Abstract
Several chemicals that are used by humans, such as pesticides and plastics, are released into the aquatic environment through wastewater and runoff and have been shown to be potent disruptors of androgen synthesis at high concentrations. Although many of these chemicals have been studied in isolation, a large amount of uncertainty remains over how fish respond to low concentrations of anti-androgenic mixtures, which more accurately reflects how such chemicals are present in the aquatic environment. In this study male fathead minnows (FHM) (Pimephales promelas) were exposed to environmentally relevant concentrations of two anti-androgens, the herbicide linuron, and the plasticizer di(2-ethylhexyl) phthalate (DEHP) individually and as part of a mixture of the two for a 28-day period. At the end of this period there was a reduction in plasma testosterone (T) concentrations in male FHM exposed to the mixture, but not in FHM exposed individually to linuron or DEHP or the control FHM. There was also a significant reduction in 17β-estradiol (E2) in the DEHP-only and mixture exposed groups as compared to the control. Contrary to what has been previously published for these two chemicals in mammals, the lower plasma T concentrations in male FHM exposed to the mixture was not a result of the inhibition of genes involved in steroidogenesis; nor due to an increase in the expression of genes associated with peroxisome proliferation. Rather, an increase in relative transcript abundance for CYP3A4 in the liver and androgen-and estrogen-specific SULT2A1 and SULT1st2 in the testes provides evidence that the decrease in plasma T and E2 may be linked to increased steroid catabolism. Feedback from the pituitary is not repressed as the relative expression of follicle stimulating hormone β-subunit mRNA transcript levels in the brain was significantly higher in both DEHP and mixture exposed FHM. In addition, luteinizing hormone β-subunit mRNA transcript levels increased but were not significant in the mixture as compared to the control. Hormone receptor mRNA transcript levels in the liver and testes were not significantly different across all four exposure groups. This study highlights the importance of assessing environmentally relevant concentrations of mixtures when determining risk to aquatic organisms.
Keywords: Phthalates, Herbicides, Anti-androgen, Fathead minnow, Endocrine disruption, Low-dose
1. Introduction
Many fish populations are continually exposed to a wide variety of contaminants that can exert deleterious effects on endocrine function. In particular, recent monitoring programs have highlighted the occurrence of anti-androgenic chemicals as part of complex mixtures in the environment (Hill et al., 2010). Compounds that inhibit androgen-signaling can act through several modes of action: (1) competitively binding to the androgen receptor (AR), thus inhibiting the transcription of androgen-dependent genes; (2) modifying the production of androgens through the inhibition of rate-limiting genes and enzymes involved in steroidogenesis; or (3) increasing the degradation of androgen precursors and testosterone (T) in the testes or liver. In all three cases, the compound(s) disrupts one or multiple biologic pathways along the hypothalamic-pituitary-gonadal axis (HPG). Previous research has shown that exposure to individual anti-androgenic compounds, such as vinclozolin and prochloraz, reduce fecundity, alter secondary sex characteristics, impact gonadal morphology, and lower plasma T concentrations (Ankley et al., 2009a, 2009b; Martinović et al., 2008). Although many of these chemicals have been studied in isolation, a large amount of uncertainty remains regarding the response of fish to low concentrations of anti-androgenic mixtures, a more accurate representation of the types of exposures that occur in the aquatic environment.
Studies that have examined the effects of mixtures of endocrine-disrupting compounds (EDCs) on fish have focused mostly on estrogenic compounds. The literature on these effects is somewhat contradictory. Whereas a number of studies have suggested an additive effect (Brian et al., 2005; Correia et al., 2007; Zhang et al., 2009), other studies have found that the mRNA expression profile of the individual chemical differs from that of the mixture in both the genes expressed and the level of expression (Filby et al., 2007; Finne et al., 2007). In addition, dose can influence genomic response with some chemicals (Clewell et al., 2011; Gentry et al., 2010; Hockley et al., 2006) but the impact of exposure dose on gene expression related to endocrine function is unknown. Differences could have a significant impact on conclusions regarding endocrine disruption potential and in environmental monitoring for endocrine disruption where the exposure from an individual or mixture of chemicals is at a concentration that is below the level in which current biomarker responses occur.
This study exposes male FHM to two anti-androgenic chemicals at concentrations reported in the environment, the herbicide linuron and di(2-ethylhexyl) phthalate (DEHP), individually and as part of a mixture to assess the changes in plasma hormone levels and mRNA expression along the HPG axis. The urea-based herbicide linuron is used on a variety of agricultural crops and inhibits photosynthesis in broadleaf weeds. It has been found in aquatic habitats up to 1 μg L−1 (ppb) (Woudneh et al., 2009). Linuron is considered a mixed-effect anti-androgen as it has been shown to competitively bind with the androgen receptor, and inhibit cytochrome P450 (CYP)17 lyase production, which in turn decreases T production in rats (Lambright et al., 2000; Wilson et al., 2009). In FHM, in vitro assays have shown that linuron competitively binds to the ARfhm (Foster, 2006), but it is still unknown whether linuron inhibits CYP17 expression. In fish, the inhibition of testosterone production from exposure to mixed-effect anti-androgens may be compound-and species-specific. Carp (Cyprinus carpio) testes and liver exposed to a similar mixed-effect anti-androgen diuron, saw no change to testosterone synthesis and metabolism, but there was a decrease in testosterone production in FHM exposed to another mixed-effect anti-androgen prochloraz (Ankley et al., 2009b; Thibaut and Porte, 2004).
DEHP is a commonly used plasticizer that is pervasive in the aquatic environment. It has been estimated to occur normally between 2 and 3 μg L−1, but has been measured at concentrations as high as 98 μg L−1 (Frömme et al., 2002). Although previous research indicated that a variety of phthalates, including DEHP, were estrogenic in mammals, it is now thought that phthalates, act mostly as anti-androgenic endocrine disruptors (Jobling et al., 1995; Liu et al., 2005). In rats, DEHP and several other phthalates have been shown to decrease fetal testis T production and reduce the expression of steroidogenic genes, such as Steroidogenic Acute Regulatory protein (StAR), CYP17 and CYP11a (Foster, 2006). DEHP has not been shown to be an androgen receptor antagonist or interfere with the genetic expression of the androgen receptor mRNA transcript (Gray et al., 2000). In fish, there has been little research performed on the effects of DEHP or phthalates in general on hormone levels or gene expression in adult male fish. DEHP has been shown to decrease 5′-reductase activity, which in turn decreases the synthesis of 5′-androsteindione and 5′-dihydrotestosterone in the testes of male carp (Thibaut and Porte, 2004). High doses of DEHP (5000 mg/kg) increased peroxisome proliferation in the testes, but has not been shown to inhibit expression of genes involved in steroidogenesis (Uren-Webster et al., 2010). In the same study, although there was no difference in the relative expression of ER1, ER2a, and ER2b mRNA transcripts in the liver and testes, there was a significant increase in vitellogenin mRNA transcripts levels, which would indicate an estrogenic effect at high-levels of DEHP exposure. Dibutyl phthalate has been shown to increase plasma T concentrations, but decrease androgen associated spiggin concentrations in male sticklebacks (Gasterosteus aculetaus) (Aoki et al., 2011). In both of these studies the relative expression of steroidogenic genes were unchanged. Therefore it is still unclear what the mechanism by which DEHP or other phthalates act on androgen synthesis and androgen responsive gene expression in fish.
