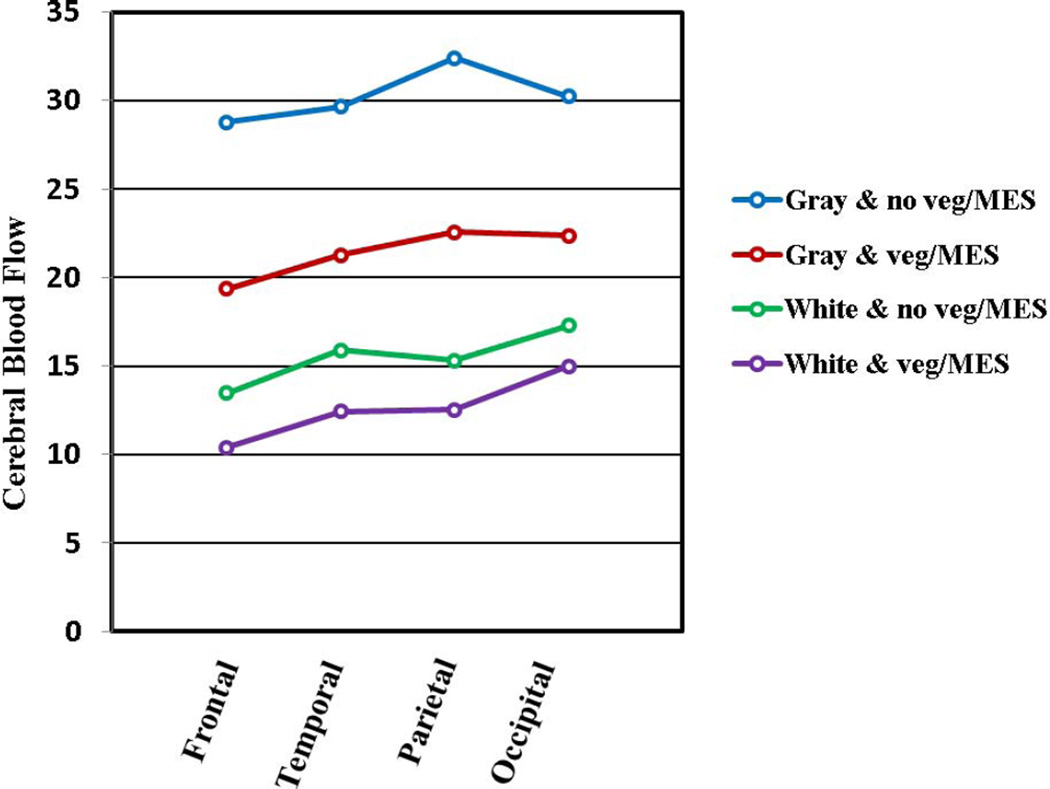
As noted in Table 1, patients without NPSLE (SLE group) commonly have vegetations (28%), cerebromicroembolism (20%), cognitive dysfunction (33%), and brain lesions (42%) suggestive of subclinical cerebroembolism. Therefore, to define better the association of vegetations with cerebromicroembolism, NPSLE, cognitive dysfunction, and brain injury, all SLE patients were stratified into those with and without vegetations . As shown in Table 2, 39 patients with vegetations compared to 37 without vegetations had 3 times more cerebromicroembolic events per hour after simultaneously adjusting for patent foramen ovale, interatrial septal aneurysm, carotid and aortic atherosclerosis, and antiphospholipid antibodies (95% CI=1.3–7.2, p=0.01), more stroke/TIA and overall NPSLE events, lower neurocognitive scores, and more brain lesions and lesion load (all p≤0.04). Also, 12 patients with vegetations and microembolism as compared to 32 patients with neither had lower cerebral blood flow (Figure 2), more stroke/TIA and NPSLE events, lower neurocognitive scores, and more cerebral infarcts and lesion load (all p≤0.03) (Supplemental Table 4).
Figure 2. Cerebral blood flow in SLE patients with vegetations and cerebromicroembolism.
