Abstract
Background
The anticancer properties of aspirin are restricted by its gastrointestinal toxicity and its limited efficacy. Therefore, we synthesized phospho-aspirin (PA-2; MDC-22), a novel derivative of aspirin, and evaluated its chemotherapeutic and chemopreventive efficacy in preclinical models of triple negative breast cancer (TNBC).
Methods
Efficacy of PA-2 was evaluated in human breast cancer cells in vitro, and in orthotopic and subcutaneous TNBC xenografts in nude mice. Mechanistic studies were also carried out to elucidate the mechanism of action of PA-2.
Results
PA-2 inhibited the growth of TNBC cells in vitro more potently than aspirin. Treatment of established subcutaneous TNBC xenografts (MDA-MB-231 and BT-20) with PA-2 induced a strong growth inhibitory effect, resulting in tumor stasis (79% and 90% inhibition, respectively). PA-2, but not aspirin, significantly prevented the development of orthotopic MDA-MB-231 xenografts (62% inhibition). Mechanistically, PA-2: 1) inhibited the activation of epidermal growth factor receptor (EGFR) and suppressed its downstream signaling cascades, including PI3K/AKT/mTOR and STAT3; 2) induced acetylation of p53 at multiple lysine residues and enhanced its DNA binding activity, leading to cell cycle arrest; and 3) induced oxidative stress by suppressing the thioredoxin system, consequently inhibiting the activation of the redox sensitive transcription factor NF-κB. These molecular alterations were observed in vitro and in vivo, demonstrating their relevance to the anticancer effect of PA-2.
Conclusions
Our findings demonstrate that PA-2 possesses potent chemotherapeutic efficacy against TNBC, and is also effective in its chemoprevention, warranting further evaluation as an anticancer agent.
Keywords: Breast cancer, Triple-negative breast cancer, Phospho-aspirin, Non-steroidal anti-inflammatory drugs, Epidermal growth factor receptor (EGFR), p53, Oxidative stress
Background
Breast cancer is the second most common cause of female cancer-related deaths, with more than one million new cases diagnosed per year throughout the world [1]. Despite advances in its early detection, breast cancer remains a significant health problem. In particular, triple negative breast cancer (TNBC) is known to be more aggressive with poor prognosis, and is frequently associated with resistance to chemotherapeutic agents. Thus, novel agents capable of inhibiting TNBC are urgently needed.
Aspirin, a prototypical non-steroidal anti-inflammatory drug (NSAID), is the most widely used anti-inflammatory medication in the world [2,3]. NSAIDs have a significant antineoplastic effect, which should be viewed, at least in part, in the context of the increasingly appreciated role of inflammation in cancer. Aspirin has been formally documented to be a chemopreventive agent against colon cancer [4,5]. Epidemiological studies also support a role of aspirin in reducing the risk of breast cancer [6]. However, gastrointestinal toxicity caused by chronic aspirin use is a significant health concern. In order to reduce the toxicity and enhance the efficacy of aspirin, we synthesized phospho-aspirin (PA-2; MDC-22; Figure 1A), which consists of aspirin chemically modified at its –COOH group, the moiety accounting for its gastrointestinal toxicity [7,8]. Indeed, as we have recently reported, the gastrointestinal toxicity of PA-2 in rats is much reduced compared to that of aspirin [9].
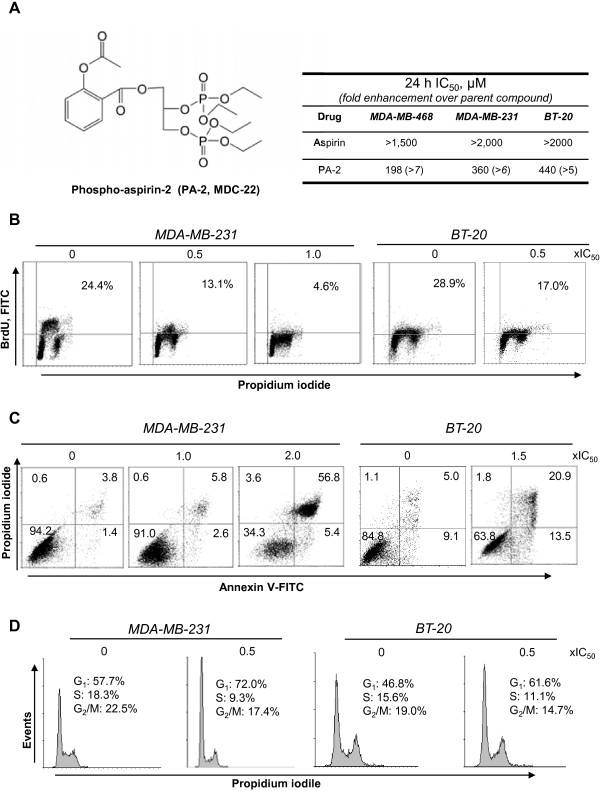
Figure 1.
Phospho-aspirin-2 inhibits the growth of TNBC cells. A: Left: Chemical structure of phospho-aspirin-2 (PA-2, MDC-22). Right: 24 h-IC50 values of PA-2 and aspirin in TNBC cell lines. B: MDA-MB-231 and BT-20 cells were treated with PA-2 for 24 h and the percentage of proliferating cells was determined by BrdU incorporation. C: MDA-MB-231 and BT-20 cells treated with PA-2 for 24 h were stained with Annexin V/PI, and the percentage of apoptotic cells was determined by flow cytometry. D: PA-2 blocks the G1/S cell cycle phase transition after 24 h treatment in MDA-MB-231 cells, determined by flow cytometry following PI staining.
The epidermal growth factor receptor (EGFR) and p53 are key molecular determinants of TNBC [10-12]. Aberrant activation of EGFR plays an important role in breast carcinogenesis via the sustained initiation of downstream cascades that promote cell survival and proliferation. Thus, EGFR is an attractive target for the development of cancer therapeutics [13]. On the other hand, the inactivation of p53, a potent tumor suppressor, is also a major contributor to breast cancer development [12]. Apart from its ability to block cell cycle progression and promote apoptosis, it is now appreciated that p53 also suppresses tumor development by modulating autophagy, cellular metabolism, angiogenesis, and metastasis [14]. This portends that the restoration of p53 function in tumors will be extremely beneficial, since it will not merely inhibit the growth of tumor cells but also obliterate the microenvironment required for tumor survival.
Herein, we examined the antineoplastic properties of PA-2 in TNBC in vitro and in vivo. PA-2 was much more potent than aspirin in inhibiting the growth of TNBC cells and strongly suppressed TNBC growth in subcutaneous and orthotopic xenograft models. Mechanistically, the antineoplastic effect of PA-2 is mediated through inhibition of EGFR, acetylation of p53 and induction of oxidative stress.
Methods
Reagents
PA-2 was provided by Medicon Pharmaceuticals, Inc., Setauket, NY. Aspirin were purchased from Sigma (St Louis, MO). For cell culture study, we prepared 500 mM stock solutions of both in DMSO. In all cell culture media, the final DMSO concentration was adjusted to 1%. All general solvents and reagents were of HPLC grade or of the highest grade commercially available. Antibodies against β-actin were from Sigma. All other antibodies were from Cell Signaling (Beverly, MA).
