Abstract
Objectives
This study aimed to determine the distributions of the age at onset (AAO) in patients with major depressive disorder (MDD) using admixture analysis and to determine the clinical differences between subgroups with different AAO.
Methods
Participants were administered the Mini-International Neuropsychiatric Interview and the Montgomery–Asberg Depression Rating Scale to obtain clinical data. Admixture analysis was performed using the STATA module DENORMIX to identify subgroups characterized by differences in AAO.
Results
The best fit model was the three-component model with the following means, standard deviations and proportions: 14.60 (3.75) years (49.1%), 29.15 (6.75) years (34.1%) and 46.96 (6.06) years (16.8%) (χ2=3.64, 2 df, P=.162). The three subgroups were divided by AAO of 22 and 40. After controlling for duration of illness, there were no significant differences between the three subgroups in terms of gender and family history. However, the early-onset subgroup was significantly more likely to report being single compared to the intermediate- and late-onset groups. The proportion of individuals meeting criteria for lifetime comorbid panic disorders and obsessive–compulsive disorder did not differ significantly between the AAO groups. However, there was a trend for higher incidence of generalized anxiety disorder in the early- and intermediate-onset compared to the late-onset subgroup (43.4% and 41.9% vs. 30.0%, P=.095). Furthermore, the early-onset group reported a higher incidence of attention-deficit/hyperactivity disorder (5.1% vs. 1.7% and 1.2%, P=.085) diagnosis, although this was also not statistically significant. In addition, there was a trend for higher rate of lifetime substance use in the early-onset group compared to the intermediate- and late-onset groups (18.9% compared to 12.4% and 10.0%, respectively; P=.061).
Conclusions
Our study identified clinically different AAO subgroups in individuals suffering from MDD. The subgroups may reflect different underlying neurobiological mechanisms involved.
1. Introduction
Depression is a highly prevalent mental disorder. The National Comorbidity Survey-Replication (NCS-R) [1] reported a 16.2% lifetime and 6.6% 12-month prevalence of major depressive disorder (MDD). However, the population of individuals diagnosed with MDD comprises significant heterogeneity in terms of their symptom severity [2,3], comorbidities [4], recurrences [5] and response to treatment. As such, the identification of clinical indicators capable of differentiating patients into more homogeneous subgroups will greatly benefit the understanding of disease pathophysiology and help guide treatment selection and development. One of the indicators proposed is the age at onset (AAO) of first depressive episode [6].
Based on data from the NCS-R [1], the median AAO for MDD is 32 years old, with a wide distribution and an interquartile range (the number of years between the 25th and 75th percentiles of the AAO) of 25 years (age 19–44). However, clinically significant depressive symptoms in older adults are viewed as a separate diagnostic entity also called late life depression when the onset is between 50 and 60 [7–10].
Previous studies have shown that early-onset MDD was associated with longer duration of illness, more recurrence, higher suicidality, greater symptom severity and more axis I comorbidity compared to late-onset MDD [11–13]. However, there has been significant discrepancy in the definition of early- versus late-onset groups. Various studies have employed AAO cutoffs that ranged from 18 to 25 years of age [11–14]. The admixture analysis of AAO was previously used in bipolar spectrum disorder and schizophrenia populations to identify cutoff age for subgroups with distinct clinical and demographic features [15–17]. Hitherto, a similar analysis has not been endeavored in individuals with MDD.
In the analysis herein, we employed the admixture analysis to model distinct AAO distributions in 531 individuals with MDD and identify the ideal AAO cutoff between early-, intermediate- and late-onset MDD. We then evaluated whether the three subgroups differed with respect to clinical and demographic variables, as well as disease prognosis and psychiatric comorbidities.
2. Methods
2.1. Patient recruitment and assessment
A total of 856 individuals presenting for evaluation and/or treatment to the Mood Disorders Psychopharmacology Unit (MDPU) at the University Health Network, University of Toronto, Toronto, Ontario, Canada, and the Cleveland Clinic Center for Mood Disorders Treatment and Research at Lutheran Hospital, Cleveland, OH, USA, were enrolled in the International Mood Disorders Collaborative Project (IMDCP). Both the MDPU and the Cleveland Clinic Center for Mood Disorders Treatment and Research are academic specialty research programs providing clinical service to adults seeking evaluation and treatment for MDD or bipolar disorder. The MDPU is exclusively an outpatient program, while the Cleveland Clinic offers both outpatient and inpatient services.