Cerebral blood flow (ml/min/100 grams of tissue) in the gray and white matter is significantly lower in 11 patients with vegetations and cerebromicroembolism as compared to 24 patients with neither across the 4 cerebral lobes and left and right hemispheres (RM ANOVA p≤0.001 for both gray and white matter). Abbreviations: Gray & no veg/MES, White & no veg/MES = gray or white matter perfusion in patients with no vegetations and no cerebromicroembolism; Gray & veg/MES, White & veg/MES = gray or white matter perfusion in patients with vegetations and cerebromicroembolism.
Independent risk factors for CVD
Libman-Sacks vegetations (OR 13.4, p<0.001), valve regurgitation, and triglyceride levels were independent risk factors for NPSLE; vegetations and cerebromicroembolism (OR 8.0, p=0.01), current smoking, non-neurologic injury score, and age at diagnosis of SLE were risk factors for neurocognitive dysfunction; vegetations (OR 5.6, p=0.004), P-selectin, and complement C4 levels were risk factors for focal brain lesions; and vegetations (OR 7.5, p<0.001), triglyceride levels, P-selectin, and C4 levels were risk factors for all 3 outcomes combined (Table 3).
Follow-up findings
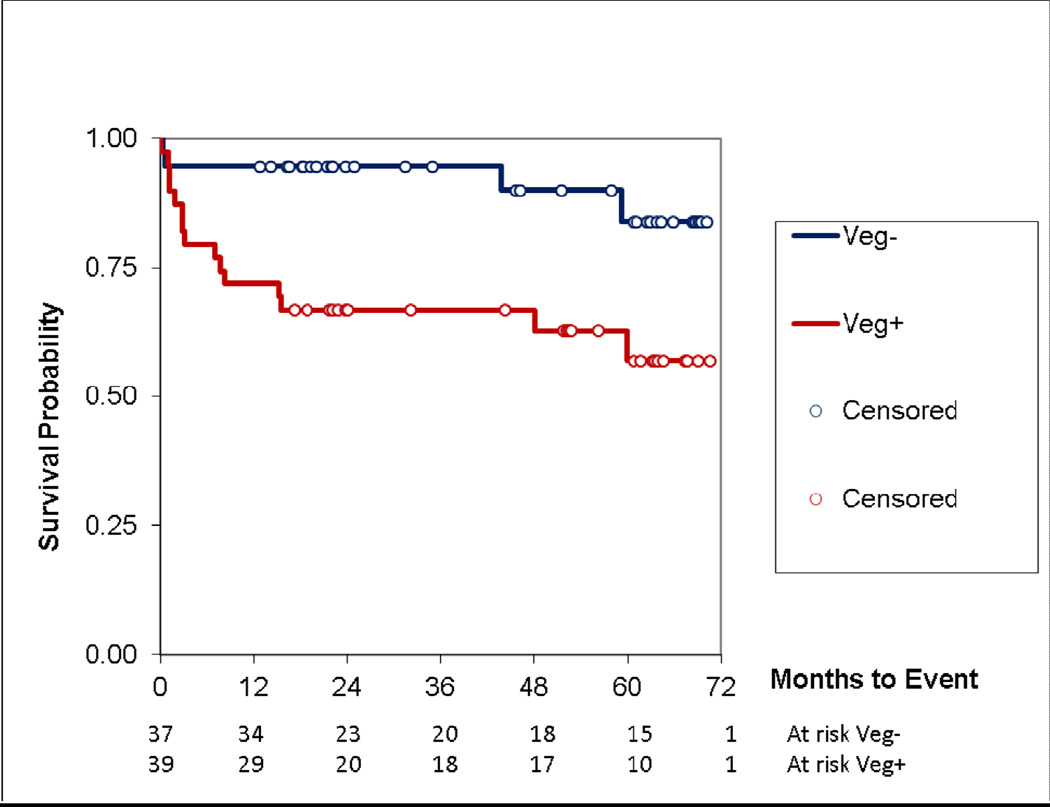
In support of the relationship of Libman-Sacks vegetations with cerebroembolism and CVD, re-evaluations in 18(78%) of 23 survivors of treated NPSLE demonstrated improvement of vegetations, cerebromicroembolism, cerebral blood flow, neurocognitive function, and brain lesion load (all p≤0.04) (Table 4, Supplemental Figures 1–5). During follow-up, 19 of 76 patients (25%) developed major clinical events. Twelve patients (16%) developed new or recurrent stroke/TIA [9/12 patients underwent re-evaluations and all had recurrent or persistent vegetations (n=8), cerebromicroembolism (n=5), or brain lesions (n=9)] (Supplemental Figures 1–5); 10 (14%) developed cognitive disability; and 7 (9%) died. Fifteen (38%) of 39 patients with vegetations developed events as compared to 4 (11%) of 37 without vegetations (p=0.008). Kaplan-Meier analysis demonstrated reduced event free survival in patients with vegetations (time to death, p=0.06; to disability, p=0.04; to stroke/TIA, p=0.003; and to combined event, p=0.007) (Figure 3). Vegetations, aortic or carotid atherosclerosis, and P-selectin were the independent predictors of the combined event by Cox proportional hazard stepwise analysis (HR 4.8, 4.1, and 1.4, respectively; all p≤0.008).