Cell culture
We used three human breast cancer cell lines: MDA-MB 231 (ER-, PR-, HER2/Neu-, EGFR+, and p53 mutant R280K), BT-20 ( ER-, PR-, HER2/Neu-, EGFR++, and p53 mutant K132G), and MDA-MB-468 (ER-, PR-, HER2/Neu-, EGFR++, and p53 mutant R2073H). All were obtained from the American Type Culture Collection (ATCC, Manassas, VA, and grown as monolayers in the specific medium and conditions suggested by ATCC. All cell lines were grown in our laboratory less than 6 months after their receipt and the cells studied were between passages 2-10.
Cell viability assay
We used an assay based on reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye (MTT), which was determined according to the manufacturer’s protocol (Promega, Madison, WI).
Cytokinetic analysis
For apoptosis, cells were seeded and treated with PA-2 for 24 h, trypsinized and stained with Annexin V-FITC (100X dilution; Invitrogen, Carlsbad, CA) and PI (0.5 μg/ml; Sigma, St Louis, MO), then analyzed by FACScaliber (BD Biosciences, San Jose, CA). To determine cell proliferation, we measured the incorporation of 5-bromo-2′-deoxyuridine (BrdU) into newly synthesized cellular DNA followed by the manufacture’s protocol (BD Biosciences), and cells were subjected to flow cytometric analysis. Cell cycle phase distribution was analyzed by flow cytometry as described [15].
Plasmid and siRNA transfection
EGFR, and SIRT1 plasmids were purchased from Addgene (Cambridge, MA). Transient transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions.
Determination of reactive oxygen and nitrogen species (RONS)
After the indicated treatment, cells were collected by trypsinization, resuspended in 10 μM of 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA Invitrogen), or MitoSox Red (Invitrogen) or dihydroethidium (DHE, Sigma), incubated at 37°C for 30 min in the dark and their fluorescence intensity was determined by flow cytometry.
Urinary F2 isoprostane assay
Urine was collected at the endpoint of treatment. Levels of F2 isoprostane and creatinine in urine were determined by ELISA (Oxford Biomedical Research, MA). F2-isoprostane values were normalized to creatinine levels.
Determination of TrxR reductase activity
After treatment, cells were lysed and TrxR activity was determined in the protein lysate using a commercially available kit, following the instructions of the manufacturer (Cayman Chemical, Ann Arbor, MI). In this assay, TrxR uses NADPH to reduce 5,5′-dithiobis-(2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid (TNB). Glutathione (oxidized and reduced) was determined by the glutathione (GSH) reductase-coupled 5,5′-dithiobis(2-nitrobenzoic acid) assay [16].
Immunoblotting
After treatment with PA-2 as indicated, cells were scraped on ice, washed with ice-cold PBS and lysed in RIPA lysis buffer (Sigma). Protein concentration was determined using the Bradford method (Bio-Rad, Hercules, CA). Electrophoresis of cell lysates were performed on 10% SDS-polyacrylamide gel electrophoresis gels and protein was transferred onto nitrocellulose membranes as described [17];
Electrophoretic Mobility Shift Assay (EMSA)
Following treatment, nuclear fractions were isolated from 2 × 106 cells as described [16]. NF-κB, or p53 EMSA was performed according to The Thermo Scientific LightShift Chemiluminescent EMSA Kit (Rockford, IL) following the instructions of the manufacturer.
Efficacy studies in nude mouse breast xenografts and orthotopic model
All animal experiments were approved by the Institutional Animal Care and Use Committee.
Treatment protocol
Female Balb/C nude mice (Charles River Laboratories, Wilmington, MA) were inoculated subcutaneously into each of their flanks with 2.5-3 × 106 TNBC cells (MDA-MB-231 or BT-20) in Matrigel (BD Biosciences, Franklin Lakes, NJ). When the tumor reached approximately 100-150 mm3, animals were randomized into the control and treatment groups (n = 10/group). For MDA-MB-231 xenografts, the animals were treated with vehicle or PA-2 120 mg/kg p.o. in corn oil 5 times/wk. For BT-20 xenografts, animals were treated with vehicle or PA-2 300 mg/kg i.p. in corn oil 5 times/wk.
Prevention protocol
Female Balb/C nude mice were treated with PA-2 120 mg/kg or ASA 40 mg/kg p.o. in corn oil (equimolar) for 1 wk. Then, the mice were inoculated into the mammary fat pad with 1.0 × 106 MDA-MB-231 cells in Matrigel. Drug treatment was continued until the end of the study. Tumor volume was calculated as [length × width × (length + width/2) × 0.56]. At the end of treatment, animals were sacrificed and tumors were removed and weighed. To calculate tumor growth inhibition, we subtracted the baseline tumor volume from the final one.
Immunohistochemical analysis
Immunohistochemical staining for Ki-67, Dmp1 and phospho-NF-κB (p-p65, activated form of NF-κB) was performed on human breast xenograft tissue samples as previously described [18]. Apoptosis was determined by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end-labeling (TUNEL) assay [19].
Statistical analysis
Results are expressed as mean ± SEM. Differences between groups were determined by one-factor analysis of variance followed by Tukey’s test for multiple comparisons. p < 0.05 was statistically significant.
Results
PA-2 inhibits the growth of human TNBC through a strong cytokinetic effect
We first compared the growth inhibitory effect of PA-2 and aspirin in a panel of TNBC cell lines. PA-2 inhibited cell growth more potently than aspirin in all the cell lines evaluated. The potency enhancement ranged between 5 and 7-fold in MDA-MB-231, MDA-MB-468, and BT-20 (Figure1A). PA-2 inhibited TNBC cell growth via a triple cytokinetic effect. In MDA-MB-231 and BT-20 cells, PA-2 a) inhibited cell proliferation by > 40% at 0.5 × IC50 and by > 80% at 1 × IC50; b) induced apoptosis by 1.6- to 12-fold over control at 1.5-and 2 × IC50; and c) suppressed the G1 to S cell cycle phase transition, leading to accumulation of cells in G1 phase by 14% at 0.5 × IC50 (Figure1B-D).
To assess the efficacy of PA-2 in vivo, we employed both subcutaneous and orthotopic TNBC xenografts in nude mice. Initially, we evaluated the chemotherapeutic effect of PA-2 on subcutaneous MDA-MB-231 and BT-20 xenografts. As shown in Figure 2A, PA-2 significantly inhibited MDA-MB-231 xenograft growth starting on day 8 of treatment until the end of the study (p < 0.001). At sacrifice, the tumor volume of vehicle was 309 ± 36 mm3 and that of PA-2 was 143 ± 16 mm3, representing a 79% tumor growth inhibition (p < 0.01). PA-2 also suppressed the growth of BT-20 xenografts (Figure 2B). After 28 days of treatment, the tumor volume of vehicle and PA-2 groups were 248 ± 27 mm3 and 157 ± 15 mm3, respectively (90% inhibition, p < 0.01).