The sample selected for this analysis consisted of 531 participants, 345 from Cleveland and 186 from Toronto. The subject age ranged from 18 to 65 years.
Individuals assessed were included in the study if they were diagnosed with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnosis of MDD as derived by the Mini-International Neuropsychiatric Interview-Plus (MINI-Plus 5.0.0). The information regarding comorbidity with generalized anxiety disorder (GAD), panic disorder, attention-deficit/hyperactivity disorder (ADHD) and obsessive–compulsive disorder (OCD) was extracted from the MINI-Plus.
Participants were excluded from the analysis here if they had a diagnosis of bipolar disorder. The AAO was defined as the age at the onset of the patients’ first episode of depression, as reported by the patient at the time of the interview.
The MDPU research platforms at both centers were approved by the Research Ethics Board of the University Health Network University of Toronto, and the Institutional Review Board of the Cleveland Clinic Foundation at Lutheran Hospital, and written informed consent was obtained from all participants enrolled in the IMDCP. Individuals who were unwilling or unable to provide informed consent or comply with study assessment were excluded.
2.2. Statistical analysis
Admixture analysis was performed using the STATA (release 8, StataCorp, College Station, TX, USA) module DENORMIX [18]. This analysis performs a decomposition of the AAO distribution into a mixture of normal components and estimates the number of components of such a mixture. The maximum likelihood estimation of the finite normal mixture was applied to determine a theoretical model that best fitted the observed distribution of AAO. The χ 128 2 goodness-of-fit test was performed, and a P value was obtained. The P value is an indicator of the degree to which each model approximated the empirical distribution function of AAO in our sample. The model with the highest P value was selected as the best fitting model. Using the fitted function, each participant’s probability of belonging to each AAO subgroup was calculated and then assigned to the subgroup to which he/she had the maximum probability of belonging. The cutoff age for each AAO subgroup is characterized by the intersection between two distribution curves.
Because we had three AAO groups, we investigated the relationship between the clinical characteristics and AAO using a multinomial logistic regression. The independent predictor variables of interest were gender, family history, marital status, lifetime psychiatric comorbidities and substance dependence. The duration of illness was used as a covariate in all of the analysis to account for the confounding effect of age. We used the duration of illness rather than the age at interview because the latter may be a too stringent correction for subjects that were assessed right after the first episode showing a very high correlation with the AAO. All statistical analyses were conducted using SPSS for Windows, Version 19.0.
3. Results
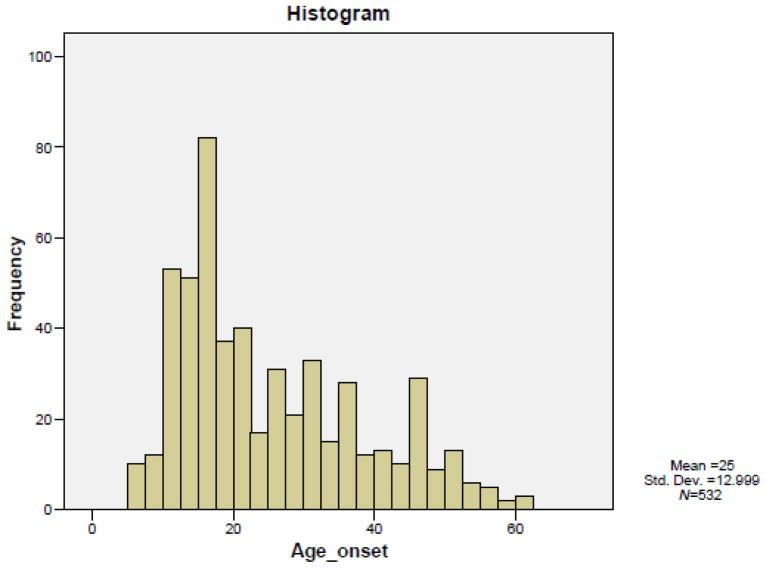
The AAO in our sample (N=531) ranged from 6 to 61 years with a mean of 25 and a standard deviation of 12.99. Our overall sample did not show a normal distribution (Fig. 1). Using the STATA module DENORMIX, the admixture analysis was performed to yield a combination of two, three or four normal theoretical distributions. The best fit model was the three-component model with the following means, standard deviations and proportions: 14.60 (3.75) years (49.1%), 29.15 (6.75) years (34.1%) and 46.96 (6.06) years (16.8%) (χ 162 2=3.64, 2 df, P=.162). In fact, the χ2 goodness-of-fit test for the four-component model was showing a significant deviation from the empirical distribution function of AAO (χ2=7. 46, 2 df, P=.024).
