Abstract
The development of new blood vessels is a crucial step in breast cancer growth, progression and dissemination, making it a promising therapeutic target. Breast cancer has a heterogeneous nature and the diversity of responsible angiogenic pathways between different tumors has been studied for many years. Inhibiting different targets in these pathways has been under investigation in preclinical and clinical studies for more than decades, among which antibody against vascular endothelial growth factor is the most studied. However, the clinical impact from antiangiogenic treatment alone or in combination with standard chemotherapeutic regimens has been relatively small till today. In this review, we summarize the most clinically relevant data from breast cancer treatment clinical trials and discuss safety and efficacy of common antiangiogenic therapies as well as biological predictive markers.
Keywords: breast cancer, antiangiogenic therapy, predictive biomarker, clinical trial
INTRODUCTION
Angiogenesis, the process of new vessel formation, has a key role in tumor growth and progression (1). It has been shown decades ago that without expanding vasculature, the tumor growth cannot exceed 2–3 mm (2). Angiogenic switch, the shift of the balance between proangiogenic and antiangiogenic in favor of proangiogenesis, applies to most and perhaps all types of solid tumors (3). However, some types of cancer are more angiogenesis dependent than others such as breast cancer. This can explain their vulnerability to ‘angiogenesis inhibitors’ identified in several preclinical and clinical studies for many years (4). Breast cancer is a heterogeneous disease and the expression level of estrogen receptor (ER), progesterone receptor and human epidermal growth factor receptor (HER-2) is among the most prominent predictive and prognostic factors. Breast cancer can also be categorized to different molecular subtypes based on expression profiling using DNA microarray (5) (i.e. luminal A, luminal B, HER-2, basal like, normal breast-tissue like and claudin-low), which show different angiogenic characteristics at gene and protein levels (6–10). This can explain the difference in clinical benefit from antiangiogenic therapies in different subsets of breast cancers.
In this review, we aim to summarize the most clinically relevant data from clinical trials of antiangiogenic agents in the treatment of breast cancer.
MAIN ANTIANGIOGENIC STRATEGIES IN BREAST CANCER
Antivascular endothelial growth factor (VEGF): VEGF is the most studied angiogenic factor and it has been shown to have a significant role in the progression and prognosis of breast cancer (11). The VEGF family consists of different isoforms and induces tumor vasculature endothelial cell survival, growth and migration through their interaction with VEGF receptors (VEGFRs).
Several points on VEGF/VEGFR interaction axis have been examined as potential antiangiogenesis targets among which ligand blockade has been the most thoroughly studied. Bevacizumab (Avastin®, Roche-Genetech) is a recombinant humanized monoclonal antibody that targets all known isoforms of VEGF-A. It has been the first and so far the most examined antiangiogenic treatment in breast cancer clinical trials (12). Bevacizumab is the only antiangiogenic drug that has shown statistically significant clinical benefits in metastatic breast cancer treatment (13).
VEGFR antagonists: VEGFRs are a family of closely related receptor tyrosine kinase (TK) involved in signal transduction cascade. Although the monoclonal antibody against the VEGFR2 external domain, ramucirumab (IMC-1121B, ImClone), has been developed and investigated (14), small molecule TK inhibitors (TKIs) that act on the intracellular domain of these receptors and block their catalytic function are more common in clinical trials. Sunitinib (Sutent®, Pfizer), sorafenib (Nexavar®, Bayer), pazopanib (Votrient®, GlaxoSmithKline) and axitinib (Inlyta®, Pfizer) are among multitargeted TKIs that have shown to have antiangiogenic effects.
Metronomic chemotherapy: Metronomic chemotherapy is defined as low-dose chemotherapy administered frequently for a long time avoiding dose-limiting side-effects such as myelosuppression. Metronomic doses of conventional regimens such as cyclophosphamide, methotrexate and 5-fluorouracil have shown to have antitumor effects through interfering with neoangiogenesis in breast cancer (15).
ANTIANGIOGENIC TREATMENT EFFICACY
Bevacizumab
The first published data came from AVF2119g Phase III trial involving second-line treatment in previously treated 462 metastatic breast cancer cases in 2005 (16). This study showed that the addition of bevacizumab to capecitabine produced a significant increase in response rates (RRs) (9.1 vs. 19.8%, P = 0.001) but it did not improve the progression-free survival (PFS) (median, 4.2 vs. 4.9 months) or the overall survival (OS) (median, 14.5 vs. 15.1 months). The second trial called E2100, an open-label trial that enrolled 722 patients with metastatic breast cancer, demonstrated that bevacizumab plus paclitaxel compared with paclitaxel alone prolonged the PFS by ∼6 months (median, 11.8 vs. 5.9 months; hazard ratios (HR) for progression, 0.60; P < 0.001) but did not affect the OS (median, 26.7 vs. 25.2 months; HR, 0.88; P = 0.16) (17). The result of this study led to Food and Drug Administration approval of bevacizumab in breast cancer treatment. Subsequent Phase III clinical trials, AVADO (18), RIBBON-1 (19) and RIBBON-2 (20) were undertaken to validate E2100. Similar to E2100, none of these trials could provide evidence of OS benefit in bevacizumab arms. PFS benefits from bevacizumab were also shown to be shorter than E2100 in the subsequent trials (Table 1).
Table 1.
Phase III trials in a metastatic setting
| Study | Arms | Patients | Overall response rate (%) | P value | Median progression-free survival (months) | HR (P value) | Overall survival (months) | HR (P value) |
|---|---|---|---|---|---|---|---|---|
| AVF2119g | Capecitabine + placebo | 230 | 9.1 | 0.001 | 4.2 | 0.98 (0.857) | 14.5 | Not reported |
| Capecitabine + bevacizumab | 232 | 19.8 | 4.89 | 15.1 | ||||
| E2100 | Paclitaxel | 326 | 22.2 | <0.0001 | 5.8 | 0.483 (<0.0001) | 25.2 | 0.88 (0.16) |
| Paclitaxel + bevacizumab | 347 | 48.9 | 11.3 | 26.7 | ||||
| AVADO | Docetaxel + placebo | 241 | 46.4 | Placebo vs. bev7.5: 0.07 | 8.1 | Placebo vs. bev7.5: 0.8 (0.045) | 31.9 | Placebo vs. bev7.5: 1.05 (0.72) |
| Docetaxel + bevacizumab (7.5 mg/kg) | 248 | 55.2 | 9.0 | 30.8 | ||||
| Docetaxel + bevacizumab (15 mg/kg) | 247 | 64.1 | Placebo vs. bev15: <0.001 | 10.0 | Placebo vs. bev15: 0.67 (<0.001) | 30.2 | Placebo vs. bev15: 1.03 (0.85) | |
| RIBBON-1 | Capecitabine + placebo | 206 | 23.6 | 0.0097 | 5.7 | 0.69 (<0.001) | 21.2 | 0.85 (0.27) |
| Capecitabine + bevacizumab | 409 | 35.4 | 8.6 | 29.0 | ||||
| Anthracycline/taxane + placebo | 207 | 37.9 | 0.0054 | 8.0 | 0.64 (<0.001) | 23.8 | 1.03 (0.83) | |
| Anthracycline/taxane + bevacizumab | 415 | 51.3 | 9.2 | 25.2 | ||||
| RIBBON-2 | Taxane or gemcitabine or capecitabine or vinorelbine (chemo) + placebo | 225 | 29.6 | 0.0193 | 5.1 | 0.78 (0.0072) | 16.4 | 0.90 (0.37) |
| Chemo + bevacizumab | 459 | 39.5 | 7.2 | 18.0 |
Other clinical trials have been conducted to evaluate the effect of addition of bevacizumab to standard therapies in different subsets of patients such as HER2-positive breast cancers in the AVEREL trial (21) or the BEVERLY-2 study (22). Most of these studies have reported limited clinical benefit even though the combination regimen has shown to be well tolerated. A number of other Phase II and III clinical trials were launched in the adjuvant setting for patients with HER2-negative and HER2-positive Stages II and III breast cancer, exploring different combinations of chemotherapy and variable durations of antiangiogenic therapy, aiming to evaluate the most efficacious and safest regimen (13).