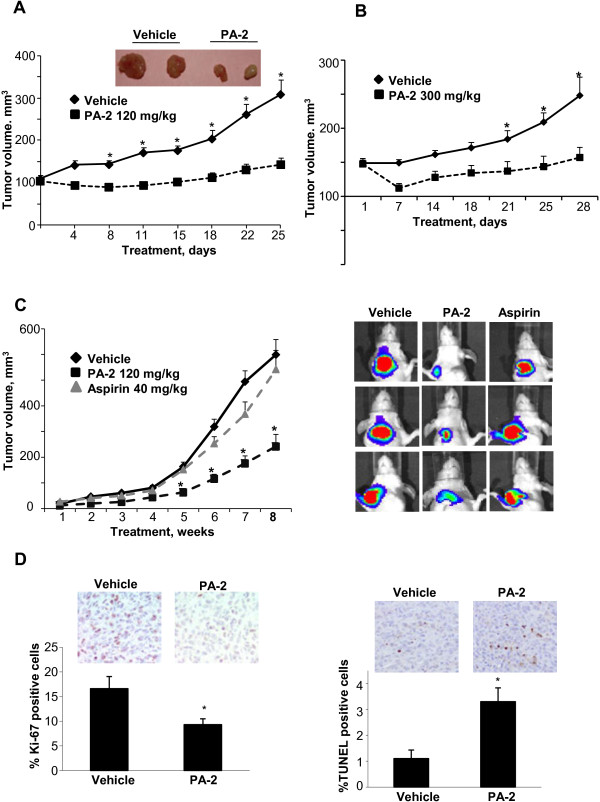
Figure 2.
Phospho-aspirin-2 inhibits the growth of TNBC xenografts. A: Chemotherapeutic effect of PA-2 on subcutaneous MDA-MB-231 xenografts in nude mice. Two representative tumors from each group are shown. *, p < 0.001, compared to vehicle; n = 10-16 tumors/group. B: Chemotherapeutic effect of PA-2 on subcutaneous BT-20 xenografts in nude mice. *, p < 0.01, compared to vehicle; n = 10-16 tumors/group. C: Chemopreventive effect of PA-2. Nude mice bearing orthotopic xenografts of MDA-MB-231 cells were treated with PA-2 or aspirin for 9 wks, starting 1 wk before cell implantation. The tumor volumes of the orthotopic MDA-MB-231 xenografts at sacrifice were determined by luciferase in vivo imaging as described in the methods section. Representative tumors from each group are shown. *, p < 0.05, compared to vehicle. D: Cytokinetic effect of PA-2 in MDA-MB-231 xenografts (treatment protocol). Left: Representative images (top) and the quantification (bottom) of Ki-67 expression in tumor sections, * p < 0.01. Right: Representative images (top) and the quantification (bottom) of TUNEL positive cells in tumor sections, * p < 0.002. All values are mean ± SEM.
We next evaluated the chemopreventive effect of PA-2 and compared it to aspirin, its parent compound. Following a prevention protocol, we treated nude mice bearing orthotopically implanted MDA-MB-231 xenografts with equimolar doses of PA-2 or aspirin starting 1 week before tumor implantation. On day 66 post-implantation, PA-2 inhibited the development of primary tumor in the mammary fat pads by 62% (p < 0.05; Figure 2C). In contrast, aspirin had no significant effect on breast tumor growth in this orthotopic model, consistent with previous findings [20].
We also determined cell proliferation and apoptosis in MDA-MB-231 xenografts in the treatment study (Figure 2A) using Ki-67 staining and TUNEL assay, respectively (Figure 2D). Compared to the vehicle, PA-2 inhibited cell proliferation by 44% (p < 0.01) and increased apoptosis by 3-fold (p < 0.002). This indicates that PA-2 also exerted a cytokinetic effect on TNBC xenografts in vivo.
PA-2 modulates the phosphorylation status of EGFR, p53 and NF-κB
To elucidate the mechanisms of action of PA-2, we performed antibody microarray analyses (Kinexus, Vancouver, Canada) on MDA-MB-231 cells treated with vehicle or PA-2 1.5 × IC50 for 2 h. This assay revealed pronounced changes in EGFR, p53 and NF-κB pathways following PA-2 treatment (Additional file 1: Table S1). Therefore, we further investigate the contribution of each of these pathways to the anti-cancer effect of PA-2.
PA-2 inhibits EGFR activation and its downstream signaling
EGFR is known to correlate with the progression of TNBC [11]. In MDA-MB-231 and BT-20 cells, PA-2 inhibited EGFR phosphorylation in a time-dependent manner, being evident as early as 1 h after treatment (Figure 3A). This observation was confirmed in vivo, where PA-2 reduced EGFR phosphorylation by 68% and 83% in MDA-MB-231 and BT-20 xenografts, respectively, compared to controls (p < 0.05, Figure 3A).
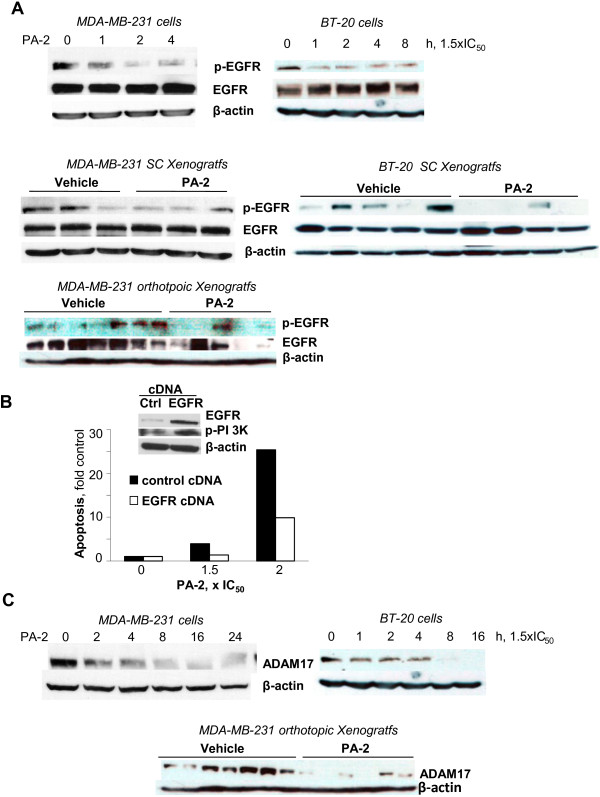
Figure 3.
Phospho-aspirin-2 inhibits EGFR phosphorylation. A: Upper: PA-2 1.5 × IC50 inhibited the expression of p-EGFR in MDA-MB-231 and BT-20 cells. Lower: PA-2 treatment inhibited the expression of p-EFGR in MDA-MB-231 and BT-20 xenografts (p < 0.05). B: Effect of PA-2 at various concentrations on apoptosis in EGFR overexpressing MDA-MB-231 cells or their mock transfected control. Western blot confirmed the overexpression of EGFR and increased levels of p-PI3K. C: PA-2 suppressed ADAM17 levels in MDA-MB-231 and BT-20 cells in vitro and in MDA-MB-231 orthotopic xenografts (p < 0.05). In all panels, immunoblots were performed with β-actin as loading control.