Fig. 1.
Observed distribution of AAO in patients with unipolar MDD.
The three subgroups were divided by AAO of 22 and40 years as the ideal cutoffs, characterized by the point at which the three curves intersected (Fig. 2). At the age of 21, the probability (55.6%) of belonging to the early-onset subgroup is higher than that of the intermediate subgroup, and at the age of 40, the probability (49.2%) of belonging to the intermediate-onset subgroup is lower than that of the late subgroup; therefore, the patients with onset at ≥ 22and ≤ 40 were included in the intermediate-onset subgroup.
Fig. 2.
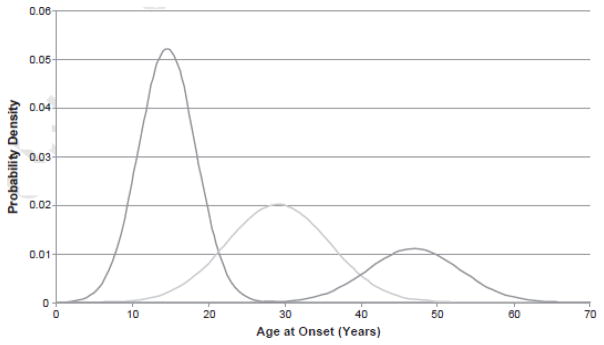
Probability density distributions. Three-component model with ideal cutoffs at 21 and 40 years of age.
When we looked at the site difference (Toronto versus Cleveland) between the two AAO distributions, we found a slight difference (χ2=6.406, 2 df, P=.041). This difference is very minor and is not likely to affect the results of our analysis; therefore, a separate analysis was not performed for the two samples.
The three subgroups differed significantly in their mean age at the time of the interview, with the early-onset group reporting a mean age of 35.8 compared to 42.7 for the intermediate group and 53.1 for the late-onset group (Pb.001), using the analysis of variance (ANOVA) [F(2/528)=78.4] (Table 1). Conversely, the early-onset group reported higher duration of illness with a mean of 21.11 years compared to 12.1 years for the intermediate group and 4.7 years for the late-onset subgroup [F(2/528)= 85.8, Pb.001].
Table 1.
Age characteristics of patients belonging to the early-, intermediate- and late-onset subgroups as identified by admixture analysis
| Age characteristics | Early onset (N=279) | Intermediate onset (N=172) | Late onset (N=80) | P valuea |
|---|---|---|---|---|
| Age at interview | ||||
| Range | 18–64 | 23–65 | 41–64 | |
| Mean (S.D.) | 35.83 (12.63) | 42.72 (9.53) | 53.18 (6.16) | <.001 |
| Age at onset | ||||
| Range | 6–21 | 22–40 | 41–61 | |
| Mean (S.D.) | 14.71 (3.63) | 30.66 (5.23) | 48.43 (4.78) | <.001 |
| Illness duration | ||||
| Mean (S.D.) | 21.11 (13.25) | 12.06 (9.33) | 4.75 (4.93) | <.001 |
P values (global) refer to ANOVA (univariate) tests.
There were no significant differences between the three subgroups in terms of gender and family history [likelihood ratio test (LRT)=0.527 and 2.715, respectively; 2 df; P=.768 and .257, respectively] (Table 2). However, the early-onset subgroup was significantly more likely to report being single (63.2%) compared to the intermediate- and late-onset subgroups (43.4% and 41.2%, respectively) (LRT= 33.195, 2 df, Pb.001) using the multinomial logistic regression. The odds ratio (OR) of the early-onset subgroup reporting being single compared to the late-onset subgroup was 5.862 [Pb.001, 95% confidence interval (CI) 2.743–12.527]. When comparing the early- with the intermediate- onset group, we also found significant difference regarding marital status (Pb.001, OR 3.422 with 95% CI 2.049–5.715). However, the difference in marital status was not significant between the intermediate- and the late-onset groups (P=.137, OR 1.713, 95% CI 0.843–3.484) (Table 3).
