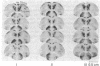
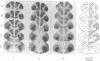
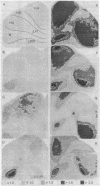
Abstract
This report describes experiments in which successful acoustic imprinting correlates with differential uptake of D-2-deoxy[14C]glucose in particular forebrain areas that are not considered primarily auditory. Newly hatched guinea chicks (Numida meleagris meleagris) were imprinted by playing 1.8-kHz or 2.5-kHz tone bursts for prolonged periods. Those chicks were considered to be imprinted who approached the imprinting stimulus (emitted from a loudspeaker) and preferred it over a new stimulus in a simultaneous discrimination test. In the 2-deoxy-D-glucose experiment all chicks, imprinted and naive, were exposed to 1.8-kHz tone bursts for 1 hr. As shown by the autoradiographic analysis of the brains, neurons in the 1.8-kHz isofrequency plane of the auditory "cortex" (field L) were activated in all chicks, whether imprinted or not. However, in the most rostral forebrain striking differences were found. Imprinted chicks showed an increased 2-deoxy-D-glucose uptake in three areas, as compared to naive chicks: (i) the lateral neostriatum and hyperstriatum ventrale, (ii) a medial magnocellular field (medial neostriatum/hyperstriatum ventrale), and (iii) the most dorsal layers of the hyperstriatum. Based on these findings we conclude that these areas are involved in the processing of auditory stimuli once they have become meaningful by experience.
Full text
PDF

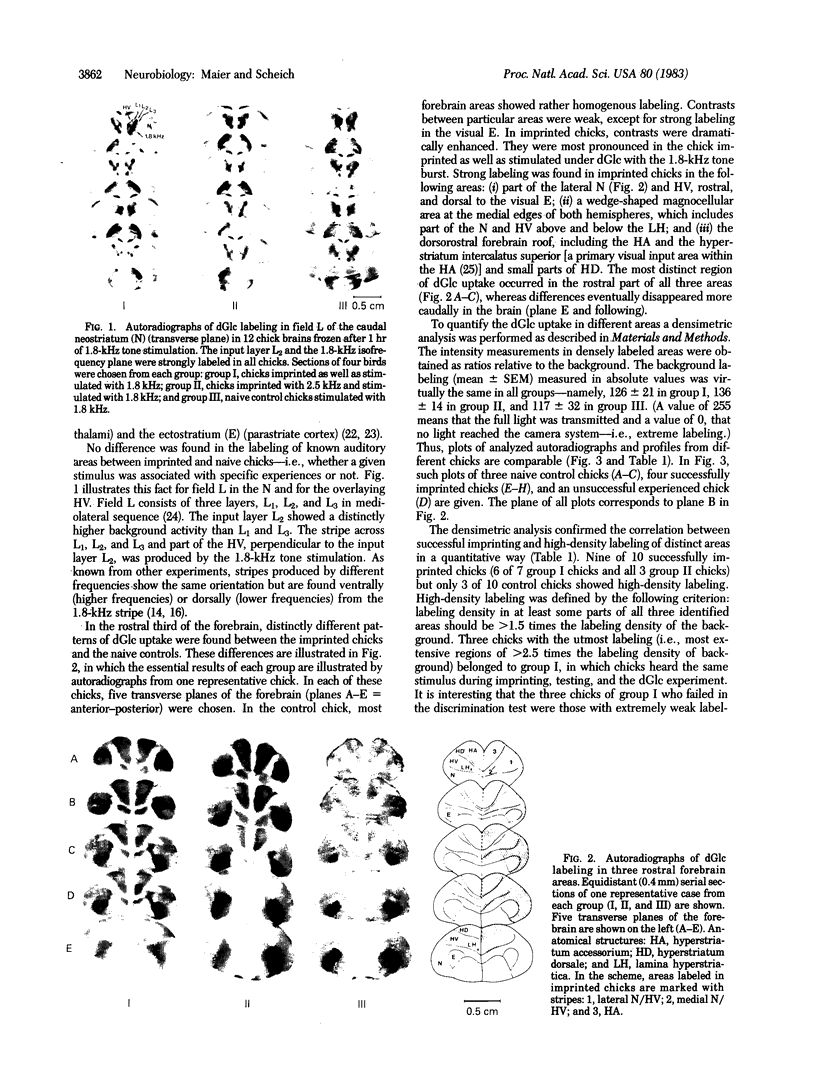


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateson P. P., Horn G., Rose S. P. Imprinting: correlations between behaviour and incorporation of (14-C) uracil into chick brain. Brain Res. 1975 Feb 7;84(2):207–220. doi: 10.1016/0006-8993(75)90976-2. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Alger B., Thompson R. F. Neuronal substrate of classical conditioning in the hippocampus. Science. 1976 Apr 30;192(4238):483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Campbell C. B., Hodos W. The concept of homology and the evolution of the nervous system. Brain Behav Evol. 1970;3(5):353–367. doi: 10.1159/000125482. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: VI. Specific embryonic experience required to maintain species-typical perception in ducklings. J Comp Physiol Psychol. 1980 Aug;94(4):579–587. doi: 10.1037/h0077691. [DOI] [PubMed] [Google Scholar]
- Horn G., McCabe B. J., Bateson P. P. An autoradiographic study of the chick brain after imprinting. Brain Res. 1979 May 25;168(2):361–373. doi: 10.1016/0006-8993(79)90176-8. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Hodos W., Nauta W. J., Revzin A. M. Neural connections of the "visual wulst" of the avian telencephalon. Experimental studies in the piegon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol. 1973 Aug;150(3):253–278. doi: 10.1002/cne.901500303. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Hodos W. Telencephalic projections of the nucleus rotundus in the pigeon (Columba livia). J Comp Neurol. 1970 Sep;140(1):35–51. doi: 10.1002/cne.901400103. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Revzin A. M. The afferent connections of the nucleus rotundus in the pigeon. Brain Res. 1966 Oct;2(4):368–377. doi: 10.1016/0006-8993(66)90006-0. [DOI] [PubMed] [Google Scholar]