The goal of this study was (1) to examine whether there is a significant change in endocrine function when male FHM are exposed to environmentally-relevant concentrations of linuron or DEHP. (2) How mixtures of these chemicals influence endocrine function and (3) to determine the mechanism by which changes in endocrine function may occur, and specifically if this is due to an inhibition of steroidogenesis or some other mechanism of action (Fig. 1). Plasma T and 17β-estradiol (E2) concentrations were measured to assess changes in hormone production. Gonadosomatic index (GSI) was measured to assess changes in testes size. The relative mRNA transcript level of luteinizing hormone β-subunit (LHβ) and follicle-stimulating hormone β-subunit (FSHβ) in the brain were measured to assess changes in the feedback response mechanism in the HPG axis that would be associated with changes in T and E2 production. The relative mRNA transcript levels of StAR, CYP11a, 3β-hydroxysteroid dehydrogenase (3β-HSD), CYP17, and 17β-HSD were measured in the testes, as all five proteins are necessary for testosterone synthesis. The relative mRNA transcript level of aromatase (CYP19a1), which is responsible for converting T to E2 was also measured in the testes to assess potential changes in E2 production. The relative mRNA transcript levels of three hormone receptors, AR, estrogen receptor (ER)1 and ER2b, were measured in both the testes and liver to assess changes to receptor expression that may be due to exposure to anti-androgenic compound(s). Increased peroxisome proliferation in the testes was assessed by measuring mRNA transcript levels of peroxisome proliferator-activated receptor-alpha (PPARα) as well as the PPARα-responsive genes acyl-coenzyme A oxidase1 (ACOX1) and enoyl-coenzyme A, hydratase/3-hydroxyacyl coenzymeA dehydrogenase (EHHADH) in the testis. Increased expression of ACOX1 and EHHADH are associated with peroxisome proliferation and has been measured previously in fish (Uren-Webster et al., 2010). Plasma hormone concentrations can partially be mediated by changes in hormone degradation rates that occur primarily in the liver, but also locally in the testes. Therefore, the relative mRNA transcript levels of phase I-metabolizing enzyme cytochrome P450 3A4 (CYP3A4), androgen-specific phase II-metabolizing enzyme sulfotransferase dehydrogenase 2A1 (SULT2A1) and UDP glucuronosyltransferase 2B15 (UGT2B15), and estrogen-specific phase II-metabolizing enzyme SULT1st2 were measured in both the testes and the liver. Finally, the mRNA transcript level of the pregnane X-receptor (PXR) was measured in the testes and liver because PXR has been shown to bind to DEHP and all four of these phase I and II enzymes are in part transcriptionally regulated by the PXR (Bélanger et al., 1998; Cooper et al., 2008; Sonoda et al., 2002; Yamazaki and Shimada, 1997).
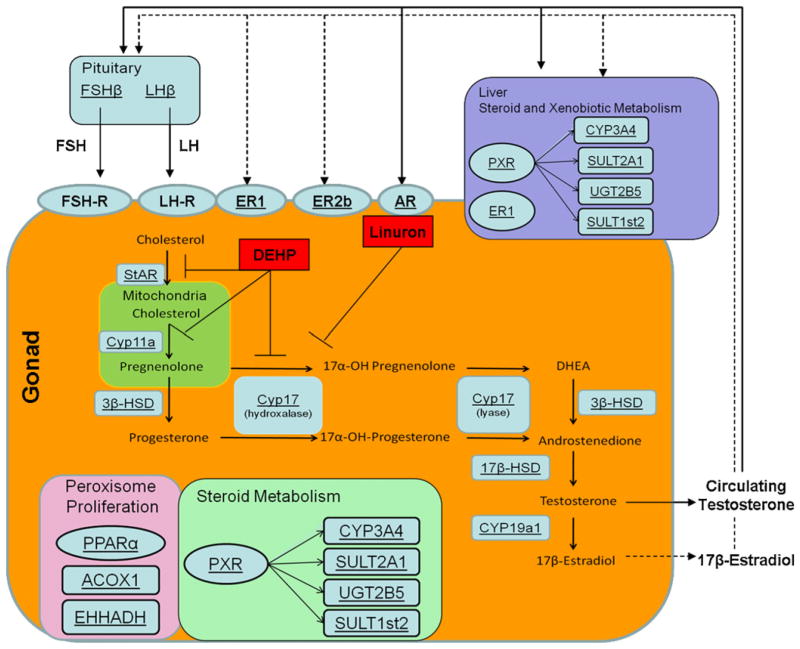
Fig. 1.
Diagram of the organs and genes involved in testosterone production and metabolism. mRNA transcript levels of genes involved in these process are underlined. Specific inhibitory targets of DEHP and linuron are shown.
2. Methods
2.1. Chemicals
Both linuron and DEHP were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). All other reagents used were of analytical grade. A 2.4 ppm stock solution of linuron, was created by dissolving 2.4 mg of linuron in 40 μL of methanol, and then pipetting this mixture into 1 L of reagent grade water. The 1 L stock solution was then added directly into 23 L of dechlorinated water in an exposure tank. For DEHP exposure, 0.288 μL of liquid DEHP stock solution (1 g/mL) purchased from Sigma–Aldrich was added to 1 L of reagent grade water, vigorously shaken for 5 min, and then added directly into 23 L of dechlorinated water in an exposure tank. For the mixture exposure, stock solutions of both linuron and DEHP were prepared as stated previously and added to 22 L of dechlorinated water in an exposure tank.
2.2. Exposure study
Adult male FHM were obtained from a laboratory culture at the Great Lakes WATER Institute (Milwaukee, WI). A total of 40 male FHM, divided evenly among the four exposures, were used in the 28-day trial. For each exposure 10 male FHM placed in a single 24 L tank filled with dechlorinated water (pH 6.8, total hardness (CaCO3) = 120), and were acclimatized for 2 days before exposure. A full-spectrum bulb provided a 16:8 h light:dark cycle, and a water temperature of 24 °C was kept throughout the 28-day experiment. Fish were fed TetraMin (Tetra, Blacksburg, VA), a commercially bought flake fish food, once daily throughout the exposure study. Male FHM were exposed to one of four treatments: control (methanol), 1 μg L−1 dose of linuron, 12 μg L−1 dose of DEHP, or 1 μg L−1 dose of linuron plus 12 μg L−1 dose of DEHP. To account for exposure to the methanol vehicle, methanol levels were made constant among all treatment groups by adding 40 μL of methanol was added to 1 L of reagent grade water and then 2.4 μL of the methanol solution was added to the control and DEHP treatments. Water exchange occurred every three days, at which time new solutions of methanol, linuron and DEHP were added. At the end of the exposure period there were 35 fish remaining (N = control: 10, linuron: 8, DEHP: 9, mixture: 8).