Figure 3. Kaplan-Meier event free survival in SLE patients with and without vegetations.
During follow-up, the event free survival from stroke/TIA, cognitive disability, or death of patients with vegetations (Veg+) was significantly lower than in those without vegetations (Veg−) (p=0.007).
DISCUSSION
This 6-year duration, fully integrated, controlled, cross-sectional and longitudinal study revealed 5 major findings: 1) NPSLE as compared to SLE patients have more Libman-Sacks vegetations, cerebromicroembolism, neurocognitive dysfunction, and focal brain lesions; 2) patients with vegetations have 3.0 times more cerebromicroembolism per hour, lower cerebral perfusion, more stroke/TIA and overall NPSLE events, greater neurocognitive dysfunction, and greater brain injury; 3) valve vegetations are strong independent risk factor for stroke/TIA and NPSLE, neurocognitive dysfunction, brain lesions, and all 3 outcomes combined; 4) vegetations, cerebromicroembolism, NPSLE, neurocognitive dysfunction, and brain perfusion and lesion load improve with anti-inflammatory and/or antithrombotic therapy; and 5) patients with vegetations have poor outcomes with reduced event-free time to stroke/TIA, cognitive disability, or death. These findings support that Libman-Sacks vegetations may generate platelet or fibrin macro-or-microemboli that occlude cerebral vessels and result in reduced cerebral perfusion, ischemic brain injury, stroke/TIA, non-focal NPSLE syndromes, neurocognitive dysfunction or disability, and contribute to death. Thus, Libman-Sacks endocarditis may be a common and under-recognized pathogenesis of embolic CVD in SLE.
Age at diagnosis of SLE, non-neurologic damage score, triglyceride levels, smoking, P-selectin (a cell adhesion molecule indicative of platelet activation and aggregation and endothelial cells activation or injury) (27), and complement C4 levels were also independent risk factors of CVD. Thus, disease duration and severity, atherogenic risk factors, platelet aggregation, and endothelial inflammation may be risk factors for valve vegetations, endothelial dysfunction, early atherosclerosis, and thrombosis, and thus, either contribute to thromboembolic CVD or are independent risk factors for CVD (28).