Table 2.
Clinical and demographic characteristics of patients belonging to the early-, intermediate- and late-onset subgroups as identified by admixture analysis
| Demographic and clinical features | Early onset (N=279) | Intermediate onset (N=172) | Late onset (N=80) | P value |
|---|---|---|---|---|
| Gender | ||||
| Female (%) | 67.0% | 62.2% | 62.5% | .523b |
| Male (%) | 33.0% | 37.2% | 37.5% | |
| Martial status | ||||
| Single (%) | 63.2% | 43.4% | 41.2% | <.001b |
| Family history of psychiatric disordersa | ||||
| % | 59.7% | 57.4% | 65.9% | .625b |
| Comorbid psychiatric disorders | ||||
| Panic disorders | 17.8% | 17.4% | 15.2% | .866b |
| OCD | 7.6% | 9.9% | 7.5% | .663b |
| GAD | 43.4% | 41.9% | 30.0% | .095b |
| ADHD | 5.1% | 1.7% | 1.2% | .085b |
| Lifetime substance use | ||||
| Alcohol use | 30.8% | 25.6% | 27.5% | .475b |
| Alcohol dependence | 20.1% | 14.5% | 16.2% | .305b |
| Alcohol abuse | 13.4% | 12.7% | 12.9% | .977b |
| Lifetime substance dependence | 18.9% | 12.4% | 10.0% | .061b |
Positive family history is defined as the presence of Asia I disorder in the patients’ family pedigree.
P values refer in LRTs.
Table 3.
Odds ratio of statistically significant clinical features
| OR (95% CI)
| |||
|---|---|---|---|
| Early vs. late onset | Intermediate vs. late onset | Early vs. Intermediate onset | |
| Martial status (single) | 5.862 (2.743–12.527)a | 1.713 (0.843–3.484) | 3.422 (2.049–5.715)a |
| GAD | 1.651 (0.900–3.030) | 1.552 (0.858–2.806) | 1.064(0.706–1.602) |
| ADHD | 4.417 (0.506–38.545) | 1.339 (0.132–13.636) | 3.298 (0.894–12.159) |
| Lifetime substance use | 1.713 (0.706–4.159) | 1.023 (0.414–2.526) | 1.675 (0.945–2.967) |
Denotes statistical significance
In terms of lifetime comorbid psychiatric diagnosis, the three groups did not differ significantly in their association with panic disorders and OCD using the multinomial logistic regression. However, a higher proportion of participants in the early- and intermediate-onset subgroups met the criteria for GAD (43.4% and 41.9% vs. 30.0%, P=.095). Additionally, a higher proportion of the early-onset group met the criteria for ADHD (5.1% vs. 1.7% and 0.8%, P=.085) compared to the intermediate- and late-onset groups, although these trends were not statistically significant (Table 2).
Moreover, a higher proportion of individuals in the early-onset subgroup met the criteria for lifetime substance use compared to the intermediate- and late-onset (18.9% compared to 12.4% and 10.0%, respectively; P=.061; Table 2). Although the early-onset subgroup reported slightly higher incidence of alcohol use (dependence and abuse) than the intermediate- and late-onset (30.8% vs. 25.6% and 27.5%), the difference was not statistically significant after adjusting for duration of illness (P=.475).
4. Discussion
The analysis herein aimed to better understand AAO as a clinical marker for MDD by determining the distribution of subgroups and comparatively analyzing their clinical and demographic features. Three AAO subgroups were identified in individuals with MDD: the early-onset group with mean AAO of 14.7 years, the intermediate-onset group with mean of 30.6 years and late-onset group with mean of 48.4 years.