After 28 days, the fish were euthanized and whole body and testes were weighed to the nearest 0.1 mg using an analytical scale. The GSI of each fish was determined by dividing the weight of the organ by the weight of the fish and then multiplying by 100. Blood was drawn and centrifuged to collect plasma, and the testes, liver and brain, which included the pituitary, were removed. Plasma, testes, liver and brain were flash frozen in liquid nitrogen and stored at −80 °C for later use.
2.3. Gene expression
RNA was extracted from gonad, liver, and brain samples using TriZol reagent (Invitrogen, Carlsbad, CA), following the instruction of the manufacturer. Samples were cleaned to remove any solvent contamination by precipitating RNA with a 1 M NaCl aqueous solution, and washing the precipitate with ethanol. RNA was then dissolved in 40 μL of water, and stored at −80 °C. RNA concentrations (ng/μL) were measured using a Nanodrop spectrophotometer (Thermo Fischer Scientific, Wilmington, DE). Degradation of RNA was assessed on a BioAnalyzer (Agilent, Santa Clara, CA). FHM testes, liver and brain RNA were DNAse treated (Promega, Madison, WI) prior to cDNA creation. For each tissue sample, cDNA was synthesized from 500 ng/μL of FHM testes, liver and brain RNA.
Partial mRNA sequences for all 20 genes were identified in the National Center for Biotechnology Information database (Table 1). Primers were designed using the PrimerQuest software from Integrated DNA Technologies (Coralville, IA), and then ordered from their site. Primer sequences are listed in Table 1. For FHM equivalent SULT2A1, SULT1st2 and UGT2B15, the genes were identified from zebrafish genome database (zebrafish SULT2st1 GenBank accession no. NM 198914, SULT1st2 GenBank accession no. NM 183347.2, UGT2B5 GenBank accession no. NM 001177345.1). These three genes have been identified as homologs to human SULT2A1 (Sugahara et al., 2003) and UGT2B15 (Huang and Wu, 2010) or in the case of SULT1st2 responsible for 17β-estradiol and estrone degradation in zebrafish (Sugahara et al., 2003).
Table 1.
List of primer sequences, function and organ measured.
| Gene | GenBank accession no. | Sequence | Organ measured |
|---|---|---|---|
| Hormone receptors | |||
| ER1 | AY775183.1 | FWD:5′-ATCACCATGATGTCCCTGCTCACA-3′ | Testes, Liver |
| REV: 5′-AGCCAAGAGCTCTCCAACAACTGA-3′ | |||
| ER2b | AY566178.1 | FWD:5′-GGTGCAAGGCTTTCTTCAAACGGA-3′ | Testes |
| REV: 5′-TTGTCAATGGTGCACTGGTTGGTG-3′ | |||
| AR | AY727529.1 | FWD:5′-AACGAGTTGGGAGAGAGGCAACTT-3′ | Testes |
| REV: 5′-ATCTCCAACCCAGAGCAAAGACCA-3′ | |||
| Steroidogenesis | |||
| StAR | DQ360497.1 | FWD:5′-AAACTGCGTGCTGGCATTTCCTAC-3′ | Testes |
| REV: 5′-TGCTCAGCTTCACTGAACGTCTCT-3′ | |||
| CYP11a | DQ360498.1 | FWD:5′-TGGTGTGCTGGCTAGCCTTCTAAT-3′ | Testes |
| REV: 5′-AGGTGATACGAGCAGCCGAGATTT-3′ | |||
| 3β-HSD | DT361291.1 | FWD:5′-GCTGTCAATGTACAAGGGACGGA-3′ | Testes |
| REV: 5′-TTGGTCTTTGGGTAGGGCATCTCA-3′ | |||
| CYP17 | AJ277867.1 | FWD:5′-AGGCTCTGGCAAAGATGGAACTCT-3′ | Testes |
| REV: 5′-AAGAACCACACCAAACTTGCCCTG-3′ | |||
| 17β-HSD | DT161033.1 | FWD:5′-TGGAGGCAAGAGCTAAAGGTGTCA-3′ | Testes |
| REV: 5′-AGAAAGGCAGCACACTCTGGATGA-3′ | |||
| CYP19α | AF288755.1 | FWD:5′-ATGTTGATCGCGGCTCCAGATACT-3′ | Testes |
| REV: 5′-TTAACCTGGACAGATGCGAGTGCT-3′ | |||
| Stimulation of testosterone production | |||
| LHβ | DQ242617.1 | FWD:5′-TCCTCCATGTGAGCCAATCAACGA-3′ | Brain |
| REV: 5′-AGACATTGGAGAACGGGCTCTTGT-3′ | |||
| FSHβ | DQ242616.1 | FWD:5′-GCAGCTGCATCACAATCGACACAA-3′ | Brain |
| REV: 5′-AGGGCAGCCTTTAAACTCGTAGGT-3′ | |||
| Peroxisome proliferation | |||
| PPARα | EU195886.1 | FWD:5′-ATGGAGCCCAAATTTCAGTTCGCC-3′ | Testes |
| REV: 5′-ATGTGCTCGATCCGTGACACGTTA-3′ | |||
| ACOX1 | DT169074.1 | FWD:5′-TTACGACCCTTCCACCCAAGAGTT-3′ | Testes |
| REV: 5′-TGTAGGCCATGACACTTTCCCTGT-3′ | |||
| EHHADH | DT190723.1 | FWD:5′-AGCCGTTATAGGTCTGGGCACAAT-3′ | Testes |
| REV: 5′-TAGCATGCCAATCACCATCTGCCT-3′ | |||
| Steroid/xenobiotic metabolism | |||
| PXR | EU153254.1 | FWD:5′-GATCATGTCGGATGAGGCCGTC-3′ | Testes, Liver |
| REV: 5′-CCGCTGCACTCCATCTTCAGG-3′ | |||
| CYP3A4 | EU332794.1 | FWD:5′-TTCCGTTCTTCGGAACGATGCTGA-3′ | Testes, Liver |
| REV: 5′-TGTGTTTGACACCCACAGGTTCAC-3′ | |||
| SULT1st2 | DT167241.1 | FWD:5′-AAACTGCAGAGGCGACCAGAGATA-3′ | Testes, Liver |
| REV: 5′-TACCTGCTTTGGGATAAGTGGCGA-3′ | |||
| SULT2A1 | DT317390.1 | FWD:5′-AGGCCGAACTGTACACGGTTCATA-3′ | Testes, Liver |
| REV: 5′-TGCCGGATTTGGGATAAGTGACGA-3′ | |||
| UGT2B5 | DT183511.1 | FWD:5′-CACTTTGCGTCCAAACATCAGGCT-3′ | Testes, Liver |
| REV: 5′-ACATGAGCCAGGGAGAATCGGAAA-3′ | |||
| Normalizer | |||
| β-Actin | EU195887.1 | FWD:5′-TCTTCCAGCCATCCTTCCTTGG-3′ | Testes, Liver, Brain |
| REV: 5′-CTGCATACGGTCAGCAATGCC-3′ | |||
Gene expression was quantified using Brilliant II SYBR Green QPCR (Stratagene, La Jolla, CA) per manufacturer’s instruction. RT-PCR was performed on the Opticon (MJ Research, Waltham, MA) with the following protocol: 1 cycle at (i) 95 °C for 10 min; 39 cycles at (ii) 95 °C for 1 min; (iii) 60 °C for 30 s. Relative QPCR expression was determined using Real-time PCR Miner (Zhao and Fernald, 2005) and normalized to the transcript levels for β-actin, as it did not vary across treatments.