Previous, often retrospective, non-controlled, or non-integrated clinical and pathologic studies support our findings. In a TTE study of 105 patients, unspecified valve disease was detected in 7(39%) of 18 patients with past stroke (29). In our 1996 controlled study of 69 patients undergoing serial TEE, Libman-Sacks endocarditis detected in 61% of patients was associated with 11% incidence of stroke/TIA and a mortality of 12% during a 5-year follow-up (13). In another TTE study, any valve abnormality detected in 44% of 71 patients was associated with past stroke/TIA (30). In 3 retrospective studies from our institution (2 using TEE), valve vegetations were associated with past stroke/TIA, non-focal NPSLE, and brain lesions on MRI (31–33). In a prospective not-controlled TTE study, Libman-Sacks vegetations detected in 11% of 342 SLE patients were associated with a higher incidence of stroke/TIA (14.8% in those with versus 3% in those without vegetations) during a 4-year follow-up (34). Using transcranial Doppler in 70 patients, 39% of 38 patients with versus none of 32 without antiphospholipid antibodies had microembolism associated with cerebrovascular ischemia and mitral valve prolapse (35). In another study (n=53), patients with versus those without NPSLE had 5.4±1.1 versus 0.3±0.8 microemboli per hour, respectively (36). Microembolism in 9% of 55 patients in one study and in 15% of 109 patients in another study was associated with cerebral infarcts and/or cognitive dysfunction (37,38). Microembolism in 10.3% of 68 patients was more common in those with than without NPSLE (25% versus 2.2%, respectively) (39). In these transcranial Doppler studies, valve disease was not assessed, but cerebromicroembolism was not associated with atherogenic risk factors, carotid atherosclerosis, and only in one study with antiphospholipid antibodies. In a post-mortem study of 50 patients, 9 of 10 patients with cerebral infarcts had Libman-Sacks endocarditis, chronic valvulitis, or left heart thrombus (40). In 57 fatal NPSLE cases, half had multiple cerebral infarcts with fibrin or platelet thromboemboli (41). In a study of 14 patients, focal white matter lesions and cerebral infarcts on pre-mortem MRI correlated highly with old and acute cerebral infarcts and microthromboemboli on histopathology (10). Eight (57%) of these 14 patients had Libman-Sacks endocarditis. In these pathologic studies, vasculitis, cerebritis, and atherosclerosis were rare. In contrast to prior studies, the present study is the largest and first fully integrated study linking Libman-Sacks vegetations with cerebromicroembolism, cerebral hypoperfusion, ischemic brain injury, stroke/TIA, overall NPSLE, neurocognitive dysfunction, and death. Improvement with current therapy of Libman-Sacks vegetations, cerebromicroembolism, brain perfusion and injury, and neurocognitive dysfunction further support a causal association of Libman-Sacks endocarditis and CVD.
The present study has potential limitations. The difficulty of testing patients at onset of CVD and before therapy, transcranial Doppler sampling for only 90 minutes, and excluding patients with renal dysfunction, and thus more aggressive disease, may have reduced the strength of the association of vegetations with cerebromicroembolism and CVD. The study in a tertiary care center may have overestimated the association of vegetations with CVD. However, patients were selected for acute CVD and not for valve vegetations, thus, a true association between vegetations and CVD is likely. A hypothetical study design where “exposure” (vegetations) precedes the “outcome” (CVD) may be limited in determining causality due to common resolution of valve vegetations over time (13) and multiplicity of potential pathogenesis of CVD. Therefore, such a design would require re-evaluations during acute CVD, as in our study design. Cerebroembolism from Libman-Sacks vegetations leading to breakdown of the blood brain barrier may be an important route of entry of antineuronal antibodies into the brain and should be investigated in future studies. Detection of focal nodularities or vegetations-like abnormalities in 2 apparently healthy controls were confirmed by a second independent observer and it is known that apparently healthy populations may have a 9%−15% prevalence of silent valve disease (13,14,42). However, we cannot exclude that such vegetations-like structures may have been mistaken with normal variants mimickers of valve masses such as atypical lamellar type of Lambl’s excrescences or less likely nodes of Aranti.
This study has several clinical implications. Libman-Sacks endocarditis is a strong independent risk factor for CVD in SLE. An increased awareness of this association should lead to a greater focus on the cardiovascular evaluation of SLE patients and CVD including use of TEE for increased detection of vegetations. Because of the semi-invasive nature of TEE, SLE patients should be carefully selected for undergoing such procedure with the highest diagnostic yield. Our study results support that is appropriate to perform TEE in SLE patients with 1) acute, recent (within 2–4 weeks), or recurrent stroke or TIA; or 2) acute, recent (within 2–4 weeks), or recurrent non-focal neurologic manifestations of confusional state, cognitive dysfunction, or seizures, if they also have focal brain abnormalities on MRI or cerebromicroembolism on transcranial Doppler. Clinical findings integrated with those of TEE, transcranial Doppler, and brain MRI should lead to a prompt and accurate diagnosis and treatment of Libman-Sacks endocarditis and CVD, which may prevent recurrence or progression of CVD. The identification of valve disease in SLE as a potential source of embolism resulting in ischemic CVD may also apply to other conditions commonly associated with valve and brain disease such as rheumatoid arthritis, primary antiphospholipid syndrome, rheumatic fever, and Behcet’s disease (42–46). Current non-standardized pharmacotherapy seems beneficial for Libman-Sacks endocarditis and CVD. However, there is a need for a randomized controlled study to determine the most appropriate pharmacotherapy (antiplatelet, anticoagulation, immunosuppressive, neuroprotective, lipid-lowering, or combined therapy) for primary and secondary prevention of potentially disabling and life threatening Libman-Sacks endocarditis and CVD.
