Abstract
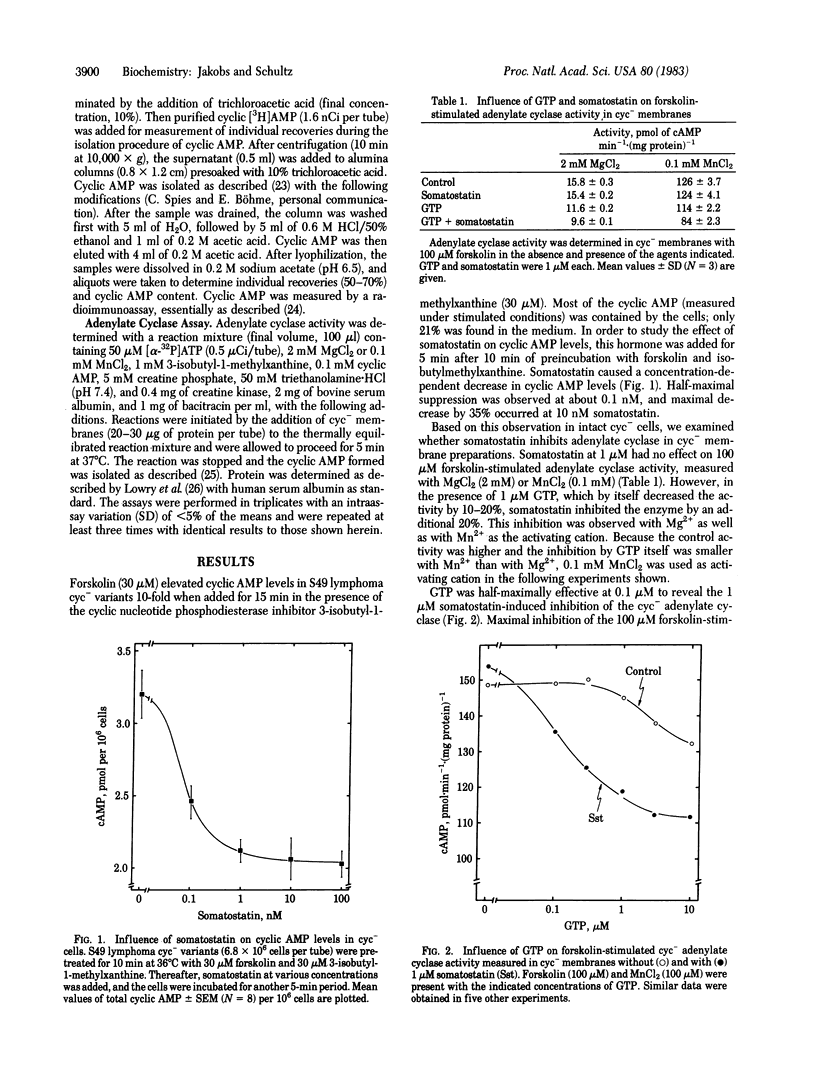
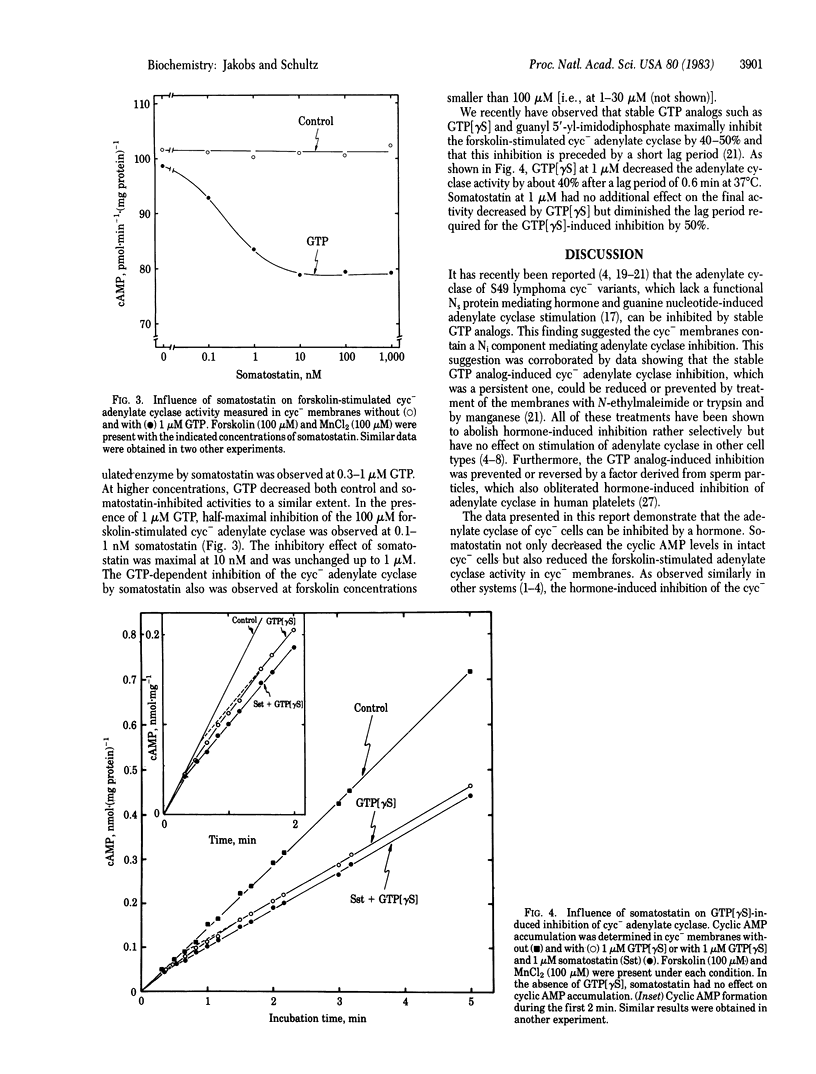
The influence of somatostatin was studied on cyclic AMP levels and adenylate cyclase activity in cyc- variants of S49 lymphoma cells. These cells are deficient in the guanine nucleotide site that mediates hormone-induced adenylate cyclase stimulation, but their cyclase can be stimulated by forskolin. Somatostatin maximally decreased the 30 microM forskolin-stimulated cyclic AMP levels by 35%. Half-maximal suppression occurred at about 0.1 nM somatostatin. Somatostatin (up to 1 microM) had no effect on the 100 microM forskolin-stimulated adenylate cyclase activity in cyc- membrane preparations when guanine nucleotides were not present. In the presence of GTP, however, which by itself caused a small decrease in activity, somatostatin maximally inhibited the enzyme by 20-25%. GTP was half-maximally effective at 0.1 microM, and half-maximal inhibition by somatostatin was observed at 0.1- 1 nM. In the presence of the stable GTP analog guanosine 5'-O-(3-thiotriphosphate) (1 microM), which decreased the stimulated activity by about 40% after a short lag period, somatostatin (1 microM) did not cause a further decrease in final activity but reduced the lag period by about 50%. The data indicate that membranes of cyc- variants contain a regulatory site that mediates both guanine nucleotide and hormone-induced inhibition of the adenylate cyclase and suggest that the mechanisms of activation and inactivation of this inhibitory site are similar to those of the stimulatory component missing in cyc-membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Schultz G., Jakobs K. H. Cholera toxin does not impair hormonal inhibition of adenylate cyclase and concomitant stimulation of a GTPase in adipocyte membranes. Biochim Biophys Acta. 1982 Oct 28;719(1):58–64. doi: 10.1016/0304-4165(82)90307-5. [DOI] [PubMed] [Google Scholar]
- Aktories K., Schultz G., Jakobs K. H. Cholera toxin inhibits prostaglandin E1 but not adrenaline-induced stimulation of GTP hydrolysis in human platelet membranes. FEBS Lett. 1982 Sep 6;146(1):65–68. doi: 10.1016/0014-5793(82)80706-0. [DOI] [PubMed] [Google Scholar]