To determine the role of EGFR inhibition in the anticancer effect of PA-2, we transiently transfected MDA-MB-231 cells with an EGFR-overexpressing plasmid, and evaluated whether PA-2-induced cell death was affected. EGFR overexpression and activation of its downstream target p-PI3K was confirmed by western blot (Figure 3B). EGFR overexpression suppressed the induction of apoptosis by PA-2. Compared to mock transfected control, EGFR-overexpressing MDA-MB-231 cells have 2.5-fold reduction in the annexin V (+) fraction after treatment with PA-2 2xIC50 (Figure 3B). This indicates that EGFR inhibition is an important mechanism of action of PA-2, and the reversal of this effect mediates drug resistance.
An important upstream regulator of EGFR phosphorylation is ADAM17, which activates EGFR through a ligand cleavage mechanism [21]. We assessed the effect of PA-2 on ADAM proteins. As shown in Figure 2C, in MDA-MB231 and BT-20 cells, PA-2 reduced the expression of ADAM17. Moreover, PA-2 suppressed the levels of ADAM17 in MDA-MB-231 orthotopic xenografts.
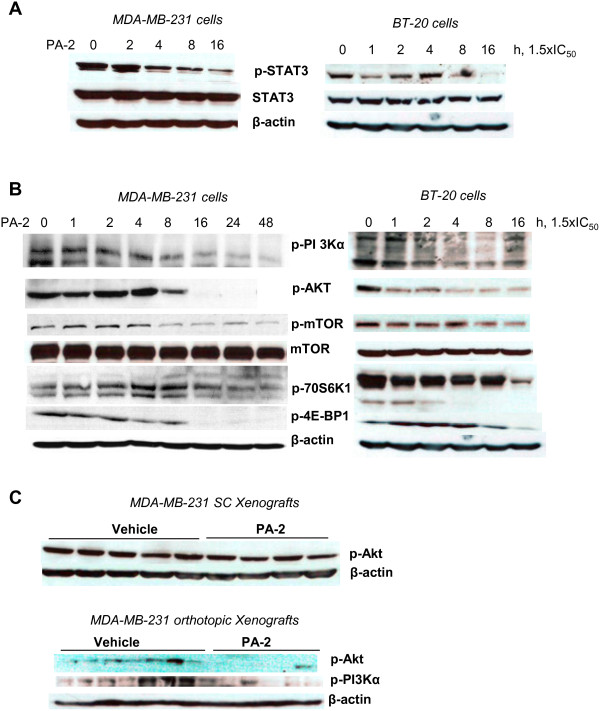
Inhibition of EGFR activation resulted in a potent inhibitory effect on its downstream signaling cascades, STAT3 and PI3K/Akt pathways. PA-2 reduced STAT3 phosphorylation in MDA-MB-231 and BT-20 cells (Figure 4A). PA-2 also suppressed the levels of p-PI3K and p-Akt in these cells in vitro (Figure 4B) and in MDA-MB-231 xenografts (Figure 4C). In addition, the downstream targets of PI3K/Akt pathway, including p-mTOR, p-4E-BP1 and p-70S6K1, were reduced after prolonged (16h) PA-2 treatment (Figure 4B). Hence, PA-2 triggered a temporal suppression of EGFR signaling cascades in TNBC.
Figure 4.
Phospho-aspirin-2 inhibits EGFR downstream signaling. A. PA-2 1.5 × IC50 inhibited STAT3 phosphorylation in MDA-MB-231 and BT-20 cells in a time-dependent manner. B: PA-2 treatment resulted in the sequential inactivation of PI3K signaling cascade, as indicated by the time-dependent reduction of the expression of p-PI3K, p-Akt, p-mTOR, p-p70S6K and p-4E-BP-1 in MDA-MB-231 and BT-20 cells. C: PA-2 reduced p-Akt expression in subcutaneous (treatment protocol) and orthotopic (prevention protocol) MDA-MB-231 xenografts. In all panels, immunoblots were performed with β-actin as loading control.
PA-2 induces acetylation of p53 and cell cycle arrest
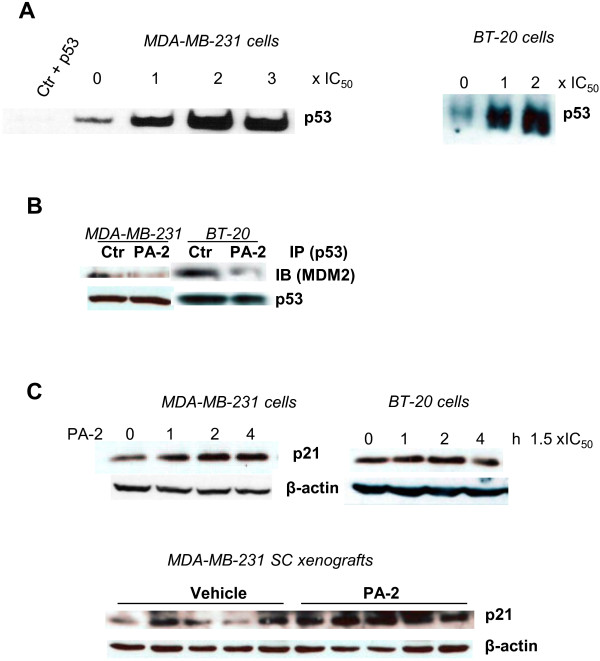
The tumor suppressor gene p53 is frequently inactivated in TNBC [22]. In MDA-MB-231 and BT-20 cells, PA-2 enhanced the DNA-binding activity of p53 in a concentration-dependent manner (Figure 5A). PA-2 did not appear to alter the nuclear shuttling of p53 (Figure 5A). On the other hand, immunoprecipitation showed that PA-2 significantly reduced the binding of p53 to murine double minute 2 (MDM2) (Figure 5B). Dissociation of p53 from MDM2, which otherwise binds to p53 and represses its transcriptional activity [23], may therefore contribute to the induction of p53 DNA binding activity by PA-2 in TNBC cells. The activation of p53 by PA-2 was consequential, as PA-2 blocked G1 to S cell cycle transition (Figure 1D) and up-regulated p21 in TNBC cells in vitro and in MDA-MB-231 xenografts (Figure 5C).
Figure 5.
Phospho-aspirin-2 induces p53 activity and p21. A: PA-2 1 × IC50-3 × IC50 increased the DNA binding activity of p53 in MDA-MB-231 and BT-20 cells, as determined by electrophoretic mobility shift assay. B: PA-2 1.5 × IC50 disrupted the interaction between p53 and MDM2 in MDA-MB-231 and BT-20 cells. Following treatment with PA-2, p53 was immunoprecipitated and the levels of MDM2 were determined. C: Upper: PA-2 1.5 × IC50 induced the expression of p21 in MDA-MB-231 and BT-20 cells. Lower: PA-2 induced the expression of p21 in MDA-MB-231 xenografts (treatment protocol).