Supplementary Material
Acknowledgements
Special thanks to Dr. Warren Laskey for his insightful review of the manuscript and to Janeen Sharrar, RN, and Julia Middendorf, RN, for their outstanding job in the coordination of this study.
Grant support: This research was funded by the grant RO1-HL04722-01-A6 by the National Institutes of Health/National Heart Lung and Blood Institute and in part by the grant 8UL1-TR000041 by the National Center for Research Resources.
Abbreviations List
- CVD
cerebrovascular disease
- SLE
systemic lupus erythematosus
- NPSLE
neuropsychiatric SLE
- TIA
transient ischemic attack
- MRI
magnetic resonance imaging
- TTE
transthoracic echocardiography
- TEE
transesophageal echocardiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hanly JG, Urowitz MB, Su L, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:529–535. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The American College of Rheumatology nomenclature and case definitions for neropsychiatric lupus erythematosus. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Luyendijk J, Steens SC, Ouwendijk WJ, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum. 2011;63:722–732. doi: 10.1002/art.30157. [DOI] [PubMed] [Google Scholar]
- 4.Sibbitt WL, Jr, Schmidt PJ, Hart BL, Brooks WM. Fluid Attenuated Inversion Recovery (FLAIR) imaging in neuropsychiatric systemic lupus erythematosus. J Rheumatol. 2003;30:1983–1989. [PubMed] [Google Scholar]
- 5.Bernatsky S, Clarke A, Gladman DD, et al. Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus. 2006;15:835–839. doi: 10.1177/0961203306073133. [DOI] [PubMed] [Google Scholar]
- 6.Efthimiou P, Blanco M. Pathogenesis of neuropsychiatric systemic lupus erythematosus and potential biomarkers. Mod Rheumatol. 2009;19:457–468. doi: 10.1007/s10165-009-0198-5. [DOI] [PubMed] [Google Scholar]
- 7.Syuto T, Shimizu A, Takeuchi Y, et al. Association of antiphosphatidylserine/prothrombin antibodies with neuropsychiatric systemic lupus erythematosus. Clin Rheumatol. 2009;28:841–845. doi: 10.1007/s10067-009-1123-1. [DOI] [PubMed] [Google Scholar]
- 8.Govoni M, Bombardieri S, Bortoluzzi A, et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 2012;51:157–168. doi: 10.1093/rheumatology/ker310. [DOI] [PubMed] [Google Scholar]
- 9.Gono T, Kawaguchi Y, Kaneko H, et al. Anti-NR2A antibody as a predictor for neuropsychiatric systemic lupus erythematosus. Rheumatology (Oxford) 2011;50:1578–1585. doi: 10.1093/rheumatology/keq408. [DOI] [PubMed] [Google Scholar]
- 10.Sibbitt WL, Jr, Brooks WM, Kornfeld M, Hart BL, Bankhursts AD, Roldan CA. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum. 2010;40:32–52. doi: 10.1016/j.semarthrit.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brey RL, Muscal E, Chapman J. Antiphospholipid antibodies and the brain: a consensus report. Lupus. 2011;20:153–157. doi: 10.1177/0961203310396748. [DOI] [PubMed] [Google Scholar]
- 12.Kozora E, West SG, Maier SF, et al. Antibodies against N-methyl-D-aspartate receptors in patients with systemic lupus erythematosus without major neuropsychiatric syndromes. J Neurol Sci. 2010;295:87–91. doi: 10.1016/j.jns.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roldan CA, Shively BK, Crawford MH. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. New Engl J Med. 1996;335:1424–1430. doi: 10.1056/NEJM199611073351903. [DOI] [PubMed] [Google Scholar]
- 14.Roldan CA, Qualls CR, Sopko KS, Sibbitt WL., Jr Transthoracic versus transesophageal echocardiography for detection of Libman-Sacks endocarditis: a randomized controlled study. J Rheumatol. 2008;35:224–229. [PubMed] [Google Scholar]