In Japan, JO19901, a Phase II clinical trial, demonstrated that E2100 results are reproducible in Japanese populations (23). Overall RR (ORR) was 74%, median OS was 35.8 months, the 1-year OS rate was 88.9% and the regimen was well tolerated.
Another large open-label single-arm trial ATHENA, involving 2251 cases was designed to evaluate first-line bevacizumab with taxane-based chemotherapy in a population mirroring everyday oncology practice (24). Safety and efficacy results from this large observational study of bevacizumab in combination with taxane-based chemotherapy was consistent with the results from the previous randomized Phase III trials, suggesting clinical benefit of this combination in routine oncology practice.
In the neoadjuvant setting for early breast cancer, the GBG44 (25) and NSABP B-40 (26) studies are among the first trials to assess the benefit of bevacizumab. Both studies were designed to assess whether combining bevacizumab with various chemotherapies would have an impact on the pathological complete response (pCR) as a putative surrogate clinical endpoint in women with non-metastatic HER-2 negative breast cancer. GBG44 randomized 1948 HER-2 negative breast cancer patients to standard epirubicin–cyclophosphamide followed by docetaxel with or without bevacizumab. The primary endpoint was set as the pCR in breast and nodes. The rates of pCR (in breast and nodes) were 14.9% in the chemotherapy arm and 18.4% in the chemotherapy plus bevacizumab arm (OR, 1.29; 95% CI, 1.02–1.65; P = 0.04). Addition of bevacizumab increased the pCR in breast regardless of nodes from 16.5 to 20.5% (P = 0.03).In a subpopulation of 663 triple-negative breast cancers (TNBCs), the pCR rate improved from 27.9 to 39.3% (P = 0.003) by addition of bevacizumab. Breast-conserving surgery rate was 61.9 vs. 62.4% (P = 1.00), respectively. The NSABP-B40 trial was designed to evaluate whether addition of bevacizumab to the regimen of capecitabine/gemcitabine plus docetaxel followed by doxorubicin plus cyclophosphamide in 1206 HER2-negative early breast cancer could change the pCR (breast alone). The addition of bevacizumab significantly increased the rate of pCR in the breast, from 28.2 to 34.5% (P = 0.02). The effect was more apparent in the hormone-receptor–positive subset (15.1% without bevacizumab vs. 23.2% with bevacizumab, P = 0.007).
Tyrosine Kinase Inhibitors
Small molecule oral TKIs are designed to target the intracellular catalytic function of the VEGFR family (VEGFR1, 2 and 3), as well as platelet-derived growth factor receptor (PDGFR) and other angiogenic growth factor receptors expressed by endothelial cells (27). Sunitinib malate and sorafenib are oral TKIs that target several receptor TKs, including VEGFRs, PDGFR, stem cell factor receptor (c-KIT) and Flt3 receptor. They have shown interesting but far less encouraging degrees of activity compared with bevacizumab when added to standard breast cancer chemotherapies or when used alone.
A Phase II multicenter study evaluating sunitinib monotherapy in 64 heavily pretreated patients with metastatic breast cancer showed activity with mostly Grade 1/2 adverse events (AEs) and Grade 3/4 transient neutropenia in one-third of the patients (28). In 2010, a multicenter Phase II trial was conducted to evaluate whether sunitinib consolidation could prolong remission after taxane-based chemotherapy in HER-2 negative metastatic breast cancer (MBC) (29). Only 28% of patients achieved the 5-month PFS endpoint after starting sunitinib and due to higher rates of toxicity (69% of Grade 3/4 toxicity), the study failed to confirm the hypothesis. A randomized Phase III trial (Sun 1107) compared single-agent sunitinib to capecitabine in pretreated MBCs with the primary end point of prolonging PFS (30). The data demonstrated an inferior outcome for sunitinib vs. capecitabine group. (Median PFS was 2.8 vs. 4.2 months and median OS was 15.3 vs. 24.6 months.) A multicenter Phase III trial was designed to evaluate the clinical benefit of addition of sunitinib to docetaxel in advanced breast cancer (31). Although the objective RR was higher with the combination compared with monotherapy (55 vs. 42%, P = 0.001), PFS was no different and AEs were also more common with the combination. Another open-label Phase III study was conducted in an advance setting to compare sunitinib plus paclitaxel vs. bevacizumab plus paclitaxel as first-line treatment for patients with HER-2 negative breast cancer (32). The median PFS was 7.4 months in the sunitinib arm vs. 9.2 months in the bevacizumab arm and bevacizumab–paclitaxel was tolerated better.
In breast cancer, single-agent activity of sorafenib has been reported to be limited in previously treated patients (33). However, significant benefits have been observed from sorafenib in combination with standard chemotherapies. Four multinational, double-blind, placebo-controlled, randomized Phase IIb screening Trials to Investigate the Efficacy of Sorafenib (TIES) were developed to evaluate sorafenib in combination with palliative treatments for patients with locally advanced or metastatic HER-2 negative breast cancer (34–37). SOLTI-0701 assessed the treatment effect of sorafenib when added to capecitabine in patients not previously treated with VEGF inhibitors. The median PFS was 6.4 months for the sorafenib arm vs. 4.1 months for the placebo arm (HR, 0.58; 95% CI, 0.41–0.81; P = 0.001), while the median OS was 22.2 months for the sorafenib arm vs. 20.9 for the placebo arm (HR, 0.86; 95% CI, 0.61–1.23; P= 0.41). The NU07B1 study evaluated the addition of sorafenib to first-line paclitaxel for HER2-negative, locally recurrent (inoperable) breast cancer or MBC patients who had not received prior chemotherapy for advanced disease. The addition of sorafenib increased the time to progression (TTP) (median, 8.1 vs. 5.6 months; HR, 0.674; 95% CI, 0.465–0.975; P = 0.0343) but the OS did not statistically differ (median, 16.8 vs.17.4 months; HR, 1.022; 95% CI, 0.715–1.461; P = 0.904). AC01B07, another TIES trial was designed to assess whether sorafenib in combination with gemcitabine or capecitabine could overcome clinical bevacizumab resistance in patients with HER-2 negative MBC. Sorafenib plus gemcitabine/capecitabine was associated with a statistically significant prolongation in PFS vs. placebo plus gemcitabine/capecitabine (3.4 vs. 2.7 months; HR, 0.65; 95% CI, 0.45–0.95; P = 0.02); the TTP was increased (median, 3.6 vs.2.7 months; HR, 0.64; 95% CI, 0.44–0.93; P = 0.02). Although the results were relatively small, it showed possible benefit of sorafenib for MBC even after bevacizumab failure. The last TIES trial, FM-B07-01, which was designed to investigate sorafenib with docetaxel and/or letrozole (based on hormone-receptor status and presence or absence of visceral disease) without prior VEGF-targeted treatment, reported no PFS benefit with the addition of sorafenib to the best standard regimen compared with placebo; the median PFS was 8.4 months in both the sorafenib and placebo arms (HR, 1.21; 95% CI, 0.91–1.62; P = 0.12). OS analysis is still ongoing. Based on TIES data, a Phase III confirmatory placebo-controlled trial, RESILENCE, has been initiated and is currently underway to evaluate the effect of a lower dose of 600 mg/day of sorafenib in combination with capecitabine on MBC (38). Other ongoing randomized trials will evaluate sorafenib in combination with other standard chemotherapy and or endocrine therapy in advanced breast cancer (13).