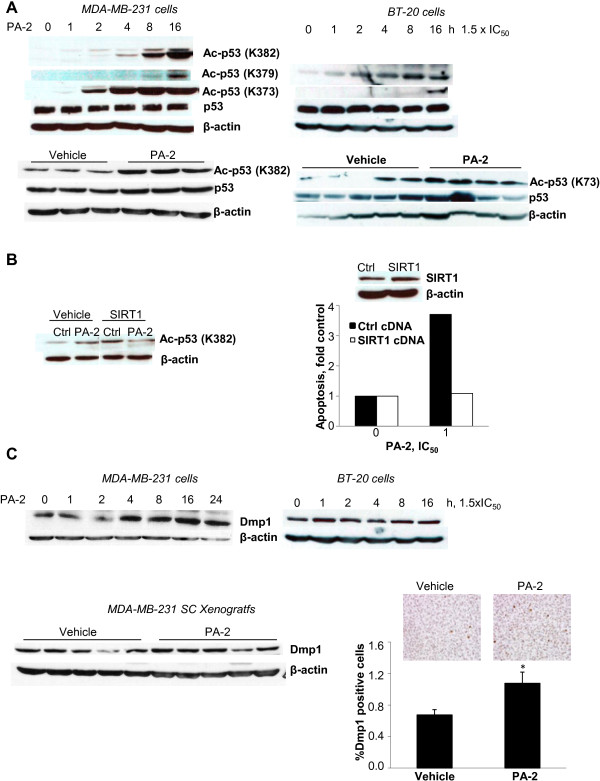
Acetylation of p53 at lysine residues is critical for its stability and transcriptional activity [24]. Given that the aspirin moiety of PA-2 contains an acetyl group capable of acetylating multiple proteins in cancer cells [25], we examined the effect of PA-2 on the acetylation status of p53. In MDA-MB-231 cells, PA-2 induced p53 acetylation at three distinct lysine residues (K373, K379 and K382) in a time-dependent manner; while in BT-20 cells PA-2 induced acetylation at K373 and K379 residues (Figure 6A). In MDA-MB-231 and BT-20 xenografts, treatment with PA-2 increased p53 acetylation at K382 and K373 residues, respectively (Figure 6A).
Figure 6.
Phospho-aspirin-2 induces p53 acetylation and Dmp1 expression. A: Upper: PA-2 induced the acetylation of p53 in MDA-MB-231 (K373, K379 and K382) and BT-20 (K373 and K379) cells. Lower: PA-2 induced p53 acetylation in MDA-MB-231 (K382) and BT-20 (K373) xenografts. B: Left panel: SIRT1 overexpression prevents p53 acetylation (K382) by PA-2. Right panel: SIRT1 overexpression attenuated PA-2-induced apoptosis in MDA-MB-231 cells. Western blot confirmed the overexpression of SIRT1. C: Upper: PA-2 1.5 x IC50 increased the expression of Dmp1 in MDA-MB-231 and BT-20 cells. Lower: PA-2 increased Dmp1 expression in xenografts, as indicated by western blot (left) and immunohistochemistry (right). Two representative tissue sections are shown. *, p < 0.02, compared to control; magnification 200X. Immunoblots were performed with β-actin as the loading control.
To further assess the role of p53 acetylation in cell death induction by PA-2, we overexpressed in MDA-MB-231 cells SIRT1, which negatively regulates p53 through its de-acetylation [26,27]. Overexpression of SIRT1 blocked the ability of PA-2 to acetylate p53 at the K382 residue (Figure 6B). Importantly, SIRT1 overexpression attenuated the induction of apoptosis in response to PA-2 by 71%, indicating that PA-2 induces apoptosis, at least in part, by a p53 acetylation-dependent mechanism (Figure 6B).
PA-2 may also regulate p53 independently of acetylation. PA-2 significantly enhanced the expression of Dmp1, a tumor suppressor that induces p53-dependent cell cycle arrest by directly binding to its promoter [28]. Such an effect is observed in TNBC cells in vitro. In MDA-MB-231 xenografts, PA-2 treatment increased Dmp1 expression by 57% (p < 0.02) compared to the control group (Figure 6C).
PA-2 induces RONS levels, inhibits the thioredoxin system and NF-κB activation
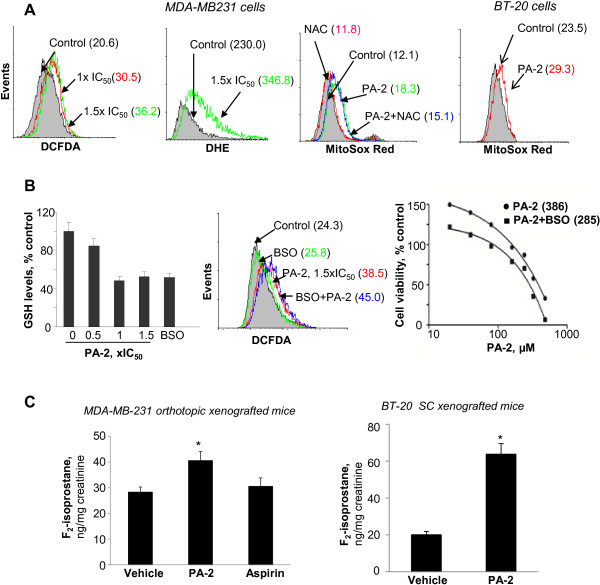
RONS play a significant role in the action of phospho-NSAIDs [29]. We determined the effect of PA-2 using various molecular probes: DCFDA (general RONS), DHE (cytoplasmic O2•−), and MitoSOX Red (mitochondrial O2•−). Compared to control, PA-2 1.5 × IC50 increased DCFDA by 76%, DHE by 51% and MitoSOX Red by 51% in MDA-MB-231 cells (Figure 7A). N-acetylcysteine (10 mM), a ROS scavenger, partly blocked ROS induction by 26% in MDA-MB-231 cells (Figure 7A). In BT-20 cells, PA-2 increased MitoSOX Red by 25%. PA-2 1-1.5 × IC50 also decreased the level of glutathione, a major cellular antioxidant. Co-incubation of PA-2 with BSO, an inhibitor of GSH synthesis, synergistically induced RONS levels and inhibited cell growth (Figure 7B).
Figure 7.
Phospho-aspirin-2 induces oxidative stress in TNBC. A: PA-2 induced RONS in MDA-MB-231 and BT-20 cells after 1h treatment, as determined by DCFDA, DHE and MitoSOX Red staining and flow cytometry. B: Left panel: GSH level was suppressed in MDA-MB-231 cells treated with various concentrations of PA-2 for 24 h, BSO as a positive control. Values are mean ± SEM. Middle panel: PA-2 and BSO synergistically induced RONS. RONS production was determined by DCFDA staining in MDA-MB-231 cells treated with PA-2 or PA-2 plus BSO for 1 h. Right panel: PA-2 and BSO synergistically inhibited cell growth. Cell growth inhibition was determined by MTT in MDA-MB-231 cells treated with PA-2 or PA-2 plus BSO for 24h. C: PA-2 increased the levels of 15-F2t-Isoprostane in 24-h urine from nude mice bearing orthotopic MDA-MB231 (p < 0.05) and subcutaneous BT-20 xenografts (p < 0.006), while aspirin had no effect. Urinary 15-F2t-Isoprostane was determined using an ELISA kit, as described in Methods.