- 15.Omdal R, Lunde P, Rasmussen K, Mellgren SI, Husby G. Transesophageal and transthoracic echocardiography and Doppler-examinations in systemic lupus erythematosus. Scand J Rheumatol. 2001;30:275–281. doi: 10.1080/030097401753180354. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. and the Committee on Prognosis Studies in SLE: Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 17.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 18.Crawford MH, Roldan CA. Quantitative Assessment of Valve Thickness in Normal Subjects by Transesophageal Echocardiography. Am J Cardiol. 2001;87:1419–1423. doi: 10.1016/s0002-9149(01)01569-7. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 20.Roldan CA, Joson J, Sharrar J, Qualls CR, Sibbitt WL., Jr Premature aortic atherosclerosis in systemic lupus erythematosus: a controlled transesophageal echocardiographic study. J Rheumatol. 2010;37:71–78. doi: 10.3899/jrheum.090665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: A report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19:943–954. doi: 10.1016/j.echo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y, Saqqur M, Asil T, et al. A combined power M-mode and single gate transcranial Doppler ultrasound microemboli signal criteria for improving emboli detection and reliability. J Neuroimaging. 2009;32:1–9. doi: 10.1111/j.1552-6569.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 23.Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum. 2004;51:810–818. doi: 10.1002/art.20692. [DOI] [PubMed] [Google Scholar]
- 24.Gasparovic CM, Roldan CA, Sibbitt WL, Jr, et al. Elevated cerebral blood flow and volume in systemic lupus measured by dynamic susceptibility contrast magnetic resonance imaging. J Rheumatol. 2010;37:1834–1843. doi: 10.3899/jrheum.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scully M, Anderson B, Lane T, et al. An automated method for segmenting white matter lesions in lupus through multilevel morphometric feature classification. Front Hum Neurosci. 2010;4:1–7. doi: 10.3389/fnhum.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman E, Wilterdink JL, Kosinski A, et al. Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial. Neurology. 2007;68:2099–2106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 27.Rouzet F, Bachelet-Violette L, Alsac JM, et al. Radiolabeled fucoidan as a p-selectin targeting agent for in vivo imaging of platelet-rich thrombus and endothelial activation. J Nucl Med. 2011;52:1433–1440. doi: 10.2967/jnumed.110.085852. [DOI] [PubMed] [Google Scholar]
- 28.Davey R, Bamford J, Emery P. The role of endothelial dysfunction in the pathogenesis of neuropsychiatric systemic lupus erythematosus. Lupus. 2010;19:797–802. doi: 10.1177/0961203309359780. [DOI] [PubMed] [Google Scholar]
- 29.Futrell N, Millikan C. Frequency, etiology, and prevention of stroke in patients with systemic lupus erythematosus. Stroke. 1989;20:583–591. doi: 10.1161/01.str.20.5.583. [DOI] [PubMed] [Google Scholar]
- 30.Morelli S, Bernardo ML, Viganego F, et al. Left-sided heart valve abnormalities and risk of ischemic cerebrovascular accidents in patients with systemic lupus erythematosus. Lupus. 2003;12:805–812. doi: 10.1191/0961203303lu468oa. [DOI] [PubMed] [Google Scholar]
- 31.Roldan CA, Gelgand EA, Qualls CR, Sibbitt WL. Valvular heart disease as a cause of cerebrovascular disease in patients with systemic lupus erythematosus. Am J Cardiol. 2005;95:1441–1447. doi: 10.1016/j.amjcard.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Roldan CA, Gelgand EA, Qualls CR, Sibbitt WL. Valvular heart disease is associated with non-focal neuropsychiatric systemic lupus erythematosus. J Clin Rheumatol. 2006;12:3–10. doi: 10.1097/01.rhu.0000200378.42836.7f. [DOI] [PubMed] [Google Scholar]