Past studies have hypothesized that individuals with a strong genetic loading of MDD (i.e., positive family history) may present with earlier AAO and more aggressive illness [11–13]. However, the lack of significant difference in family history between the early- and late-onset subgroups may suggest limited genetic effect on the AAO of MDD, but it may also point to a complex interaction between environmental and genetic factors in precipitating MDD. On the other hand, Korten at al. (2012) found a significant association between positive family history and early onset applying the arbitrary cutoff age of 40. The difference between our study and the Korten study is likely to be the effect of the arbitrary cutoff age in that study [19].
After adjusting for duration of illness, a significantly larger proportion of individuals with early-onset MDD reported being single at the time of the interview compared to the intermediate- and late-onset subgroups. This may suggest that patients with early-onset MDD are more socially isolated. Rohde et al. [20] showed that adolescents demonstrated increased internalizing behaviors, emotional dependence and subsyndromal depressive symptoms after their first major depressive episode. However, the association between early onset and lower social functioning needs to be tested considering different social outcomes, and it is difficult to ascertain whether this is due to the increased severity of disease in the early-onset subgroup or the fact that the onset of disease at a time of puberty and growth caused significant impact on the development of appropriate social functioning. On the other hand, the fact that the early onset subjects were younger at the time of the assessment may be responsible for the association.
There was also a trend towards higher incidence of lifetime substance use in the early-onset group. Additionally, the early-onset group had higher incidence of lifetime alcohol dependence, although this was not statistically significant (P=.305). This finding is congruent with the growing evidence for an underlying genetic link between major depression and addiction [21–23].
Finally, although the AAO of MDD did not have an effect on the incidence of comorbid OCD and panic disorders, there was a trend towards higher comorbidity with GAD and ADHD diagnosis in the early-onset subgroup compared to late-onset subgroup, although this trend was not statistically significant. The ADHD comorbidity may suggest a higher cognitive burden in early-onset depression, but we did not have cognitive measures to confirm this association.
To our knowledge, this is the first study to implement the admixture analysis to a large clinical sample of individuals with MDD. The database was constructed using information collected from standardized interviews and is easily replicable. Unfortunately, because the interviews were performed at variable points during the patients’ illness course, there may be a recall bias in reporting of symptom history and number of episodes. However, the retrospective assessment of the AAO is a limitation since it is sensitive to the duration of illness; therefore, the outcomes associated with early-onset depression should be investigated in first- episode depression samples where the recall bias is minimal.
5. Conclusion
Our study identified clinically different AAO subgroups in individuals suffering from MDD. The subgroups may have different response to treatment, and further studies are needed to discern the interactions between genetics and life events in determining the onset of MDD. MDD patients with early AAO are more likely to report decreased marital rates and social functioning, more substance use problems and more psychiatric comorbidities. Clinicians should be vigilant regarding timely diagnosis of these patients as they may benefit from earlier detection along with more aggressive management employing medications and psychotherapy as well as close follow-up. Future research should investigate whether the subgroups respond differently to antidepressants and psychotherapies.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Determinants of persistence of major depressive episodes in the general population. J Affect Disord. 2004;81:231–40. doi: 10.1016/j.jad.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Rytsälä HJ, Melartin TK, Leskela US, Sokero TP, Lestela-Mielonen PS, Isometsa ET. Functional and work disability in major depressive disorder. J Nerv Ment Dis. 2004;193:189–95. doi: 10.1097/01.nmd.0000154837.49247.96. [DOI] [PubMed] [Google Scholar]