To assess the effect of PA-2 on RONS in vivo, we measured urinary 15-F2t-isoprostane, a marker of oxidative stress [30,31], in the mice bearing TNBC xenografts. In orthotopic MDA-MB-231 xenografts (Figure 2C), 15-F2t-isoprostane levels on day 25 were 28.7 ± 3.3 ng/mg creatinine in controls and 40.9 ± 2.2 ng/mg creatinine in the PA-2 group, representing a nearly 40% increase (p < 0.05) (Figure 7C). In contrast, aspirin had no significant effect (p = 0.6). In BT-20 xenografts, PA-2 treatment increased 15-F2t-isoprostane levels by over 3-fold over the control (p < 0.006). Hence, PA-2, but not aspirin, induced RONS in vivo.
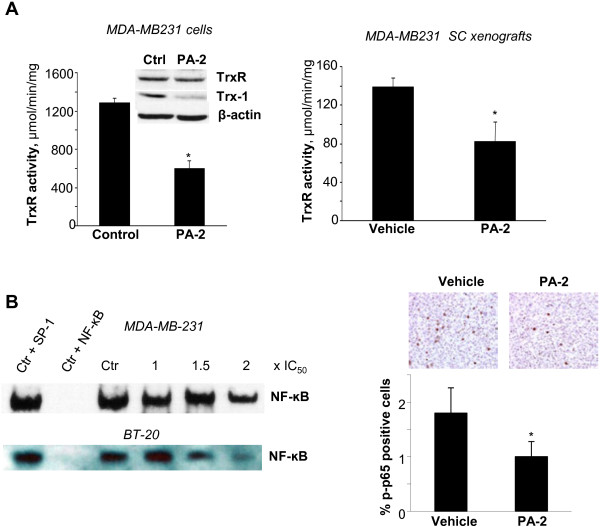
The thioredoxin (Trx) system, composed of thioredoxin reductase (TrxR) and Trx-1, plays an important role in redox homeostasis by reducing oxidized proteins; the latter is overexpressed in TNBC [32]. PA-2 inhibited TrxR activity in MDA-MB-231 cells in cell culture and MDA-MB-231 xenografts by 54% and 41%, respectively (p < 0.02-0.04), without affecting its levels (Figure 8A). PA-2 also significantly reduced Trx-1 levels in MDA-MB-231 cells after 1h treatment with 1.5 × IC50 PA-2 (Figure 8A). These results indicate that PA-2 targets major components of the Trx system.
Figure 8.
Phospho-aspirin-2 suppresses the thioredoxin (Trx) system and activation of NF-кB. A: Left panel: PA-2 1.5 × IC50 reduced TrxR activity in MDA-MB-231 cells after 1 h treatment. *, p < 0.02, compared to control. Immunoblots of TrxR and Trx-1 showed that PA-2 reduced the expression of Trx-1 in MDA-MB-231 cells. Right panel: PA-2 reduced TrxR activity in the protein lysates from MDA-MB-231 xenografts (treatment protocol) from animals treated with vehicle or PA-2 for 25 days. *, p < 0.04, compared to vehicle. B: Left panel: PA-2 inhibited constitutive NF-κB activation. EMSA for NF-κB of nuclear fractions isolated from MDA-MB-231 (upper) and BT-20 (lower) cells after 4 h treatment with or without PA-2 1.5 × IC50. To determine the specificity of the NF-κB transcription factor-DNA complex, the control nuclear fraction was incubated in the presence of 100-fold molar excess of unlabeled oligonucleotide containing the consensus sequence for either the specific (+NF-κB) or an unspecific (+AP-1) transcription factor. Right panel: NF-κB (p-p65) levels from MDA-MB-231 tumors, determined by immunohistochemistry using an anti-p-p65 antibody, were reduced in PA-2 treated group compared to the vehicle control. The percentage of p-p65-positive cells in various fields was determined and averaged for each xenograft. *, p < 0.0009, compared to vehicle. Representative images are shown; magnification 200X. All values are mean ± SEM.
The Trx system is closely linked with the NF-κB signaling pathway. Trx-1 enhances DNA binding of NF-κB by reducing the intermolecular Cys62 -S-S- bond of its p50 subunit [33]. We thus examined the effect on PA-2 on NF-κB activation. Consistent with its inhibitory effect on Trx-1 expression, PA-2 also inhibited NF-κB-DNA binding in a concentration-dependent manner in MDA-MB-231 and BT-20 cells (Figure 8B). A similar inhibition was observed in MDA-MB-231 xenografts. PA-2 reduced the levels of activated p-p65 by 44% (p < 0.009), compared to the control group (Figure 8B).
Discussion
Our data demonstrate that PA-2, a novel derivative of aspirin, effectively inhibits TNBC in preclinical models and is much more potent than aspirin, its parent compound. The anticancer activity of PA-2 is associated with a pronounced effect on a) EGFR activation; b) p53 acetylation and c) RONS induction. These signaling effects of PA-2 culminate in the substantial inhibition of cell proliferation and induction of apoptosis, the net effect of which is a strong reduction in TNBC xenograft growth in vivo.
Efficacy and safety are the prime considerations in the evaluation of anticancer agents [34], and the latter is especially paramount in chemoprevention. Extensive epidemiological and clinical evidence supports a beneficial role of aspirin in the prevention of breast, colon, and lung cancers [35]; however, its gastrointestinal toxicity is limiting. Our results demonstrated that PA-2, with the novel phospho-modification, is superior to aspirin both in terms of efficacy in TNBC, as well as safety [9]. PA-2, but not aspirin, administered in a chemoprevention protocol slowed the development of orthotopic MDA-MB-231 xenografts.
PA-2 is highly efficacious when used in the treatment setting and its chemotherapeutic effect is even stronger than its chemopreventive effect. PA-2 potently inhibits TNBC in vivo, almost completely arresting the growth of both MDA-MB-231 and BT-20 xenografts. Consistent with its strong chemotherapeutic efficacy, PA-2 induced a profound cytokinetic effect involving inhibition of cell proliferation and induction of apoptosis. Hence, PA-2 is a promising anticancer candidate that merits further evaluation.
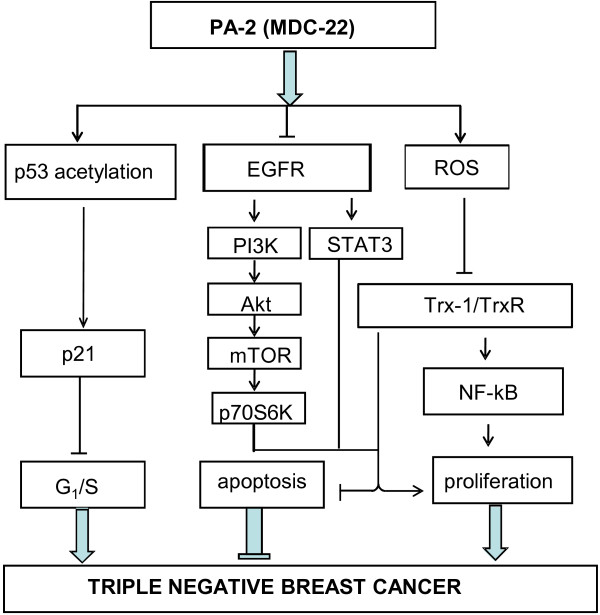
Our work identified EGFR, p53 and RONS as the major signaling mechanisms (Figure 9) involved in eliciting the growth inhibitory effect of PA-2. EGFR is overexpressed in 16–48% of breast cancers and its expression is associated with poor prognosis [36,37]. In particular, EGFR is frequently overexpressed in TNBC, a subset of breast cancer that is characterized by their unique molecular profile, aggressive behavior and distinct patterns of metastasis [38]. PA-2 is an inhibitor of EGFR phosphorylation in vitro and in vivo. The inhibitory effect of PA-2 appears to be mediated by a novel mechanism involving the inhibition of ADAM17, a major protease that controls availability of EGFR ligands [39]. A key consequence of PA-2-induced inhibition of EGFR is the suppression of pro-survival STAT3 and PI3K and the sequential inactivation of their downstream signaling propagation, thereby causing growth inhibition and cell death.