- 33.Roldan CA, Gelgand EA, Qualls CR, Sibbitt WL. Valvular heart disease by transthoracic echocardiography is associated with focal brain injury and central neuropsychiatric systemic lupus erythematosus. Cardiology. 2007;108:331–337. doi: 10.1159/000099104. [DOI] [PubMed] [Google Scholar]
- 34.Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM. Libman-Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med. 2007;120:636–642. doi: 10.1016/j.amjmed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Rademacher J, Sohngen D, Specker C, Janda I, Sitzer M. Cerebral microembolism, a disease marker for ischemic cerebrovascular events in the antiphospholipid syndrome of systemic lupus erythematosus? Acta Neurol Scand. 1999;99:356–361. doi: 10.1111/j.1600-0404.1999.tb07364.x. [DOI] [PubMed] [Google Scholar]
- 36.Kumral E, Evyapan D, Keser G, et al. Detection of microemboli signals in patients with neuropsychiatric lupus erythematosus. Eur Neurol. 2002;47:131–135. doi: 10.1159/000047970. [DOI] [PubMed] [Google Scholar]
- 37.Dahl A, Omdal R, Waterloo K, et al. Detection of cerebral embolic signals in patients with systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 2006;77:774–779. doi: 10.1136/jnnp.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantú-Brito C, Baizabal-Carvallo JF, Alonso-Juárez M, García-Ramos G. The clinical significance of microembolic signals in patients with systemic lupus erythematosus. Neurol Res. 2010;32:134–138. doi: 10.1179/016164109X12478302362699. [DOI] [PubMed] [Google Scholar]
- 39.Azarpazhooh MR, Mokhber N, Orouji E, et al. Microembolic signals in patients with systemic lupus erythematosus. Can J Neurol Sci. 2010;37:371–375. doi: 10.1017/s0317167100010271. [DOI] [PubMed] [Google Scholar]
- 40.Devinsky O, Petito CK, Alonso DR. Clinical and neuropathological findings in systemic lupus erythematosus: the role of vasculitis, heart emboli, and thrombotic thrombocytopenic purpura. Ann Neurol. 1988;23:380–384. doi: 10.1002/ana.410230411. [DOI] [PubMed] [Google Scholar]
- 41.Ellis SG, Verity MA. Central nervous system involvement in systemic lupus erythematosus: a review of neuropathologic findings in 57 cases, 1955–1977. Semin Arthritis Rheum. 1979;8:212–221. doi: 10.1016/s0049-0172(79)80009-8. [DOI] [PubMed] [Google Scholar]
- 42.Roldan CA, DeLong C, Qualls CR, Crawford MH. Characterization of valvular heart disease in rheumatoid arthritis by transesophageal echocardiography and clinical correlates. Am J Cardiol. 2007;100:496–502. doi: 10.1016/j.amjcard.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 43.Hanly JG, Fisk JD, McCurdy G, Fougere L, Douglas JA. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol. 2005;32:1459–1456. [PubMed] [Google Scholar]
- 44.Turiel M, Muzzupappa S, Gottardi B, Crema C, Sarzi-Puttini P, Rossi E. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus. 2000;9:406–412. doi: 10.1191/096120300678828532. [DOI] [PubMed] [Google Scholar]
- 45.Kiliç A, Unüvar E, Tatli B, et al. Neurologic and cardiac findings in children with Sydenham chorea. Pediatr Neurol. 2007;36:159–164. doi: 10.1016/j.pediatrneurol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Cho BS, Kim HS, Oh SJ, et al. Comparison of the clinical manifestations, brain MRI and prognosis between neuroBechet’s disease and neuropsychiatric lupus. Korean J Intern Med. 2007;22:77–86. doi: 10.3904/kjim.2007.22.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.