- 4.Unick GJ, Snowden L, Hastings J. Heterogeneity in comorbidity between major depressive disorder and generalized anxiety disorder and its clinical consequences. J Nerv Ment Dis. 2009;197:215–24. doi: 10.1097/NMD.0b013e31819d954f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruijshaar ME, Hoeymans N, Bijl RV, Spijker J, Essink-Bot ML. Levels of disability in major depression — findings from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) J Affect Disord. 2003;77:53–64. doi: 10.1016/s0165-0327(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 6.Copeland JR, Beekman AT, Braam AW, Dewey ME, Delespaul P, Fuhrer R, et al. Depression among older people in Europe: the EURODEP studies. World Psychiatry. 2004;3:45–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Gallo JJ, Lebowitz BD. The epidemiology of common late-life mental disorders in the community: themes for the new century. Psychiatr Serv. 1999;50:1158–66. doi: 10.1176/ps.50.9.1158. [DOI] [PubMed] [Google Scholar]
- 8.Kivela SL, Pahkala K, Laippala P. Prevalence of depression in an elderly population in Finland. Acta Psychiatr Scand. 1988;78:401–13. doi: 10.1111/j.1600-0447.1988.tb06358.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Shinkai S. Correlates of cognitive impairment and depressive symptoms among older adults in Korea and Japan. Int J Geriatr Psychiatry. 2005;20:576–86. doi: 10.1002/gps.1313. [DOI] [PubMed] [Google Scholar]
- 10.Steffens DC, Skoog I, Norton MC, Hart AD, Tschanz JT, Plassman BL, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry. 2000;57:601–7. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- 11.Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, et al. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res. 2004;129:127–40. doi: 10.1016/j.psychres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Alpert JE, Fava M, Uebelacker LA, Nierenberg JA, Worthington JJ, III, Rosenbaum JF. Patterns of axis I comorbidity in early-onset versus late-onset major depressive disorder. Biol Psychiatry. 1999;46:202–10. doi: 10.1016/s0006-3223(99)00017-7. [DOI] [PubMed] [Google Scholar]
- 13.Klein DN, Schatzberg AF, McCullough JP, Dowling F, Goodman D, Howland RH, et al. Age of onset in chronic major depression: relation to demographic and clinical variables, family history, and treatment response. J Affect Disord. 1999;55:149–57. doi: 10.1016/s0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 14.Parker G, Roy K, Hadzi-Pavlovic D, Mitchell P, Wilhelm K. Distinguishing early and late onset non-melancholic unipolar depre sion. J Affect Disord. 2003;74:131–8. doi: 10.1016/s0165-0327(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 15.Javaid N, Kennedy JL, De Luca V. Ethnicity and age at onset in bipolar spectrum disorders. CNS Spectr. 2011:16. doi: 10.1017/S1092852912000296. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Tozzi F, Manchia M, Galwey NW, Severino G, et al. Admixture analysis of age at onset in bipolar disorder. Psychiatry Res. 2011;185:27–32. doi: 10.1016/j.psychres.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Panariello F, O’Driscoll L, de Souza RP, Tiwari A, Manchia M, Kennedy J, et al. Age at onset in Canadian schizophrenia patients: admixture analysis. Schizophr Res. 2010;122:278–9. doi: 10.1016/j.schres.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Kolenikov S. DENORMIX: Stata module to perform decomposition of normal mixture. 2001 http://ideas.repec.org/c/boc/bocode/s416605.html.
- 19.Korten NC, Comijs HC, Lamers F, Penninx BW. Early and late onset depression in young and middle aged adults: differential symptomatology, characteristics and risk factors? Affect Disord. 2012;138(3):259–67. doi: 10.1016/j.jad.2012.01.042. [Epub 2012 Feb 26] [DOI] [PubMed] [Google Scholar]
- 20.Rohde P, Lewinsohn PM, Seeley JR. Are adolescents changed by an episode of major depression? J Am Acad Child Adolesc Psychiatry. 1994;33:1289–98. doi: 10.1097/00004583-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Nurnberger JI, Jr, Foroud T, Flury L, Su J, Meyer ET, Hu K, et al. Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiatry. 2001;158:718–24. doi: 10.1176/appi.ajp.158.5.718. [DOI] [PubMed] [Google Scholar]
- 22.Lyons MJ, Schultz M, Neale M, Brady K, Eisen S, Toomey R, et al. Specificity of familial vulnerability for alcoholism versus major depression in men. J Nerv Ment Dis. 2006;194:809–17. doi: 10.1097/01.nmd.0000244480.78431.49. [DOI] [PubMed] [Google Scholar]
- 23.Gokturk C, Schultze S, Nilsson KW, von Knorring L, Oreland L, Hallman J. Serotonin transporter (5-HTTLPR) and monoamine oxidase (MAOA) promoter polymorphisms in women with severe alcoholism. Arch Women’s Ment Health. 2008;11:347–55. doi: 10.1007/s00737-008-0033-6. [DOI] [PubMed] [Google Scholar]