Figure 9.
Proposed mechanism for the anticancer effect of phospho-aspirin-2 in TNBC. PA-2 inhibits TNBC through (i) inhibition of EGFR phosphorylation and attenuation of downstream signaling cascades (STAT3 and PI3K/Akt); (ii) acetylation of p53, which enhances p53 DNA-binding activity, p21 expression and cell cycle arrest; and (iii) induction of oxidative stress and alteration of the Trx system; which culminate in the inhibition of cell proliferation and induction of apoptosis in TNBC.
Mutations of p53 are exceptionally frequent in TNBC (>80%) and its inactivation predicts poor survival in TNBC patients [10,12]. Activation-inactivation of p53 depends on a repertoire of post-translational modifications [40], including phosphorylation and acetylation. p53 acetylation was found to be indispensable for its activation, as it destabilizes the p53-MDM2 interaction, thereby abrogating MDM2-mediated transcription repression [24]. Here, we show that PA-2 acetylates p53 in vitro and in vivo and disrupts its association with MDM2, which in turn, enhances p53-DNA binding activity. Forced deacetylation of p53 partly attenuated cell death induction by PA-2, indicating an important role of p53 acetylation in mediating the effect of PA-2. PA-2 additionally regulates p53 independently of acetylation. Dmp1 is a transcription factor that physically interacts with p53 [41]. Dmp1-p53 binding antagonizes the ubiquitination of p53 byMDM-2 and promotes the nuclear translocation of p53 [28]. PA-2 enhanced the expression of Dmp1 in TNBC cells and in xenografts, which may further contribute to the robust p53 activation triggered by PA-2.
The induction of oxidative stress plays a key role in the anticancer effect of structurally related phospho-NSAIDs [9,42]. PA-2 induces oxidative stress in TNBC cells in culture and in xenografts by compromising cellular antioxidant defense mechanisms. First, PA-2 suppressed the Trx system, with both TrxR and Trx-1 affected in a significant way. TrxR and Trx-1 are critical components of the cellular redox system [43]. Trx-1 reduces client proteins oxidized by RONS, with itself undergoing oxidation, while TrxR regenerates the reduced Trx-1. In TNBC cells and xenografts, PA-2 inhibited TrxR activity and decreased the expression of Trx-1. Second, PA-2 significantly reduced intracellular levels of GSH, the major chemical antioxidant of mammalian cells. Induction of oxidative stress has major repercussions on redox- and Trx-dependent signaling cascades, as exemplified by the inhibitory effect on NF-кB. NF-кB is constitutively active in TNBC [44], and its aberrant activation is linked to inflammation and cancer. Induction of oxidative stress and the subsequent inhibition of NF-кB by PA-2 may mediate part of its growth inhibitory effect.
It is of interest that PA-2 has superior efficacy compared to conventional aspirin, the starting compound for its synthesis. The reasons for this difference are not entirely clear, but as a new chemical entity, PA-2 is expected to have distinct properties from its parent compound. Perhaps the most relevant property of these two compounds is their ability to induce oxidative stress, an effect that accounts for much of the anticancer efficacy of phospho-NSAIDs, including phospho-aspirin [29]. Indeed, PA-2 caused robust oxidative stress in mice, reflected in enhanced urinary levels of the biomarker F2-isoprostane. In contrast, aspirin failed to produce such an effect, and this difference explains to a large extent their differential efficacy. Additional mechanistic differences might also contribute, especially with regards to effects on cell signaling cascades.
Conclusions
Our work indicates that PA-2 possesses chemotherapeutic and chemopreventive efficacy against TNBC in preclinical models, and establishes inhibition of EGFR, acetylation of p53 and induction of oxidative stress as critical mediators of its mechanism of action (Figure 9). The multi-targeted nature of PA-2 towards the dysregulated signaling cascades in TNBC further suggests the notion that PA-2 may be a promising therapeutic option, either alone or in combination with other therapies.
Abbreviations
ADAM: A disintegrin and metalloproteinase; Akt: Protein kinase B; EGFR: Epidermal growth factor receptor; ER: Estrogen receptor; mTOR: Mammalian target of rapamycin; NSAID: Nonsteroidal anti-inflammatory drug; NF-ΚB: Nuclear factor kappa-light-chain-enhancer of activated B cells; PA-2: Phospho-aspirin-2; PI3K: Phosphoinositide 3-kinase; PR: Progesterone receptor; RONS: Reactive oxygen and nitrogen species; SIRT1: Sirtuin; STAT3: Signal transducer and activator of transcription 3; Trx-1: Thioredoxin-1; TrxR: Thioredoxin reductase; SC: Subcutaneous.
Competing interests
The authors have nothing to disclose except for BR, who has an equity position in Medicon Pharmaceuticals, Inc. and NO who is an employee for the same.
Authors’ contributions
LH conceived the study, participated in its design, carried out most of the in vitro and in vivo studies, analyzed the data and participated in the preparation of the manuscript. YS participated in the study design, performed the orthotopic transplantation of breast cancer cells and involved in the drafting the manucript; CCW, GGM, KWC participated in the study design, data analyses and writing of the manuscript. KV synthesized batches of PA-2, participated in the study design and data analysis. NA performed the luciferase imaging of breast tumors, participated in data collection and data analysis. NO performed the immunohistochemical analyses, participated in data collection and data analysis. BR participated in the study design, supervised the work, analyzed data and participated in writing the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Antibody microarray analysis on phosphor-aspirin treated MDA-MB-231 cells. TNBC MDA-MB-231 cells treated with vehicle or PA-2 1.5 × IC50 for 2 h were performed antibody microarray analysis by Kinexus (Vancouver, CA).
Contributor Information
Liqun Huang, Email: liqun.huang@stonybrookmedicine.edu.
Chi C Wong, Email: chichun.wong@stonybrookmedicine.edu.
Gerardo G Mackenzie, Email: gerardo.mackenzie@stonybrookmedicine.edu.
Yu Sun, Email: yu.sun@stonybrookmedicine.edu.
Ka Wing Cheng, Email: kawing.cheng@stonybrookmedicine.edu.
Kvetoslava Vrankova, Email: k.vrankova@googlemail.com.
Ninche Alston, Email: alston@pharm.stonybrook.edu.
Nengtai Ouyang, Email: nengtai.ouyang@stonybrookmedicine.edu.
Basil Rigas, Email: basil.rigas@stonybrookmedicine.edu.