- Karten H. J. The ascending auditory pathway in the pigeon (Columba livia). II. Telencephalic projections of the nucleus ovoidalis thalami. Brain Res. 1968 Oct;11(1):134–153. doi: 10.1016/0006-8993(68)90078-4. [DOI] [PubMed] [Google Scholar]
- Karten H. J. The organization of the ascending auditory pathway in the pigeon (Columba livia). I. Diencephalic projections of the inferior colliculus (nucleus mesencephali lateralis, pars dorsalis). Brain Res. 1967 Nov;6(3):409–427. doi: 10.1016/0006-8993(67)90055-8. [DOI] [PubMed] [Google Scholar]
- Kohsaka S. I., Takamatsu K., Aoki E., Tsukada Y. Metabolic mapping of chick brain after imprinting using [14C]2-deoxyglucose technique. Brain Res. 1979 Aug 31;172(3):539–544. doi: 10.1016/0006-8993(79)90585-7. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G., Bateson P. P. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981 Jan 26;205(1):29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Andersen R. A., Motter B. C. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci. 1981 Nov;1(11):1218–1225. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst B. E., O'Connor T. A., Miller J. M. Response plasticity of neurons in auditory cortex of the rhesus monkey. Exp Brain Res. 1977 Sep 28;29(3-4):393–404. doi: 10.1007/BF00236178. [DOI] [PubMed] [Google Scholar]
- Ridley R. M., Ettlinger G. Visual discrimination performance in the monkey: the activity of single cells in infero-termporal cortex. Brain Res. 1973 May 30;55(1):179–182. doi: 10.1016/0006-8993(73)90497-6. [DOI] [PubMed] [Google Scholar]
- Salzen E. A., Parker D. M., Williamson A. J. A forebrain lesion preventing imprinting in domestic chicks. Exp Brain Res. 1975 Dec 22;24(2):145–157. doi: 10.1007/BF00234060. [DOI] [PubMed] [Google Scholar]
- Salzen E. A., Parker D. M., Williamson A. J. Forebrain lesions and retention of imprinting in domestic chicks. Exp Brain Res. 1978 Jan 18;31(1):107–116. doi: 10.1007/BF00235808. [DOI] [PubMed] [Google Scholar]
- Scheich H., Bonke B. A., Bonke D., Langner G. Functional organization of some auditory nuclei in the guinea fowl demonstrated by the 2-deoxyglucose technique. Cell Tissue Res. 1979;204(1):17–27. doi: 10.1007/BF00235161. [DOI] [PubMed] [Google Scholar]
- Scheich H., Bonke B. A. Tone-versus FM--induced patterns of excitation and suppression in the 14-C-2-deoxyglucose labeled auditory "cortex" of the guinea fowl. Exp Brain Res. 1981;44(4):445–449. doi: 10.1007/BF00238839. [DOI] [PubMed] [Google Scholar]
- VAN TIENHOVEN A., JUHASZ L. P. The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol. 1962 Apr;118:185–197. doi: 10.1002/cne.901180205. [DOI] [PubMed] [Google Scholar]