2.4. Testosterone and 17β-estradiol sampling protocol
Plasma T and E2 in males was analyzed using competitive enzyme immunoassay kits (Cayman Chemical, Ann Arbor, MI) following the instruction of the manufacturer. Absorbance readings were performed on the Wallac 1420 Explorer (PerkinElmer, Irvine, CA) at 405 nm for 1 s. Concentration values were calculated by comparing absorbance of samples to a standard curve. Samples were analyzed in duplicate, and re-assayed if the coefficient of variance between duplicates was above 20%.
2.5. Statistics
All data from the experiment were tested for normality using a Kolmogorov–Smirnov test, and homogeneity of variance using Levene’s test. In cases where the QPCR data or log2 transformed data met parametric assumptions, one-way analysis of variance (ANOVA) was used to test for differences across groups, while differences between groups were determined using Tukey test. For the cases in which the data did not conform to parametric assumptions, a nonparametric Kruskal–Wallis test (KW) was used to test for differences across groups, and a Dunn’s test was used to determine differences among groups. Differences were considered significant at p < 0.05. Data are presented as mean ± standard error of the mean. All statistical analyses were conducted using PASW v.18 (SPSS, Chicago, IL).
3. Results
3.1. Testosterone, 17β-estradiol production and gonado-somatic index
There was no difference in the plasma testosterone concentration for male FHM exposed to either linuron-only or DEHP-only as compared to the control. Conversely, male FHM exposed to the mixture had significantly lower levels of plasma testosterone as compared to the control, linuron-only and DEHP-only exposed FHM (KW p = 0.003, df = 3, Chi square = 14.115; Dunn post hoc mixture vs. control: p = 0.009, mixture vs. linuron-only: p = 0.002, mixture vs. DEHP-only: p < 0.001) (Fig. 2A).There was also a significant decrease in plasma E2 in DEHP-only and mixture exposed male FHM as compared to the control and linuron-only exposed FHM (KW p = 0.004, df = 3, Chi square = 13.515; Dunn post hoc DEHP vs. control p = 0.055; DEHP vs. linuron p = 0.018; mixture vs. control: p = 0.011; mixture vs. linuron p = 0.001. There was no difference in plasma E2 in the linuron-only exposure as compared to the control (Fig. 2B). GSI did not differ among the four groups (p > 0.05).
Fig. 2.
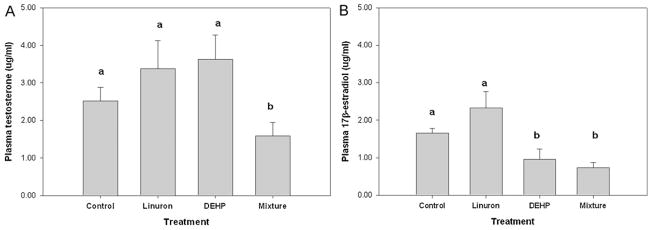
(A) Plasma testosterone and (B) plasma 17β-estradiol concentrations (μg/mL) in male FHM exposed to the control, linuron, DEHP, or the mixture. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8. Testosterone KW p = 0.003; Dunn post hoc mixture vs. control: p = 0.009, mixture vs. linuron p = 0.002, mixture vs. DEHP p < 0.001. 17β-estradiol KW p = 0.004, df = 3, Chi square = 13.515; Dunn post hoc DEHP vs. control p = 0.055; DEHP vs. linuron p = 0.018; mixture vs. control: p = 0.011; mixture vs. linuron p = 0.001.
3.2. LHβ and FSHβ mRNA transcript expression
There was no difference in the relative expression of LHβ among the four groups (p > 0.05) (Fig. 3A). Although not statistically significant, there was a trend toward increased relative mRNA levels of LHβ in male FHM exposed to the mixture as compared to the control, and a trend downward in linuron-only exposed FHM. There was a significant increase in the relative expression of FSHβ in male FHM exposed to the mixture as compared to the control and linuron-only FHM (ANOVA: p < 0.001, df = 28, F = 11.741, Tukey mixture vs. control: p = 0.001; mixture vs. linuron-only: p < 0.001). There was also a significant increase in the expression of FSHβ in male FHM exposed to DEHP-only as compared to the control and linuron-only FHM. (Tukey: DEHP vs. control: p = 0.017, DEHP-only vs. linuron-only: p = 0.005) (Fig. 3B). There was no difference in the relative expression of FSHβ between the control- and linuron-only male FHM.
Fig. 3.
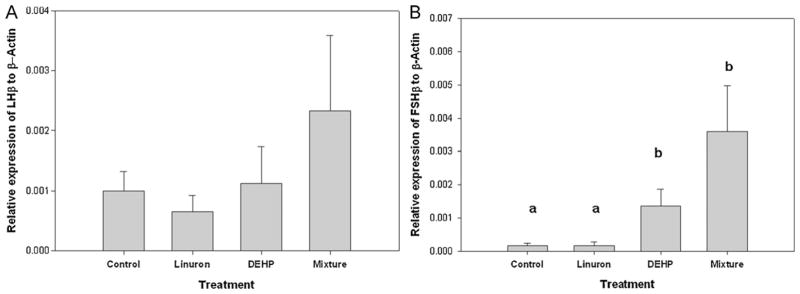
Relative mRNA transcripts expression of (A) LHβ and (B) FSHβ mRNA transcripts in brain of male FHM exposed to the control, linuron, DEHP, or the mixture. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8. ANOVA: p < 0.001, Tukey DEHP vs. control: p = 0.017, DEHP vs. linuron: p = 0.005, mixture vs. control: p = 0.001; mixture vs. linuron: p < 0.001.
3.3. Relative expression of steroidogenic enzyme mRNA transcripts
In this study, there was no difference in the relative expression of StAR, 3β-HSD, CYP11a, CYP17, 17β-HSD or CYP19a1 among all four exposure groups (p > 0.05). Although there was variation in the relative expression of all six genes among all groups, it was not significant (Fig. 4A–F).
Fig. 4.