Pazopanib, which targets VEGFRs, PDGFR and c-Kit, has been evaluated in combination with lapatinib (Tykerb®, GlaxoSmithKline), an oral inhibitor of epidermal growth factor receptor and HER2, as first-line therapy in patients with HER2-positive advanced breast cancer in a Phase II trial (39). This multicenter study showed that a combination of pazopanib with lapatinib did not improve the progressive disease rate (PDR), which was set as the primary endpoint (39). Week-12 PDRs were 36.2% in the combination arm vs. 38.9% for lapatinib alone (P = 0.37) although the RR had increased (36.2% in the combination arm vs. 22.2% in the monotherapy arm). There was no significant difference in the PFS (HR, 1.30; 95% CI, 0.76–2.23; P = 0.314) or the OS (HR, 0.91; 95% CI, 0.50–1.65; P = 0.75). Another Phase II randomized trial evaluated lapatinib plus pazopanib vs. lapatinib in patients with HER2+ inflammatory breast cancer (40). The lapatinib–pazopanib combination compared with lapatinib alone was associated with a higher ORR (45 vs. 29% in cohort 1 and 58 vs.47% in cohort 2) but no increase in the PFS (in cohort 1, the median PFS was 16.1 vs. 14.3 weeks, in cohort 2, the median PFS was 16.0 weeks in the lapatinib arm, 16.0 weeks in the lapatinib–pazopanib arm and 11.4 weeks in the pazopanib arm). The OS also was similar for the lapatinib alone and combination arms (median OS of 14.7 and 16.2 months, respectively, in cohort 1), while the OS could not be estimated for the combination arm in cohort 2.
Axitinib is an oral, potent and selective TKI of VEGFRs. A multicenter randomized, double-blind, Phase II study of axitinib plus docetaxel compared with docetaxel plus placebo as first-line therapy for patients with MBC, in which the TTP was set as the primary endpoint, showed that the median TTP was a month longer in the combination arm than in the placebo arm (8.1 vs. 7.1 months), but this difference was not statistically significant (HR, 1.24; 95% CI, 0.82–1.87; P = 0.156) (41). In a predefined subgroup analysis, the difference in the median TTP between the combination and placebo arms was greatest in patients who had received prior adjuvant chemotherapy (9.2 vs. 7.0 months; HR, 1.62; 95% CI, 0.93–2.81; P = 0.043).
Other multitargeted TKIs such as cediranib (Recentin™, AstraZeneca) and vandetanib (Zactima™, AstraZeneca) have been shown to have limited activity and increased toxicity when combined with fulvestrant or docetaxel (42,43).
Metronomic Chemotherapy
Antiangiogenic effect of metronomic chemotherapies has been widely studied in preclinical models (15). It is presumed that dose-dense chemotherapies mainly target proliferating tumor cells, while the main targets of continuous or frequent metronomic chemotherapy are the ‘activated’ endothelial cells of the newly forming tumor vasculature. It has been shown that the level of bone-marrow-derived circulating endothelial progenitor cells (CEPs) that contribute to neoangiogenesis in tumors reduces during metronomic chemotherapy while the CEP count rebounds during the intervals after the conventional maximum tolerated dose (44). Metronomic cyclophosphamide has been reported to increase the level of endogenous antiangiogenic factor thrombospondin 1, and metronomic cyclophosphamide and methotrexate in combination have been shown to reduce the serum VEGF level in breast cancer patients (45). Although metronomic chemotherapies with standard regimens such as cyclophosphamide, methotrexate, oral fluoropyrimidines and targeted agent like trastuzumab or ER antagonist have been studied in Phase II trials in breast cancer, metronomic chemotherapy plays an important role as the partner regimen with antiangiogenic agents (46). A Phase II trial of bevacizumab in conjunction with metronomic cyclophosphamide and methotrexate in Stage IV breast cancer demonstrated a promising result in terms of ORR and TTP and lower AEs (47). Several Phase III trials evaluating metronomic chemotherapy with bevacizumab in both early and advanced breast cancer and one in TNBC patients without antiangiogenic therapy are in progress in different parts of the world (12). Although the data of the ongoing trials are necessary to evaluate the efficacy of metronomic chemotherapy regimens in combination with antiangiogenic agents, weekly (metronomic-like) paclitaxel used in the E2100 trial in comparison with once-every-3-week docetaxel in the AVADO trial might explain the better result observed in the E2100 trial.
ANTIANGIOGENIC TREATMENT SAFETY
Addition of bevacizumab or TKIs to other chemotherapy regimens has usually been correlated with additional overall toxicity (48).
In Phase III trials in a metastatic setting, the incidence rates of Grade ≥3 bevacizumab-associated AEs (i.e. hypertension, proteinuria, bleeding event, arterial or venous thromboembolism, and left ventricular systolic dysfunction) were typically higher in the bevacizumab arms compared with control arms though they are usually well tolerated (13). However, the extent and frequency of AEs varied by chemotherapy partner. In RIBBON-1, the incidence rate of Grade 3–5 AEs was 34.3% in the bevacizumab arm vs.15.0% in the placebo arm among the anthracycline-receiving group, while it was 35.4 vs. 21.9% in the capecitabine–receiving group (19). Overall risk rate of developing high-grade congestive heart failure with bevacizumab compared with controls without bevacizumab is reported as 4.47% (95% CI, 1.84–12.19; P = 0.001) (49). In most of Phase III trials in the metastatic setting, the incidence of AEs resulting in discontinuation appears to be the same in bevacizumab vs. control arms (50) although, in RIBBON-1, chemotherapy partner seemed to have an impact on this incidence. While 24.4% of discontinuation occurred with bevacizumab vs. 7.8% with placebo in the taxane-receiving group, 14.3% occurred with bevacizumab vs.4.0% with placebo in the anthracycline-receiving group (19).