- Aktories K., Schultz G., Jakobs K. H. Inactivation of the guanine nucleotide regulatory site mediating inhibition of the adenylate cyclase in hamster adipocytes. Naunyn Schmiedebergs Arch Pharmacol. 1982 Dec;321(4):247–252. doi: 10.1007/BF00498508. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M. Bimodal regulation of adenylate cyclase. FEBS Lett. 1982 Feb 22;138(2):157–163. doi: 10.1016/0014-5793(82)80431-6. [DOI] [PubMed] [Google Scholar]
- Cote T. E., Grewe C. W., Tsuruta K., Stoof J. C., Eskay R. L., Kebabian J. W. D-2 dopamine receptor-mediated inhibition of adenylate cyclase activity in the intermediate lobe of the rat pituitary gland requires guanosine 5'-triphosphate. Endocrinology. 1982 Mar;110(3):812–819. doi: 10.1210/endo-110-3-812. [DOI] [PubMed] [Google Scholar]
- Gespach C., Dupont C., Bataille D., Rosselin G. Selective inhibition by somatostatin of cyclic AMP production in rat gastric glands: demonstration of a direct effect on the parietal cell function. FEBS Lett. 1980 Jun 2;114(2):247–252. doi: 10.1016/0014-5793(80)81126-4. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Scheer A. G., Smith M. M. Differential modification of the interaction of cardiac muscarinic cholinergic and beta-adrenergic receptors with a guanine nucleotide binding component(s). Mol Pharmacol. 1982 May;21(3):570–580. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Heindel J. J., Williams E., Robison G. A., Strada S. J. Inhibition of GH1 rat pituitary tumor cell adenylyl cyclase activity by somatostatin. J Cyclic Nucleotide Res. 1978 Dec;4(6):453–462. [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):113–119. doi: 10.1007/BF00500275. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Gehring U., Gaugler B., Pfeuffer T., Schultz G. Occurrence of an inhibitory guanine nucleotide-binding regulatory component of the adenylate cyclase system in cyc- variants of S49 lymphoma cells. Eur J Biochem. 1983 Feb 15;130(3):605–611. doi: 10.1111/j.1432-1033.1983.tb07192.x. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H. Inhibition of adenylate cyclase by hormones and neurotransmitters. Mol Cell Endocrinol. 1979 Dec;16(3):147–156. doi: 10.1016/0303-7207(79)90023-6. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Lasch P., Minuth M., Aktories K., Schultz G. Uncoupling of alpha-adrenoceptor-mediated inhibition of human platelet adenylate cyclase by N-ethylmaleimide. J Biol Chem. 1982 Mar 25;257(6):2829–2833. [PubMed] [Google Scholar]