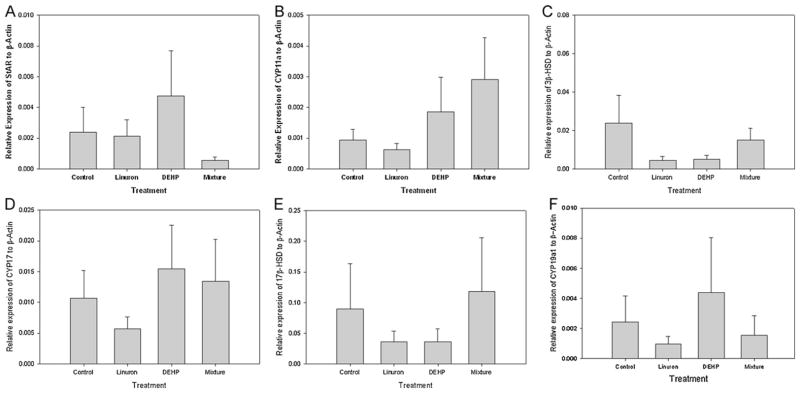
Relative mRNA transcripts expression of genes involved in steroidogenesis in testis of male FHM exposed to the control, linuron, DEHP, or the mixture. (A) StAR, (B) CYP11a, (C) 3β-HSD, (D) CYP17, (E) 17β-HSD, (F) CYP19a1. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8.
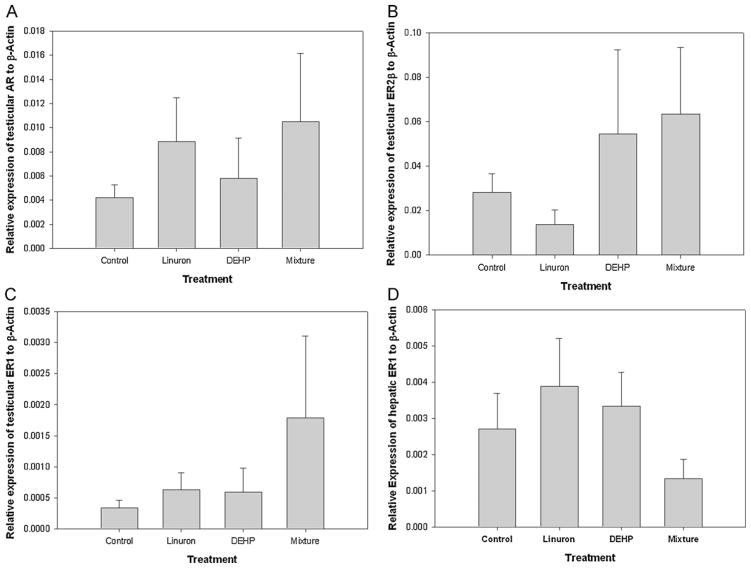
3.4. Relative expression of hormone receptor mRNA transcripts
There was no difference in the relative expression of AR, ER1, and ER2β mRNA transcripts in the testes of FHM across all treatments (p > 0.05). There was no difference in the relative expression of ER1 mRNA transcripts in the liver of FHM across all treatments (p > 0.05). Although there was variation in the relative expression of all three genes among all groups, it was not significant (Fig. 5A–D).
Fig. 5.
Relative mRNA transcripts expression of testes (A) AR, (B) ER2β, (C) ER1, and (D) hepatic ER1 of male FHM exposed to the control, linuron, DEHP, or the mixture. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8.
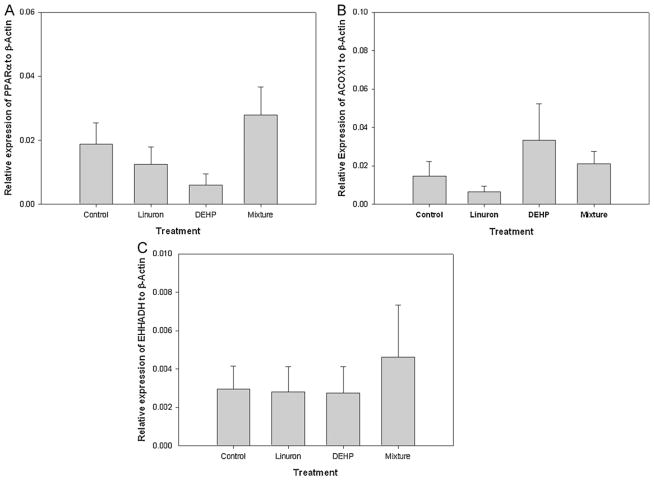
3.5. Peroxisome proliferation
Increased peroxisome proliferation was assessed by measuring relative mRNA transcript levels of PPARα as well as PPARα-responsive genes, ACOX1 and EHHADH. There was no difference in the relative expression of PPAR; among the four exposure groups (p > 0.05). Although not statistically significant, there was a trend toward decreased relative mRNA levels of PPARα in male FHM exposed to DEHP-only as compared to the control (Fig. 6A). There was no difference in the relative expression of either ACOX1 or EHHADH among the four exposure groups (p > 0.05) (Fig. 6B and C).
Fig. 6.
Relative mRNA transcripts expression of genes involved in peroxisome proliferation in testis of male FHM exposed to the control, linuron, DEHP, or the mixture: (A) PPARα, (B) ACOX1, (C) EHHADH. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8.
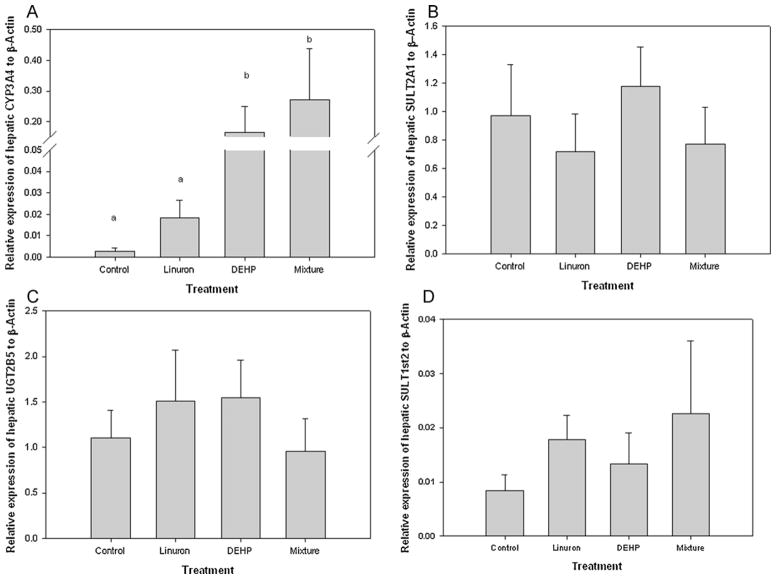
3.6. Phase I and II metabolism in the liver and testes
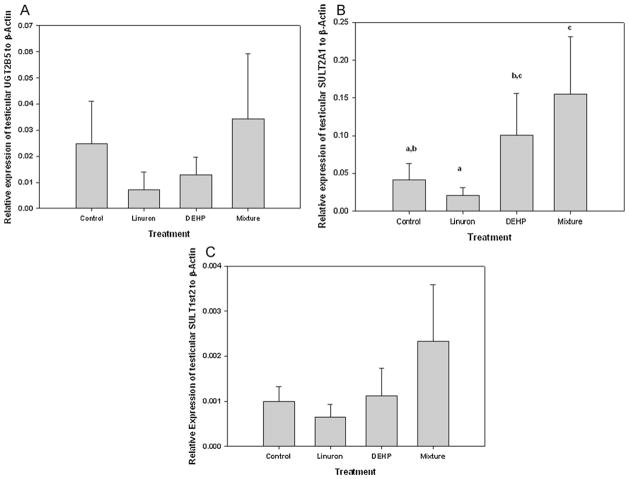
In the liver, there was a significant increase in the relative expression of CYP3A4 in DEHP-only and mixture exposed male FHM as compared to the control and linuron-only exposed FHM male (ANOVA: p = 0.004, df = 24, F = 6.068; Tukey: DEHP-only vs. control p = 0.033; mixture vs. control p = 0.007; mixture vs. linuron-only: p = 0.035) (Fig. 7A). Although there was an increase in the relative expression of hepatic CYP3A4 in male FHM exposed to linuron-only, it was not significant from that of the control (p > 0.05). There was no difference in the relative expression of either hepatic SULT2A1, UGT2B5, or SULT1st2 among all groups (p > 0.05) (Fig. 7B–D).