In the Phase III SUN 1064 trial, the frequencies of common AEs of any grade and of Grade 3 or 4 were higher with the combination of sunitinib with docetaxel (31). The only Grade 3 or 4 AE that occurred significantly more frequently with the combination compared with monotherapy was hand-foot syndrome (17 vs. 1%, respectively, P < 0.001). Neutropenia was the most common Grade 3/4 AE (combination, 46%; monotherapy, 44%). Treatment discontinuations due to toxicity were more frequent with the combination compared with docetaxel alone (29 vs. 21%).
The most common AE related to sorafenib treatment during the TIES studies was hand-foot skin reaction (HFSR) (33). Incidence rate of any grade of HFSR ranged from 36% (FM-B07-01) to 90% (SOLTI-0701) and that of Grade 3 HFSR, which is the most severe grade, was reported to range from 13% (FM-B07-01) to 44% (SOLTI-0701). Discontinuation due to AEs was higher in the sorafenib arm compared with the placebo group in all TIES trials (20 vs. 9% in SOLTI-0701, 21 vs. 6% for AC01B07, 22 vs. 6% in NU07B1 and 22 vs. 11% in FM-B07-01) (33).
PREDICTIVE MARKERS
Until today, for metastatic breast cancer, the most promising clinical benefit of involving antiangiogenic therapies plus chemotherapy has been improved PFS—a surrogate clinical endpoint- or pCR—a putative surrogate clinical endpoint—but no benefit for the OS. Many investigators have undertaken subgroup analysis to find predictive markers of future overall clinical benefit by allowing more refined patient selection and individualization for antiangiogenic therapies. Most of the data available today are from retrospective analyses of the prospective randomized trials. A common biomarker that has been studied is the levels of tumor-associated VEGF or circulating plasma VEGF to predict patient outcome. In a trial evaluating vinerolbine and bevacizumab in an advanced setting, plasma VEGF levels were measured at the baseline (51). The median TTP was 3.7 months for patients with VEGF > median (32.6 pg/ml) and 9.3 months for patients with VEGF ≤ median. Retrospective biomarker analyses of plasma and tumor DNA/RNA from available samples of the AVADO trial showed that patients with high plasma VEGF-A or VEGFR-2 concentrations at baseline appeared to benefit more from bevacizumab than those with lower VEGF-A or low VEGFR-2 concentrations (52) (Table 2). The VEGF enzyme-linked immunosorbent assay used in this analysis had greater sensitivity for shorter vs. longer isoforms of VEGF-A, i.e. VEGF111 and VEGF121. Data from AVEREL, a Phase III trial to evaluate bevacizumab in combination with trastuzumab plus docetaxel for HER-2 positive advanced breast cancers, showed that a larger bevacizumab treatment effect was observed in patients with high baseline VEGF-A than in those with low VEGF-A [HR, 0.83 (low) vs.0.70 (high); interaction P = 0.80] (21). Plasma VEGF and VEGFR-2 analysis from the BEATRICE trial, which is a study to evaluate bevacizumab adjuvant therapy in combination with chemotherapy in TNBCs, showed that high baseline plasma VEGFR-2 (median cut-off) had a potential predictive value for bevacizumab efficacy [HR, 1.24 (low) vs. 0.61 (high); P = 0.029] (53) (Table 2). The median cut-off for plasma VEGF did not show predictive value for bevacizumab efficacy and, with a third quartile cut-off, there was a more pronounced but non-significant differentiation between treatments [HR, 0.92 (low) vs. 0.64 (high); interaction P = 0.355]. Retrospective analysis on the E2100 trial included the investigation of five VEGF and two VEGFR-2 polymorphisms (54). Two VEGF genotypes (VEGF-2578AA and VEGF-1154AA) were significantly associated with improved OS in the bevacizumab plus paclitaxel group (interaction for treatment effect P = 0.023 and P = 0.001, respectively) (Table 2). These two polymorphisms had no prognostic effects on the OS in the paclitaxel plus placebo group. Two additional genotypes (VEGF-634CC and VEGF-1498TT) showed strong correlation with less Grade 3/4 hypertension in the combination arm when compared with all other genotypes combined (P = 0.005 and P = 0.022, respectively). In another correlative study of E2100, the ability for tumor VEGFA amplification to predict outcome was conducted (55). Patients with tumor VEGFA amplification had significantly worse median PFS (7.8 vs. 8.3 months; P = 0.040) and median OS (20.2 vs. 25.3 months; P = 0.013) than those whose tumors did not exhibit amplification (Table 3). While TNBC or HER-2 subtype patients with VEGFA amplification had inferior OS in comparison with the group without amplification (P = 0.047), this categorization did not change the OS in the luminal A subtype (P = 0.321). Bevacizumab-induced hypertension or an increase in antihypertensive medication required during bevacizumab treatment has been shown to be associated with extended PFS or OS in other malignancies. Grade 3 or 4 hypertension was significantly associated with increased duration of OS, compared with patients who had no hypertension (38.7 vs. 25.3 months, P = 0.002) in the E2100 trial (54).
Table 2.
Predictive biomarkers in Phase III trials
| Study | Standard treatment | Experiment regimen | Groups | Patients | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| E2100 | Paclitaxel | Paclitaxel + bevacizumab | VEGF-2578 AA vs. CA + CC | 180 | 0.58 (0.36–0.93) | 0.023 |
| VEGF-1154 AA vs. GA vs. GG | 180 | 0.62 (0.46–0.83) | 0.001 | |||
| AVADO | Docetaxel + placebo | Docetaxel + bevacizumab 7.5 | VEGF-A ≤ median | 127 | 0.96 (0.62–1.48) | 0.014 |
| VEGF-A > median | 128 | 0.52 (0.33–0.81) | ||||
| Docetaxel + placebo | Docetaxel + bevacizumab 15 | VEGF-A ≤ median | 139 | 0.86 (0.56–1.32) | 0.08 | |
| VEGF-A > median | 126 | 0.49 (0.31–0.76) | ||||
| AVADO | Docetaxel + placebo | Docetaxel + bevacizumab 7.5 | VEGFR-2 ≤ median | 133 | 1.10 (0.73–1.67) | 0.032 |
| VEGFR-2 > median | 122 | 0.46 (0.28–0.74) | ||||
| Docetaxel + placebo | Docetaxel + bevacizumab 15 | VEGFR-2 ≤ median | 134 | 075 (0.49–1.16) | 0.255 | |
| VEGFR-2 > median | 131 | 0.54 (0.35–0.85) | ||||
| AVEREL | Docetaxel + trastuzumab | Docetaxel + trastuzumab + bevacizumab | VEGF-A ≤ median | 81 | 0.83 (0.50–1.36) | 0.80 |
| VEGF-A > median | 80 | 0.70 (0.43–1.14) | ||||
| BEATRICE | Taxane/anthracycline | Taxane/anthracycline + bevacizumab | VEGFR-2 ≤ median | 586 | 1.24 (0.82–1.89) | 0.029 |
| VEGFR-2 > median | 586 | 0.61 (0.39–0.97) |
VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Table 3.