- Jakobs K. H., Saur W., Schultz G. Reduction of adenylate cyclase activity in lysates of human platelets by the alpha-adrenergic component of epinephrine. J Cyclic Nucleotide Res. 1976 Nov-Dec;2(6):381–392. [PubMed] [Google Scholar]
- Jakobs K. H., Schultz G. Different inhibitory effect of adrenaline on platelet adenylate cyclase in the presence of GTP plus cholera toxin and of stable GTP analogues. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):121–127. doi: 10.1007/BF00500276. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Farfel Z., Bourne H. R. Genetic analysis of hormone-sensitive adenylate cyclase. Adv Cyclic Nucleotide Res. 1980;13:1–37. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mellwig K. P., Jakobs K. H. Inhibition of platelet adenylate cyclase by ADP. Thromb Res. 1980 Apr 1;18(1-2):7–17. doi: 10.1016/0049-3848(80)90166-8. [DOI] [PubMed] [Google Scholar]
- Propst F., Hamprecht B. Opioids, noradrenaline and GTP analogs inhibit cholera toxin activated adenylate cyclase in neuroblastoma x glioma hybrid cells. J Neurochem. 1981 Feb;36(2):580–588. doi: 10.1111/j.1471-4159.1981.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Rouleau D., Barden N. Inhibition of anterior pituitary prostaglandin-stimulated adenylyl cyclase activity by somatostatin. Can J Biochem. 1981 Apr;59(4):307–310. doi: 10.1139/o81-042. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Stiles G. L., Lefkowitz R. J. Hormone-sensitive adenylate cyclase. Delineation of a trypsin-sensitive site in the pathway of receptor-mediated inhibition. J Biol Chem. 1982 Jun 10;257(11):6287–6291. [PubMed] [Google Scholar]
- Vinicor F., Higdon G., Clark C. M., Jr Effects of somatostatin on the hepatic adenylate cyclase system in the rat. Endocrinology. 1977 Oct;101(4):1071–1077. doi: 10.1210/endo-101-4-1071. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]