Fig. 7.
Relative mRNA transcripts expression of hepatic (A) CYP3A4, (B) SULT2A1, (C) SULT1st2, (D) UGT2B5 in male FHM exposed to the control, linuron, DEHP, or the mixture. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8. CYP3A4 ANOVA: p = 0.004, df = 24, F = 6.068; Tukey: DEHP vs. control p = 0.033; mixture vs. control p = 0.007; mixture vs. linuron: p = 0.035.
In the testes, there was no amplification of CYP3A4 in any of the exposures. There was a significant increase in the relative expression of SULT2A1 in mixture exposed male FHM as compared to the control and linuron-only exposed male FHM (KW p = 0.027, df = 3, Chi square = 8.760; Dunn post hoc: mixture vs. control p = 0.044; mixture vs. linuron p = 0.011). There was also a significant difference between DEHP-only and linuron-only exposed FHM (Dunn post hoc: p = 0.032) (Fig. 8A). There was a no difference in the relative expression of testicular UGT2B5 among treatments (p > 0.05). Although there was an increase in the relative expression of UGT2B5 in male FHM exposed to the mixture it was not significant (Fig. 8B). There was no difference in the expression of SULT1st2 among exposures (p > 0.05) (Fig. 8C).
Fig. 8.
Relative mRNA transcripts expression of (A) SULT2A1, (B) SULT1st2, (C) UGT2B5, (D) PXR in testis of male FHM exposed to the control, linuron, DEHP, or the mixture. Error bars represent ± SEM. N = control: 10, linuron: 8, DEHP: 9, mixture: 8. SULT2A1 KW p = 0.027, Dunn post hoc: DEHP vs. linuron p = 0.032, mixture vs. control p = 0.044; mixture vs. linuron p = 0.011). PXR ANOVA p = 0.001, Tukey mixture vs. control p = 0.023; mixture vs. linuron p < 0.001; mixture vs. DEHP p = 0.012.
The relative expression of PXR was compared among exposure groups to assess whether changes in T and E2 were potentially a PXR-mediated response. There was no difference in the relative expression of hepatic PXR among any groups (p > 0.05). There was a significant increase in the expression of testicular PXR in male FHM exposed to the mixture as compared to all other groups (ANOVA p = 0.001, Tukey mixture vs. control p = 0.023; mixture vs. linuron-only p < 0.001, mixture vs. DEHP-only p = 0.012).
4. Discussion
4.1. Changes to plasma E2 and T concentrations and genes along the HPG axis
This paper highlights the need for studies examining the impacts of low concentrations of environmentally relevant mixtures on aquatic organisms. In this study, DEHP appears to have an anti-estrogenic effect as both DEHP-only and mixture-exposed male FHM had significantly decreased plasma E2 concentrations. To our knowledge the decrease in plasma E2 has not been previously reported in male fish exposed to any phthalate. This decrease was in conjunction with a significant increase in FSHβ in the brain which would indicate that the HPG feedback mechanism was not altered during exposure. Although there was an increase in the relative expression of CYP19a1 in DEHP-only exposed male FHM it was not significant. There was also a decrease in plasma T concentrations in the mixture-exposed male FHM that was not seen in the control or individual exposures. Plasma T concentrations in the linuron-only and DEHP-only exposed male FHM were slightly higher. A similar increase in plasma T concentrations was seen in male sticklebacks exposed to dibutyl phthalate (Aoki et al., 2011). Unfortunately plasma E2 concentrations and FSHβ were not measured in that study. Although there was no difference in LHβ among treatment groups, this in part was due to the large variability in the relative expression of LHβ across treatments.
In this study, there was no change the relative expression of genes associated with steroidogenesis across all groups. Although previous reports in mammals have shown that both linuron and DEHP, individually and as part of a mixture, inhibit the expression of genes involved in steroidogenesis, this appears to not be true in fish (Aoki et al., 2011; Hotchkiss et al., 2004; Rider et al., 2008; Uren-Webster et al., 2010).
There was no difference in the relative mRNA transcript levels of genes associated with hormone receptor expression in the testes (AR, ER1, and ER2b) or the liver (ER1), and corresponds to previous research in zebrafish injected with DEHP (Uren-Webster et al., 2010). That said, it cannot be discounted that one or both of the compounds are acting at the protein level. Since linuron has been shown to competitively bind to the ARfhm in vitro, one possibility is that it was acting to inhibit testosterone synthesis at the protein level.
In this study, the effect of decreased T and E2 concentrations on male FHM reproduction is unknown. Although there was no change in the relative expression of 17β-HSD, CYP19a1, AR, ER1 or ER2b, there was no histological work performed to assess whether Leydig and Sertoli cells were degraded.
4.2. Peroxisome proliferation
Exposure to high concentrations of DEHP has been shown to disrupt spermatogenesis in zebrafish and mammals action, and is associated with increased peroxisome proliferation via PPARα signaling pathway (Onorato et al., 2008; Uren-Webster et al., 2010). This study did not find any difference in the relative mRNA transcript levels of the PPARα or the peroxisome proliferation associated ACOX1 or EHHADH, among all treatments. There was no histological work performed to assess whether there was an increased number of peroxisomes in the Leydig and Sertoli cells.
4.3. Phase I and II metabolism in testes and liver
Phase I and II enzymes are responsible for the degradation and clearance of xenobiotic compounds, including DEHP and linuron (Cooper et al., 2008; Hurst and Waxman, 2004; Takeuchi et al., 2008). Induction of hepatic Phase I and II enzymes are also responsible for steroid oxidative hydroxylation and conjugation and have been shown to increase T and E2 metabolism and clearance (see You, 2004 for review). In this study hepatic expression of CYP3A4 was significantly increased in DEHP-only and mixture exposed male FHM, and could be associated with increased E2 and T metabolism. DEHP has been shown to increase CYP3A4 expression, as well as increase testosterone degradation in rats (Cooper et al., 2008; Fan et al., 2004). In addition, adult male rats exposed to high concentrations of DEHP showed an increase in plasma E2 and a decrease in phase I and II enzymes associated with E2 metabolism (Corton et al., 1997; Eagon et al., 1994). In this study, there was no difference among the exposure groups in the relative expression of androgen-specific SULT2A1 and UGT2B15, and estrogen-specific SULT1st2 in the liver, which corresponds with other studies in carp exposed to either DEHP or diuron (Thibaut and Porte, 2004). This would indicate that if increased steroid metabolism is taking place in the liver, CYP3A4 and possibly some other phase I and II enzyme(s) are responsible.