Clinical implication of VEGF gene amplification sub-analysis of the E2100 trial
| Study | Population (number) | Group | Patients | Median progression-free survival (months) | P-value | Overall survival (months) | P-value |
|---|---|---|---|---|---|---|---|
| E2100 | All (722) | Paclitaxel vs. paclitaxel + bevacizumab | 326 | 5.8 | <0.0001 | 25.2 | 0.16 |
| 347 | 11.3 | 26.7 | |||||
| Analyzable for VEGFA | VEGFA amplified/borderline (amp/BA+) vs. VEGFA normal/deleted (amp/BA−) | 52 | 7.8 | 0.040 | 20.2 | 0.013 | |
| Amplification (324) | 272 | 8.3 | 25.3 | ||||
| Paclitaxel + bevacizumab (157) | VEGFA amp/BA+ vs. amp/BA− | 24 | 10.5 | 0.010 | 21.0 | 0.042 | |
| 133 | 11.3 | 25.6 | |||||
| VEGFA amp/BA+ (52) | Paclitaxel vs. paclitaxel + bevacizumab | 28 | 5.7 | 0.438 | 16.9 | 0.973 | |
| 24 | 10.5 | 21.0 | |||||
| VEGFA amp/BA− (272) | Paclitaxel vs. paclitaxel + bevacizumab | 139 | 5.5 | 6.29 × 10−5 | 24.8 | 0.472 | |
| 133 | 11.3 | 25.6 |
In a Phase II clinical study to evaluate sunitinib with an anthracycline and a taxane in an advanced setting, plasma levels of the soluble biomarkers VEGF, soluble VEGFR-2 (sVEGFR-2), soluble VEGFR-3 (sVEGFR-3) and soluble KIT (sKIT) were measured before and in 2-week intervals during the first cycle, in 4-week intervals during cycle 2 and 3, the first day of subsequent cycles and at the end of the treatment (28). The mean plasma level of all four biomarkers significantly decreased in the first cycle of sunitinib treatment (P < 0.00005). However, only decreases in sKIT levels by ≥50% at the start or end of the last treatment cycle compared with an sKIT level <50% decrease showed statistically significant association with longer TTP (median, 22.1 vs. 10.1 weeks; P < 0.0001) and OS (median, 62.6 vs. 36.0 weeks; P = 0.0194). To the best of our knowledge, no biomarker analysis data are available from TIES trials.
Other factors such as the previous chemotherapy regimen have also shown predictive value. An axitinib Phase II trial showed a higher median TTP among patients who had received prior adjuvant chemotherapy (9.2 vs. 7.0 months; P = 0.043) (41). Also in the E2100 trial, patients receiving prior adjuvant taxane appeared to receive the greatest benefit from addition of bevacizumab (median PFS 12 vs. 3 months; HR, 0.46; 95% CI, 0.30–0.71) (56). In the MERiDiAN trial, a prospective clinical study that has started recruitment, patients are stratified according to baseline plasma VEGF-A concentrations before randomization to the same regimen as in E2100 (57).
IMAGING FOR ASSESSMENT OF RESPONSE TO ANTIANGIOGENIC TREATMENT
Response Evaluation Criteria in Solid Tumors (RECIST) are widely accepted criteria being adopted in clinical trials for categorizing patients into responding, stable or progressing groups during treatment (58). Although magnetic resonance imaging (MRI) is the standard technique applied in RECIST for breast cancer, other techniques such as positron emission tomography–computed tomography are being considered as part of routine methods of tumor response assessment mostly when targeted therapies are being used (59). These therapies cause microenvironment changes prior to morphological changes that can be assessed by anatomical imaging. Dynamic contrast-enhanced MRI (DCE-MRI) that can monitor changes in microvascular structure as well as function (60), is yet to be routinely used in multicenter clinical trials due to lack of standardization (61). However, in some early phase trials using antiangiogenic therapies multiparametric DCE-MRI data have shown considerable promise (61–65).
DCE-MRI at baseline and during treatment was performed in a pilot trial on 20 patients with inflammatory and locally advanced breast cancers, who were treated with bevacizumab plus doxorubicin and docetaxel (62). It was shown that the inflow rate constant from vascular space to the tumor had decreased by 58% between baseline and cycle 4 (P < 0.0001) but only by 12.4% from cycle 4 to cycle 7 (P = 0.76). This could be interpreted that the overall vascular permeability change due to treatment effect occurred in the earlier course of therapy. Further MR analytic methods were retrospectively compared using the data from the same pilot trial to determine the strongly associated parameter or combination of parameters with response to bevacizumab therapy alone or in combination with chemotherapy (63). It was reported that none of the assessed methods could predict clinical response after cycle 1 but heuristic slope wash-in could differentiate responders and non-responders at cycle 4 (P = 0.009), implying that DCE-MRI can provide reliable information to evaluate the effects of bevacizumab. In an ongoing, Phase II, non-randomized, open-label investigator-led study from Oxford, where only a single infusion of bevacizumab was given 2 weeks before starting neoadjuvant chemotherapy, preliminary imaging results illustrated the considerable heterogeneity in patient responses to bevacizumab from significant reduction in permeability and blood flow over the extent of the tumor to a large central necrotic core and little or no change in the tumor vasculature when compared with the baseline MRI (64). Although imaging results were the vascular pattern change over this 2-week period only, they were in accordance with expression fold changes in hypoxia, and proliferation signatures obtained from core needle biopsy performed at the same time of MRI assessment. In a study on 49 inoperable breast cancer patients where patients were randomized to receive preoperatively either docetaxel alone or the combination of docetaxel and bevacizumab, DCE-MRI showed a greater decrease in contrast distributed area with the combination treatment compared with docetaxel alone at the end of cycle 1 (P = 0.024), which could be the result of a greater decrease in tumor blood perfusion by the addition of bevacizumab (65).
Normalizing tumor vasculature by antiangiogenic treatment modifies blood perfusion in tumors. In a French study, 40 breast cancer patients were selected to undergo 2-deoxy-2-[fluorine-18]fluoro-d-glucose PET/CT a week after biopsy samples of each tumor was taken to assess the Ki67 index of proliferation and immunostaining for CD34 (endothelial marker) and CD105 (proliferating endothelial marker) and before any treatment started (66). The standardized uptake value maximal index (SUVmax), reflecting tumor metabolism, correlated strongly and positively with the expression of Ki67 (r = +0.69; P = 0.0001) and tumor blood flow correlated positively with the expression of CD34 and CD105 (P = 0.016 and P = 0.007, respectively). Diffuse optical spectroscopic imaging (DOSI) is a non-invasive functional imaging modality that uses near infrared light and is capable of measuring tissue concentrations of metabolic parameters such as oxygenated hemoglobin and deoxygenated hemoglobin, which are directly related to tumor vascular characteristics (67). A retrospective study at University of California, Irvine on 41 primary breast cancer patients who received neoadjuvant chemotherapy showed that pre-therapy tumor tissue oxygen saturation (stO2) is the single best DOSI-derived predictor of the pCR (68). Although there was an insufficient number of tumors treated with bevacizumab that achieved the pCR to statistically compare stO2 values in this treatment subgroup, other imaging analysis on tumor hemoglobin might be of great value in assessing tumor response to neoadjuvant antiangiogenic therapies.