Acknowledgement
Grant support: NIH grants R01-CA139453, R01CA13945402 and 1N01CN43302WA22 and HHSN261201000109C.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Rinsema TJ. One hundred years of aspirin. Med Hist. 1999;43(4):502–507. doi: 10.1017/S0025727300065728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Hegedus R. Aspirin–the first hundred years. Biologist (London) 2000;47(5):269–271. [PubMed] [Google Scholar]
- Baron JA. What now for aspirin and cancer prevention? J Natl Cancer Inst. 2004;96(1):4–5. doi: 10.1093/jnci/djh027. [DOI] [PubMed] [Google Scholar]
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- Marshall SF, Bernstein L, Anton-Culver H, Deapen D, Horn-Ross PL, Mohrenweiser H, Peel D, Pinder R, Purdie DM, Reynolds P, Stram D, West D, Wright WE, Ziogas A, Ross RK. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst. 2005;97(11):805–812. doi: 10.1093/jnci/dji140. [DOI] [PubMed] [Google Scholar]
- Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, Thaiparambil J, Coward L, Gorman G, Li Y, Sani B, Hobrath JV, Maxuitenko YY, Reynolds RC. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2009;2(6):572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Cheng KW, Xie G, Zhou D, Zhu CH, Constantinides PP, Rigas B. Carboxylesterases 1 and 2 hydrolyze phospho-nonsteroidal anti-inflammatory drugs: relevance to their pharmacological activity. J Pharmacol Exp Ther. 2012;340(2):422–432. doi: 10.1124/jpet.111.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Mackenzie G, Ouyang N, Sun Y, Xie G, Johnson F, Komninou D, Rigas B. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;162(7):1521–1533. doi: 10.1111/j.1476-5381.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasco M, Shami S, Crook T. The p53 pathway in breast cancer. Breast Cancer Res. 2002;4(2):70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59(21):5475–5478. [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancey JE, Freidlin B. Targeting epidermal growth factor receptor–are we missing the mark? Lancet. 2003;362(9377):62–64. doi: 10.1016/S0140-6736(03)13810-X. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Zhao W, Mackenzie GG, Murray OT, Zhang Z, Rigas B. Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect. Carcinogenesis. 2009;30(3):512–519. doi: 10.1093/carcin/bgp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, Johnson F, Komninou D, Kopelovich L, Rigas B. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139(4):1320–1332. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104(8):2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR){delta} expression in APCmin/+ mice proportionally to their tumor inhibitory effect: Implications for the role of PPAR{delta} in carcinogenesis. Carcinogenesis. 2006;27(2):232–239. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- Rigas B, Kozoni V. The novel phenylester anticancer compounds: Study of a derivative of aspirin (phoshoaspirin) Int J Oncol. 2008;32(1):97–100. [PubMed] [Google Scholar]
- Likhite V. Central Reginoal Meeting of the American Chemical Society. Cleveland, Ohio; 2009. Aspirin and Breast Cancer: Studies In Mice. [Google Scholar]
- Baumgart A, Seidl S, Vlachou P, Michel L, Mitova N, Schatz N, Specht K, Koch I, Schuster T, Grundler R, Kremer M, Fend F, Siveke JT, Peschel C, Duyster J, Dechow T. ADAM17 regulates epidermal growth factor receptor expression through the activation of Notch1 in non-small cell lung cancer. Cancer Res. 2010;70(13):5368–5378. doi: 10.1158/0008-5472.CAN-09-3763. [DOI] [PubMed] [Google Scholar]
- Soussi T, Lozano G. p53 mutation heterogeneity in cancer. Biochem Biophys Res Commun. 2005;331(3):834–842. doi: 10.1016/j.bbrc.2005.03.190. [DOI] [PubMed] [Google Scholar]
- Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol. 2009;1(6):a000950. doi: 10.1101/cshperspect.a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimuthu S, Chivukula RS, Alfonso LF, Moridani M, Hagen FK, Bhat GJ. Aspirin acetylates multiple cellular proteins in HCT-116 colon cancer cells: identification of novel targets. Int J Oncol. 2011;39(5):1273–1283. doi: 10.3892/ijo.2011.1113. [DOI] [PubMed] [Google Scholar]
- Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao TP. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21(22):6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, Huber LJ. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26(1):28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja P, Maglic D, Kai F, Sugiyama T, Kendig RD, Frazier DP, Willingham MC, Inoue K. Critical roles of DMP1 in human epidermal growth factor receptor 2/neu-Arf-p53 signaling and breast cancer development. Cancer Res. 2010;70(22):9084–9094. doi: 10.1158/0008-5472.CAN-10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Huang L, Mackenzie GG, Rigas B. Oxidative stress mediates through apoptosis the anticancer effect of phospho-nonsteroidal anti-inflammatory drugs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther. 2011;338(3):775–783. doi: 10.1124/jpet.111.183533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10(8):1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- Tacconelli S, Capone ML, Patrignani P. Measurement of 8-iso-prostaglandin F2alpha in biological fluids as a measure of lipid peroxidation. Methods Mol Biol. 2010;644:165–178. doi: 10.1007/978-1-59745-364-6_14. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol. 2008;81(Spec No 1):S57–S68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20(15):3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Cheng KW, Rigas B. Preclinical predictors of anticancer drug efficacy: critical assessment with emphasis on whether nanomolar potency should be required of candidate agents. J Pharmacol Exp Ther. 2012;341(3):572–578. doi: 10.1124/jpet.112.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, Thun M. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Kataoka A, Ohno S, Murakami S, Kinoshita J, Hachitanda Y. Prognostic and predictive value of epidermal growth factor receptor in recurrent breast cancer. Clin Cancer Res. 2002;8(11):3454–3460. [PubMed] [Google Scholar]
- Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71(1):67–75. doi: 10.1023/A:1013397232011. [DOI] [PubMed] [Google Scholar]
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2008;13(2):181–194. doi: 10.1007/s10911-008-9084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walerych D, Napoli M, Collavin L, Del Sal G. The rebel angel: mutant p53 as the driving oncogene in breast cancer. Carcinogenesis. 2012;33(11):2007–2017. doi: 10.1093/carcin/bgs232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier DP, Kendig RD, Kai F, Maglic D, Sugiyama T, Morgan RL, Fry EA, Lagedrost SJ, Sui G, Inoue K. Dmp1 physically interacts with p53 and positively regulates p53′s stability, nuclear localization, and function. Cancer Res. 2012;72(7):1740–1750. doi: 10.1158/0008-5472.CAN-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68(20):8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y, Nakamura H, Yodoi J, Kato K, Noguchi S. High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin Cancer Res. 2005;11(23):8425–8430. doi: 10.1158/1078-0432.CCR-05-0449. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Taguchi Y, Ito-Kureha T, Semba K, Yamaguchi N, Inoue J. NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat Commun. 2013;4:2299. doi: 10.1038/ncomms3299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibody microarray analysis on phosphor-aspirin treated MDA-MB-231 cells. TNBC MDA-MB-231 cells treated with vehicle or PA-2 1.5 × IC50 for 2 h were performed antibody microarray analysis by Kinexus (Vancouver, CA).