Although phase II metabolism of steroids mainly occurs in the liver, it can also occur in the testes (Chatterjee et al., 1994; Strott, 2002). The decrease in plasma T concentrations in male FHM exposed to the mixture may be due to the synergistic action from the two anti-androgenic compounds that were not seen in the individual exposures. In male rats, SULT2A1 is inhibited by the AR, presumably allowing for increased levels of androgens (Chatterjee et al., 1987, 1994). Linuron has been shown to competitively bind to the AR in FHM in vitro (Wilson et al., 2004), which in turn could allow for induction of SULT2A1 by PXR, PPARα or some other nuclear factor that was not assessed. PXR has been shown to induce SULT2A1 expression in mammalian prostate, and in turn modulate endogenous hormone levels (Hurst and Waxman, 2004; Sonoda et al., 2002; Zhang et al., 2010). One hypothesis is that this same function is happening in the testes of the FHM exposed to the mixture. Due to the limited amount of plasma that can be collected from FHM (10–20 μL/fish), this study did not assess the concentrations of sulphated steroids or xenobiotics. Therefore, further research is needed to confirm this hypothesis.
4.4. Other factors that may play a role in hormone concentrations
In this study, plasma T and E2 concentrations and mRNA expression were measured at the end of the 28-day exposure period. The difference in plasma hormone concentrations seen among the exposure groups may be influenced by the time of sampling. Previous studies have shown that the relative expression of steroidogenic genes and both plasma T and E2 concentrations can change over the time during single and multiple exposure events (Ankley et al., 2007, 2009b; Martinović et al., 2008). Differences between the individual- and mixture-exposed male FHM may be due to the increased overall dosage of combining two anti-androgenic compounds in the mixture. Previous dose-response studies in fish exposed to phthalates have not shown a decrease in plasma T or inhibition of steroidogenesis with increasing dose (Aoki et al., 2011; Uren-Webster et al., 2010). As for linuron, a similar mixed-effect anti-androgen prochloraz did show a dose-responsive decrease in plasma T concentrations, but the effective dose was much higher (300 ppb) then used in this study (Ankley et al., 2009b). Therefore it cannot be discounted that time of sampling as well as the increased dose-level played a role in the differences in plasma T and E2 concentrations among exposure groups.
5. Conclusion
Our results demonstrate that the effects from exposure to a mixture of chemicals can have an impact that is greater than that seen from the individual exposures. In addition, mixtures have a different impact than either chemical alone. Therefore, due to this potential additive or synergistic effect, the individual compounds that make-up the mixture can occur at lower concentrations then previously tested and still have an endocrine disrupting effect. Although individual exposure studies can offer important insight into the specific mechanisms of action of a particular chemical, they fail to elucidate additive or synergistic effects that the chemical may have as part of an environmentally relevant mixture. Therefore it is imperative that low-level exposures to environmentally relevant mixtures be used in risk assessment of endocrine disrupting chemicals.
Acknowledgments
This research was funded by NIH grant. (No. P30ES004184-24).
Abbreviations
- FHM
fathead minnow
- DEHP
di(2-ethylhexyl) phthalate
- T
testosterone
- AR
androgen receptor
- ER
estrogen receptor
- HPG
hypothalamic-pituitary-gonadal axis
- EDCs
endocrine-disrupting compounds
- CYP
cytochrome P450
- StAR
Steroidogenic Acute Regulatory protein
- GSI
gonadosomatic index
- LHβ
luteinizing hormone β-subunit
- FSHβ
follicle-stimulating hormone β-subunit
- 3β-HSD
3β-hydroxysteroid
- 17β-HSD
17β-hydroxysteroid
- CYP19a1
aromatize
- PPARα
peroxisome proliferator-activated receptor-alpha
- ACOX1
acyl-coenzyme A oxidase1
- EHHADH
enoyl-coenzyme A hydratase/3-hydroxyacyl coenzyme A dehydrogenase
- SULT2A1
sulfotransferase dehydrogenase 2A1
- SULT2st1
sulfotransferase dehydrogenase 1st2
- UGT2B15
UDP glucuronosyltransferase 2B15
- ANOVA
pregnane X-receptor PXR
- KW
one-way analysis of variance Kruskal Wallis
References
- Ankley GT, Jensen KM, Kahl MD, Makynen EA, Blake LS, Greene KJ, Johnson RD, Villeneuve DL. Ketoconazole in the fathead minnow (Pimephales promelas) reproductive toxicity and biological compensation. Environ Toxicol Chem. 2007;26:1214–1223. doi: 10.1897/06-428r.1. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bencic DC, Breen MS, Collette TW, Conolly RB, Denslow ND, Edwards SW, Ekman DR, Garcia-Reyero N, Jensen KM, Lazorchak JM, Martinovic D, Miller DH, Perkins EJ, Orlando EF, Villeneuve DL, Wang RL, Watanabe KH. Endocrine disrupting chemicals in fish: developing exposure indicators and predictive models of effects based on mechanism of action. Aquat Toxicol. 2009a;92:168–178. doi: 10.1016/j.aquatox.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bencic DC, Cavallin JE, Jensen KM, Kahl MD, Makynen EA, Martinovic D, Mueller ND, Wehmans LC, Villeneuve DL. Dynamic nature of alterations in the endocrine system of fathead minnows exposed to the fungicide prochloraz. Toxicol Sci. 2009b;112:344–353. doi: 10.1093/toxsci/kfp227. [DOI] [PubMed] [Google Scholar]
- Aoki KA, Harris CA, Katsiadaki I, Sumpter JP. Evidence suggesting that di-n-butyl phthalate has antiandrogenic effects in fish. Environ Toxicol Chem. 2011;30:1338–1345. doi: 10.1002/etc.502. [DOI] [PubMed] [Google Scholar]
- Bélanger A, Hum DW, Beaulieu M, Lévesque E, Guillemette C, Tchernof A, Bélanger G, Turgeon D, Dubois S. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J Steroid Biochem Mol Biol. 1998;65:301–310. doi: 10.1016/s0960-0760(97)00183-0. [DOI] [PubMed] [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfà A, Marcomini A, Sumpter JP. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee B, Majumdar D, Ozbilen O, Roy AK. Molecular cloning and characterization of the cDNA for the androgen repressible SMP-2 in the rat liver. J Biol Chem. 1987;262:822–825. [PubMed] [Google Scholar]
- Chatterjee B, Song CS, Kim JM, Roy AK. Androgen and estrogen sulfo-transferases of the rat liver. Chem Biol Interact. 1994;92:273–279. doi: 10.1016/0009-2797(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Clewell HJ, Thomas RS, Kenyon EM, Hughes MF, Adair BM, Gentry PR, Yager JW. Concentration- and time-dependent genomic changes in the mouse urinary bladder following exposure to arsenate in drinking water for up to twelve weeks. Toxicol Sci. 2011;(July) doi: 10.1093/toxsci/kfr199. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Cooper BW, Cho TM, Thompson PM, Wallace AD. Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol Sci. 2008;103:268–277. doi: 10.1093/toxsci/kfn047. [DOI] [PubMed] [Google Scholar]