Contrast-enhanced ultrasound (CEUS) has been studied in solid tumors and is considered to be applicable for the detection of the vascular architecture of breast cancers (69). This technique has been applied in evaluating response in neoadjuvant chemotherapy by assessing blood perfusion changes (70). A French multicenter study on 539 patients with solid tumor, including 61 patients with metastatic breast cancer, described the standardization technique for dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic treatments in solid tumors (71). Sonazoid® is a microbubble contrast that has been studied in hepatocarcinomas (72). A preliminary study among 10 breast cancer patients has shown that CEUS using Sonazoid® can be used to assess response to bevacizumab in combination with taxanes (73). Although well-designed clinical studies are more than necessary to evaluate the accuracy of imaging modalities, assessing tumor vascularity and oxygenation before and during antiangiogenic therapies by new imaging modalities such as photoacoustic mammography (74) might be a useful tool in providing predictive markers for better selecting patients for antiangiogenic therapies.
HORMONE-RECEPTOR STATUS IN PATIENT SELECTION
Identifying subgroups that benefit from a specific therapy such as anti-HER2 or hormone-receptor antagonists is not a novel concept in breast cancer. Although angiogenesis is clearly described to be one of the fundamental hallmarks of all cancers (75), the angiogenic pathways involved might be different between different subtypes in breast cancer. Data from GBG 44 showed variations in treatment effect predominantly to be related to a potential differential activity of bevacizumab according to hormone-receptor status (25). While hormone positive patients did not show much benefit from addition of bevacizumab (pCR, 7.7 vs. 7.8%, P = 1.00), among triple-negative tumors, the rates of the pCR were 27.9% in the group that received chemotherapy and 39.3% in the group that received chemotherapy plus bevacizumab (P = 0.003). However, in the NSABP B-40 trial the increase in the pCR was higher in the hormone-receptor positive subset after addition of bevacizumab (11.1% without bevacizumab vs. 16.8% with bevacizumab, P = 0.03) (26). The differences in the results from these two trials could be explained by differences in HER2-negativity criteria and in the therapy regimens they received. Subgroup analysis of the ATHENA trial showed a median TTP of 7.2 months (95% CI, 6.6–7.8) in the TNBC subgroup, of 10.6 months in patients with non-TNBC and of 9.7 months in the overall population (76). The ORR in the TNBC subgroup was reported to be 49% vs. 56% in the non-TNBC subgroup though the safety profile of bevacizumab in patients with TNBC was qualitatively similar to that in the overall population. These data along with the result from plasma biomarkers from the BEATRICE study suggest the necessity of considering biological and chemosensitivity differences in different breast cancer subtypes.
CONCOMITANT CHEMOTHERAPY REGIMEN
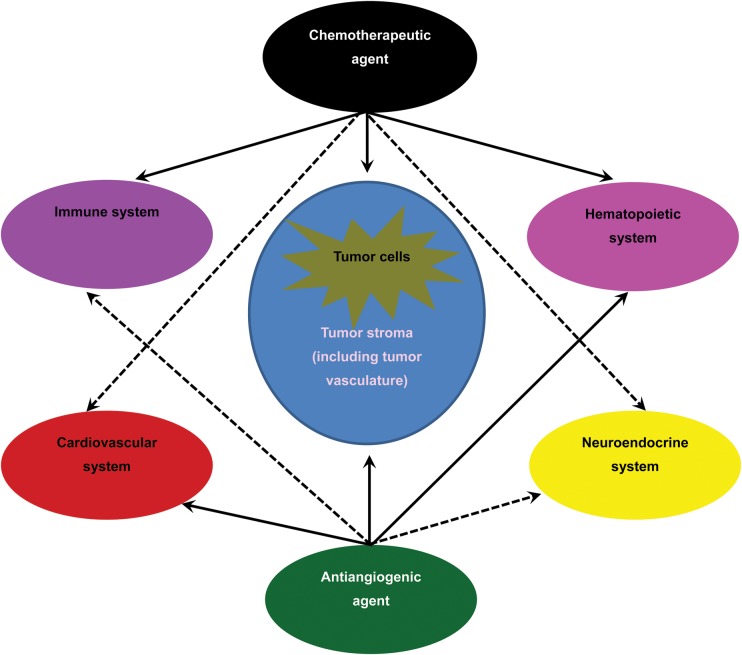
Hypothetical dose–response curves of various metronomic cyclophosphamide effects suggesting different antiangiogenic, immune stimulation or suppression, myelosuppression and tumor cell cytotoxicity effects in relation to different doses have not been thoroughly explored for other chemotherapeutic agents (77). It is probable that the sequence of these effects changes with different drugs with or without an antiangiogenic agent (Fig. 1) At the same time, clinical trial data suggest that the type of chemotherapy used in combination with bevacizumab have an impact on the added clinical benefit (50,78). Although the theory of tumor blood vessel ‘normalization’ has been well studied in cancer models (3), it is yet to be clinically validated in breast cancer. On the other hand, there is a report that bevacizumab treatment reduced intratumoral delivery of docetaxel (instead of increasing it if tumor vessel normalization had occurred) (79). In addition, it is shown that chemotherapy drugs have variable effects on bone marrow and mobilizing progenitor endothelial cells which can provide niche to tumor cells (80). Although blocking of this mobilization can be reduced by using some of the angiogenesis inhibitors, future clinical studies are needed to find the best combination. On the other hand, as discussed earlier, dose and schedule of concomitant chemotherapy are important factors for tolerance and AE prevalence. Although preclinical and Phase II clinical studies have shown promising results, data from ongoing Phase III trials evaluating oral metronomic chemotherapy regimens may help find some answers to the above-mentioned discrepancies (12).
Figure 1.
Various effects of chemotherapeutic agents and antiangiogenic agents. Different chemotherapeutic agents induce different side effects and sometimes cause complications based on the different system they affect in the body. Although the main target of antiangiogenic agents is endothelial cells, other systems beside the cardiovascular system are impacted by these therapies as well. Bevacizumab as well as tyrosine kinase inhibitors not only can modulate the immune system in a positive way but also can cause harmful effects such as reduction of T-cell proliferation and cytokine production (ref.81). The sequence and order of these effects might change in different combinations of chemotherapy and antiangiogenic agents, leading to different efficacies and side effects. Bold arrows show main effects. Dashed arrows show minor or possible effects.
CONCLUSION
Combination of angiogenesis inhibitors with standard chemotherapy regimens in metastatic breast cancer so far has resulted in modest clinical efficacy. Small molecule antiangiogenic TKIs have not shown efficacy in breast cancer treatment till today. The role of antiangiogenic therapies in improving clinical outcome in the adjuvant setting is yet to be thoroughly evaluated. Translational research and identification of biological predictive markers from past clinical trials could bring more insight with regard to optimizing the therapeutic index of the agents studied with or without chemotherapy. This could also help in designing future studies and selecting most likely winning patients and establishing clear clinical benefit for antiangiogenic treatment in breast cancer. Imaging modalities can be useful by providing information regarding response evaluation. Future antiangiogenesis trials should be more regimen-, dose- and patient-specific as these treatments act like targeted therapies in breast cancer and need to be more individualized.