- Correia AD, Freitas S, Scholze M, Goncalves JF, Booij P, Lamoree MH, Mañanós E, Reis-Henriques MA. Mixtures of estrogenic chemicals enhance vitellogenic response in sea bass. Environ Health Perspect. 2007;115 (Suppl 1):115–121. doi: 10.1289/ehp.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JC, Bocos C, Moreno ES, Merritt A, Cattley RC, Gustafsson JA. Peroxisome proliferators alter the expression of estrogen-metabolizing enzymes. Biochimie. 1997;79:151–162. doi: 10.1016/s0300-9084(97)81508-8. [DOI] [PubMed] [Google Scholar]
- Eagon PK, Chandar N, Epley MJ, Elm MS, Brady EP, Rao KN. Di(2-ethylhexyl)phthalate-induced changes in liver estrogen metabolism and hyperplasia. Int J Cancer. 1994;58:736–743. doi: 10.1002/ijc.2910580519. [DOI] [PubMed] [Google Scholar]
- Fan LQ, You L, Brown-Borg H, Brown S, Edwards RJ, Corton JC. Regulation of phase I and phase II steroid metabolism enzymes by PPAR alpha activators. Toxicol. 2004;204:109–121. doi: 10.1016/j.tox.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Filby AL, Thorpe KL, Maack G, Tyler CR. Gene expression profiles revealing the mechanisms of anti-androgen- and estrogen-induced feminization in fish. Aquat Toxicol. 2007;81:219–231. doi: 10.1016/j.aquatox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Finne EF, Cooper GA, Koop BF, Hylland K, Tollefsen KE. Toxicogenomic responses in rainbow trout (Oncorhynchus mykiss) hepatocytes exposed to model chemicals and a synthetic mixture. Aquat Toxicol. 2007;81:293–303. doi: 10.1016/j.aquatox.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Frömme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002;36:1429–1438. doi: 10.1016/s0043-1354(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Gentry PR, McDonald TB, Sullivan DE, Shipp AM, Yager JW, Clewell HJ., 3rd Analysis of genomic dose-response information on arsenic to inform key events in a mode of action for carcinogenicity. Environ Mol Mutagen. 2010;51:1–14. doi: 10.1002/em.20505. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DoTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Hill EM, Evans KL, Horwood J, Rostkowski P, Oladapo FO, Gibson R, Shears JA, Tyler CR. Profiles and some initial identifications of (anti)androgenic compounds in fish exposed to wastewater treatment works effluents. Environ Sci Technol. 2010;44:1137–1143. doi: 10.1021/es901837n. [DOI] [PubMed] [Google Scholar]
- Hockley SL, Arlt VM, Brewer D, Giddings I, Phillips DH. Time-and concentration-dependent changes in gene expression induced by benzo(a)pyrene in two human cell lines, MCF-7 and HepG2. BMC Genomics. 2006;7:260. doi: 10.1186/1471-2164-7-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, Gray LE., Jr A mixture of the antiandrogens linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004;71:1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Huang H, Wu Q. Cloning and comparative analyses of the zebrafish Ugt repertoire reveal its evolutionary diversity. PLoS ONE. 2010;5(e9144) doi: 10.1371/journal.pone.0009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Environmental phthalate monoesters activate pregnane X receptor-mediated transcription. Toxicol Appl Pharmacol. 2004;199:266–274. doi: 10.1016/j.taap.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103:582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, Gray LE., Jr Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicology. 2000;56:389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Gene expression pro-filing following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- Martinović D, Blake LS, Durhan EJ, Greene KJ, Jensen KM, Kahl MD, Makynen EA, Villeneuve DL, Ankley GT. Reproductive toxicity of vinclozolin in the fathead minnow: confirming an anti-androgenic mode of action. Environ Toxicol Chem. 2008;27:478–488. doi: 10.1897/07-206R.1. [DOI] [PubMed] [Google Scholar]
- Onorato TM, Brown PW, Morris PL. Mono-(2-ethylhexyl) phthalate increases spermatocyte mitochondrial peroxiredoxin 3 and cyclooxygenase 2. J Androl. 2008;29:293–303. doi: 10.2164/jandrol.107.003335. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS, Gray LE., Jr A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl. 2008;31:249–262. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci USA. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strott CA. Sulfonation and molecular action. Endocrinol Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Yang YS, Liu CC, Pai TG, Liu MC. Sulphonation of dehydroepiandrosterone and neurosteroids: molecular cloning, expression, and functional characterization of a novel zebrafish SULT2 cytosolic sulphotransferase. Biochem J. 2003;375:785–791. doi: 10.1042/BJ20031050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Yabushita H, Matsuda T, Kojima H. In vitro screening for aryl hydrocarbon receptor agonistic activity in 200 pesticides using a highly sensitive reporter cell line, DR-EcoScreen cells, and in vivo mouse liver cytochrome P450-1A induction by propanil, diuron and linuron. Chemosphere. 2008;74:155–165. doi: 10.1016/j.chemosphere.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Thibaut R, Porte C. Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J Steroid Biochem Mol Biol. 2004;92:485–494. doi: 10.1016/j.jsbmb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Uren-Webster TM, Lewis C, Filby AL, Paull GC, Santos EM. Mechanisms of toxicity of di(2-ethylhexyl) phthalate on the reproductive health of male zebrafish. Aquat Toxicol. 2010;99:360–369. doi: 10.1016/j.aquatox.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Cardon MC, Thornton J, Korte JJ, Ankley GT, Welch J, Gray LE, Jr, Hartig PC. Cloning and in vitro expression and characterization of the androgen receptor and isolation of estrogen receptor alpha from the fathead Minnow (Pimephales promelas) Environ Sci Technol. 2004;38:6314–6321. doi: 10.1021/es049771j. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright CR, Furr JR, Howdeshell KL, Gray LE., Jr The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. Toxicol Lett. 2009;186:73–77. doi: 10.1016/j.toxlet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Woudneh MB, Ou Z, Sekela M, Tuominen T, Gledhill M. Pesticide multiresidues in waters of the Lower Fraser Valley, British Columbia, Canada Part I. Surface water. J Environ Qual. 2009;38:940–947. doi: 10.2134/jeq2007.0524. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shimada T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997;346:161–169. doi: 10.1006/abbi.1997.0302. [DOI] [PubMed] [Google Scholar]
- You L. Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem Biol Interact. 2004;147:233–246. doi: 10.1016/j.cbi.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang B, Cheng Q, Ou Z, Lee JH, Xu M, Kochar U, Ren S, Huang M, Pflug BR, Xie W. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology. 2010;151:5721–5729. doi: 10.1210/en.2010-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kong FX, Wang SH, Yu Y, Zhang M. Vitellogenin induction by a mixture of steroidal estrogens in freshwater fishes and relevant risk assessment. Environ Toxicol. 2009;24:484–491. doi: 10.1002/tox.20453. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1045–1062. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]