Funding
This study was partly supported by the Innovative Techno-Hub for Integrated Medical Bio-imaging of the Project for Developing Innovation Systems, from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Funding to pay the Open Access publication charges for this article was provided by Department of Breast Surgery, Graduate School of Medicine, Kyoto University.
Conflict of interest statement
Masakazu Toi has received compensation for a consultant role, honoraria and research funding from Chugai Pharmaceuticals.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–15. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 5.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rody A, Karn T, Liedtke C, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender RJ, Mac Gabhann F. Expression of VEGF and semaphorin genes define subgroups of triple negative breast cancer. PLoS One. 2013;8:e61788. doi: 10.1371/journal.pone.0061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–65. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro-Silva A, Ribeiro do Vale F, Zucoloto S. Vascular endothelial growth factor expression in the basal subtype of breast carcinoma. Am J Clin Pathol. 2006;125:512–8. doi: 10.1309/D744-C4NM-15J3-B00D. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Antiangiogenesis agents. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 7th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 2865–82. [Google Scholar]
- 12.Kerbel RS. Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast cancer. J Mammary Gland Biol Neoplasia. 2012;17:229–39. doi: 10.1007/s10911-012-9266-0. [DOI] [PubMed] [Google Scholar]
- 13.Mackey JR, Kerbel RS, Gelmon KA, et al. Controlling angiogenesis in breast cancer: a systematic review of anti-angiogenic trials. Cancer Treat Rev. 2012;38:673–88. doi: 10.1016/j.ctrv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Mackey J, Gelmon K, Martin M, et al. TRIO-012: a multicenter, multinational, randomized, double-blind phase III study of IMC-1121B plus docetaxel versus placebo plus docetaxel in previously untreated patients with HER2-negative, unresectable, locally recurrent or metastatic breast cancer. Clin Breast Cancer. 2009;9:258–61. doi: 10.3816/CBC.2009.n.044. [DOI] [PubMed] [Google Scholar]
- 15.Munoz R, Shaked Y, Bertolini F, Emmenegger U, Man S, Kerbel RS. Anti-angiogenic treatment of breast cancer using metronomic low-dose chemotherapy. Breast. 2005;14:466–79. doi: 10.1016/j.breast.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 17.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 18.Pivot X, Schneeweiss A, Verma S, et al. Efficacy and safety of bevacizumab in combination with docetaxel for the first-line treatment of elderly patients with locally recurrent or metastatic breast cancer: results from AVADO. Eur J Cancer. 2011;47:2387–95. doi: 10.1016/j.ejca.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–60. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 20.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–93. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 21.Gianni L, Romieu GH, Lichinitser M, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–25. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 22.Pierga JY, Petit T, Delozier T, et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. 2012;13:375–84. doi: 10.1016/S1470-2045(12)70049-9. [DOI] [PubMed] [Google Scholar]
- 23.Aogi K, Masuda N, Ohno S, et al. First-line bevacizumab in combination with weekly paclitaxel for metastatic breast cancer: efficacy and safety results from a large, open-label, single-arm Japanese study. Breast Cancer Res Treat. 2011;129:829–38. doi: 10.1007/s10549-011-1685-x. [DOI] [PubMed] [Google Scholar]
- 24.Smith IE, Pierga JY, Biganzoli L, et al. First-line bevacizumab plus taxane-based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open-label study in 2,251 patients. Ann Oncol. 2011;22:595–602. doi: 10.1093/annonc/mdq430. [DOI] [PubMed] [Google Scholar]
- 25.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 26.Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–20. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–79. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 28.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 29.Wildiers H, Fontaine C, Vuylsteke P, et al. Multicenter phase II randomized trial evaluating antiangiogenic therapy with sunitinib as consolidation after objective response to taxane chemotherapy in women with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2010;123:463–9. doi: 10.1007/s10549-010-1066-x. [DOI] [PubMed] [Google Scholar]
- 30.Barrios CH, Liu MC, Lee SC, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat. 2010;121:121–31. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergh J, Bondarenko IM, Lichinitser MR, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–9. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 32.Robert NJ, Saleh MN, Paul D, et al. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer. 2011;11:82–92. doi: 10.1016/j.clbc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gradishar WJ. Sorafenib in locally advanced or metastatic breast cancer. Expert Opin Investig Drugs. 2012;21:1177–91. doi: 10.1517/13543784.2012.689824. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J, Segalla JG, Roché H, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–91. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 35.Gradishar WJ, Kaklamani V, Sahoo TP, et al. A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer. 2013;49:312–22. doi: 10.1016/j.ejca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Schwartzberg LS, Tauer KW, Hermann RC, et al. Sorafenib or placebo with either gemcitabine or capecitabine in patients with HER-2-negative advanced breast cancer that progressed during or after bevacizumab. Clin Cancer Res. 2013;19:2745–54. doi: 10.1158/1078-0432.CCR-12-3177. [DOI] [PubMed] [Google Scholar]
- 37.Mariani G, Burdaeva O, Roman L, et al. A double-blind, randomized phase IIb study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with docetaxel and/or letrozole in patients with metastatic breast cancer (MBC): FM-B07-01 Trial. Eur J Cancer. 2011;14(Suppl. 2):10.t al. [Google Scholar]
- 38.Baselga J, Costa F, Gomez H, et al. A phase 3 tRial comparing capecitabinE in combination with SorafenIb or pLacebo for treatment of locally advanced or metastatIc HER2-Negative breast CancEr (the RESILIENCE study): study protocol for a randomized controlled trial. Trials. 2013;14:228. doi: 10.1186/1745-6215-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston SR, Gómez H, Stemmer SM, et al. A randomized and open-label trial evaluating the addition of pazopanib to lapatinib as first-line therapy in patients with HER2-positive advanced breast cancer. Breast Cancer Res Treat. 2013;137:755–66. doi: 10.1007/s10549-012-2399-4. [DOI] [PubMed] [Google Scholar]
- 40.Cristofanilli M, Johnston SR, Manikhas A, et al. A randomized phase II study of lapatinib + pazopanib versus lapatinib in patients with HER2+ inflammatory breast cancer. Breast Cancer Res Treat. 2013;137:471–82. doi: 10.1007/s10549-012-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rugo HS, Stopeck AT, Joy AA, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol. 2011;29:2459–65. doi: 10.1200/JCO.2010.31.2975. [DOI] [PubMed] [Google Scholar]
- 42.Hyams DM, Chan A, de Oliveira C, et al. Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a randomized Phase II study. Invest New Drugs. 2013;31:1345–54. doi: 10.1007/s10637-013-9991-2. [DOI] [PubMed] [Google Scholar]
- 43.Boér K, Láng I, Llombart-Cussac A, et al. Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized Phase II study. I Invest New Drugs. 2012;30:681–7. doi: 10.1007/s10637-010-9538-8. [DOI] [PubMed] [Google Scholar]
- 44.Mancuso P, Colleoni M, Calleri A, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–9. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colleoni M, Orlando L, Sanna G, et al. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol. 2006;17:232–8. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 46.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 47.Burstein HJ, Spigel D, Kindsvogel K, et al. Metronomic chemotherapy with and without bevacizumab for advanced breast cancer: a randomized phase II trial. Breast Cancer Res Treat. 2005;94(Suppl. 1):S6. [Google Scholar]
- 48.Giovannini M, Aldrighetti D, Zucchinelli P, Belli C, Villa E. Antiangiogenic strategies in breast cancer management. Crit Rev Oncol Hematol. 2010;76:13–35. doi: 10.1016/j.critrevonc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Choueiri TK, Mayer EL, Je Y. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29:632–8. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 50.Rossari JR, Metzger-Filho O, Paesmans M, et al. Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol. 2012;2012:417673. doi: 10.1155/2012/417673. Epub 12 September 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burstein HJ, Chen YH, Parker LM, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res. 2008;14:7871–7. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]
- 52.Miles DW, de Haas SL, Dirix LY, et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer. 2013;108:1052–60. doi: 10.1038/bjc.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–42. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 54.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–8. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider BP, Gray RJ, Radovich M, et al. Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res. 2013;19:1281–9. doi: 10.1158/1078-0432.CCR-12-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derleth C, Mayer IA. Antiangiogenic therapies in early-stage breast cancer. Clin Breast Cancer. 2010;10(Suppl. 1):E23–31. doi: 10.3816/CBC.2010.s.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rugo HS. Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning? J Clin Oncol. 2012;30:898–901. doi: 10.1200/JCO.2011.38.5492. [DOI] [PubMed] [Google Scholar]
- 58.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–9. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 59.Cheng J, Lei L, Xu J, et al. 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med. 2013;54:333–40. doi: 10.2967/jnumed.112.111963. [DOI] [PubMed] [Google Scholar]
- 60.Tateishi U, Miyake M, Nagaoka T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging-prospective assessment. Radiology. 2012;263:53–63. doi: 10.1148/radiol.12111177. [DOI] [PubMed] [Google Scholar]
- 61.O'Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167–77. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 62.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–77. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 63.Thukral A, Thomasson DM, Chow CK, et al. Inflammatory breast cancer: dynamic contrast-enhanced MR in patients receiving bevacizumab-initial experience. Radiology. 2007;244:727–35. doi: 10.1148/radiol.2443060926. [DOI] [PubMed] [Google Scholar]
- 64.Mehta S, Hughes NP, Buffa FM, et al. Assessing early therapeutic response to bevacizumab in primary breast cancer using magnetic resonance imaging and gene expression profiles. J Natl Cancer Inst Monogr. 2011;2011:71–4. doi: 10.1093/jncimonographs/lgr027. [DOI] [PubMed] [Google Scholar]
- 65.Baar J, Silverman P, Lyons J, et al. A vasculature-targeting regimen of preoperative docetaxel with or without bevacizumab for locally advanced breast cancer: impact on angiogenic biomarkers. Clin Cancer Res. 2009;15:3583–90. doi: 10.1158/1078-0432.CCR-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cochet A, Pigeonnat S, Khoury B, et al. Evaluation of breast tumor blood flow with dynamic first-pass 18F-FDG PET/CT: comparison with angiogenesis markers and prognostic factors. J Nucl Med. 2012;53:512–20. doi: 10.2967/jnumed.111.096834. [DOI] [PubMed] [Google Scholar]
- 67.O'Sullivan TD, Leproux A, Chen JH, et al. Optical imaging correlates with magnetic resonance imaging breast density and reveals composition changes during neoadjuvant chemotherapy. Breast Cancer Res. 2013;15:R14. doi: 10.1186/bcr3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ueda S, Roblyer D, Cerussi A, et al. Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Res. 2012;72:4318–28. doi: 10.1158/0008-5472.CAN-12-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan Z, Quan J, Yunxiao Z, Jian C, Zhu H, Liping G. Diagnostic value of contrast-enhanced ultrasound parametric imaging in breast tumors. J Breast Cancer. 2013;16:208–13. doi: 10.4048/jbc.2013.16.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao X, Xue J, Zhao B. Potential application value of contrast-enhanced ultrasound in neoadjuvant chemotherapy of breast cancer. Ultrasound Med Biol. 2012;38:2065–71. doi: 10.1016/j.ultrasmedbio.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 71.Lassau N, Chapotot L, Benatsou B, et al. Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: the French multicenter Support for Innovative and Expensive Techniques Study. Invest Radiol. 2012;47:711–6. doi: 10.1097/RLI.0b013e31826dc255. [DOI] [PubMed] [Google Scholar]
- 72.Kudo M, Matsui O, Sakamoto M, et al. Role of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in the management of hepatocellular carcinoma: consensus at the Symposium of the 48th Annual Meeting of the Liver Cancer Study Group of Japan. Oncology. 2013;84(Suppl. 1):21–7. doi: 10.1159/000345885. [DOI] [PubMed] [Google Scholar]
- 73.Ito T, Mizuno H, Iiboshi Y, et al. Angiogenic effect of bevacizumab and paclitaxel in metastatic breast cancer: evaluation by contrast-enhanced ultrasonography using Sonazoid®. Cancer Res. 2012;72(24 Suppl.) Abstract nr P4-03-07. [Google Scholar]
- 74.Kitai T, Torii M, Sugie T, et al. Photoacoustic mammography: initial clinical results. Breast Cancer. 2012 doi: 10.1007/s12282-012-0363-0. doi:10.1007/s12282-012-0363-0. [DOI] [PubMed] [Google Scholar]
- 75.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 76.Thomssen C, Pierga JY, Pritchard KI, et al. First-line bevacizumab-containing therapy for triple-negative breast cancer: analysis of 585 patients treated in the ATHENA study. Oncology. 2012;82:218–27. doi: 10.1159/000336892. [DOI] [PubMed] [Google Scholar]
- 77.Emmenegger U, Francia G, Shaked Y, Kerbel RS. In: Angiogenesis Inhibition (Recent Results in Cancer Research) Liersch R, Berdel WE, Kessler T, editors. Berlin: Springer; 2010;. pp. 165–83. ‘Metronomic chemotherapy: principles and lessons learned from applications in the treatment of metastatic prostate cancer’. [Google Scholar]
- 78.Miles DW, Diéras V, Cortés J, Duenne AA, Yi J, O'Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;11:10–29. doi: 10.1093/annonc/mdt276. [DOI] [PubMed] [Google Scholar]
- 79.Van der Veldt AA, Lubberink M, Bahce I, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 80.Roodhart JM, Langenberg MH, Vermaat JS, et al. Late release of circulating endothelial cells and endothelial progenitor cells after chemotherapy predicts response and survival in cancer patients. Neoplasia. 2010;12:87–94. doi: 10.1593/neo.91460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heine A, Held SA, Bringmann A, Holderried TA, Brossart P. Immunomodulatory effects of anti-angiogenic drugs. Leukemia. 2011;25:899–905. doi: 10.1038/leu.2011.24. [DOI] [PubMed] [Google Scholar]