Abstract
We have developed a rapid screening assay that allows us to estimate the alkaline phosphatase content of mouse L-M cell colonies immobilized on polyester cloth. This permitted the identification and isolation of two mutant clones with increased constitutive alkaline phosphatase activity and six clones that fail to express this activity when treated with dibutyryl cyclic AMP. Both of the strains with increased constitutive activity have basal enzymatic activities that are 6- to 7-fold higher than the activity of the parental strain. The extents to which the cyclic nucleotide further induces alkaline phosphatase in these two strains are different, however, indicating that they represent two classes of mutants. Studies using amino acids and synthetic peptides as alkaline phosphatase inhibitors suggest that only one alkaline phosphatase isoenzyme predominates, in both the parental and the mutant cell lines, with or without induction by cyclic nucleotide. Comparison to mouse tissues indicates that our cell lines express an isozyme resembling that found in kidney and bone. The six clones that fail to express alkaline phosphatase activity when treated with dibutyryl cyclic AMP also have extremely low basal levels of the enzyme. All of these mutant strains continue to synthesize protein when treated with dibutyryl cyclic AMP and undergo growth cessation and morphological changes in the presence of this agent. Thus, the mutations all appear to affect factors specific to the expression of alkaline phosphatase activity rather than factors that affect general cellular responsiveness or permeability to dibutyryl cyclic AMP. The characterization of these strains may help elucidate mechanisms of eukaryotic membrane protein biogenesis, enzyme induction, and regulation of gene expression by cyclic nucleotides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doellgast G. J., Fishman W. H. Inhibition of human placental-type alkaline phosphatase variants by peptides containing L-leucine. Clin Chim Acta. 1977 Mar 15;75(3):449–454. doi: 10.1016/0009-8981(77)90365-5. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Autoradiographic detection of animal cell membrane mutants altered in phosphatidylcholine synthesis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5192–5196. doi: 10.1073/pnas.77.9.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Mutants of Chinese hamster ovary cells with altered membrane phospholipid composition. Replacement of phosphatidylinositol by phosphatidylglycerol in a myo-inositol auxotroph. J Biol Chem. 1980 May 25;255(10):4474–4480. [PubMed] [Google Scholar]
- Esko J. D., Raetz C. R. Replica plating and in situ enzymatic assay of animal cell colonies established on filter paper. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1190–1193. doi: 10.1073/pnas.75.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone G. L., Heath E. C. Role of protein glycosylation in the cAMP-mediated induction of alkaline phosphatase in mouse L-cells. J Biol Chem. 1981 Feb 10;256(3):1404–1411. [PubMed] [Google Scholar]
- Firestone G. L., Heath E. C. The cyclic AMP-mediated induction of alkaline phosphatase in mouse L-cells. J Biol Chem. 1981 Feb 10;256(3):1396–1403. [PubMed] [Google Scholar]
- Gabrielson E. G., Scoggin C., Puck T. T. Phosphorylation changes induced by cAMP derivatives in the CHO cell and selected mutants. Exp Cell Res. 1982 Nov;142(1):63–68. doi: 10.1016/0014-4827(82)90409-8. [DOI] [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J Biol Chem. 1966 Jun 10;241(11):2516–2522. [PubMed] [Google Scholar]
- Gottesman M. M., LeCam A., Bukowski M., Pastan I. Isolation of multiple classes of mutants of CHO cells resistant to cyclic AMP. Somatic Cell Genet. 1980 Jan;6(1):45–61. doi: 10.1007/BF01538695. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Tin A. W., Sussman H. H. Regulation of alkaline phosphatase expression in human choriocarcinoma cell lines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):323–327. doi: 10.1073/pnas.76.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
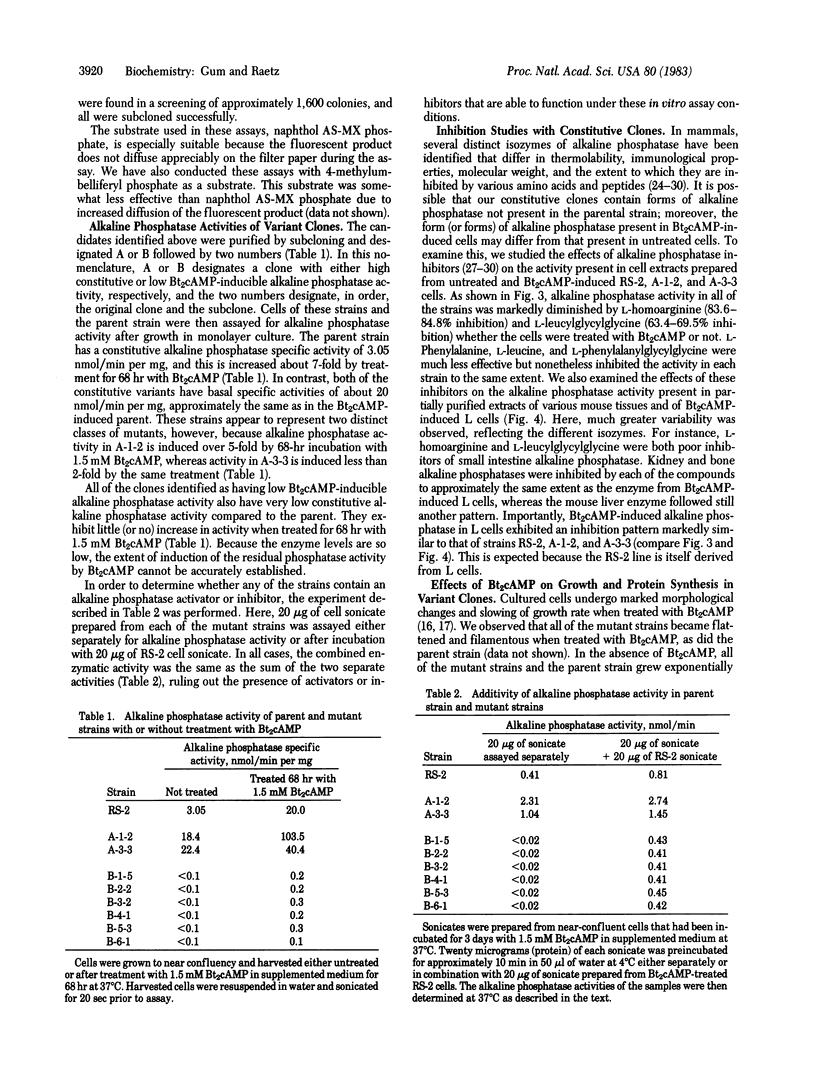
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li A. P., O'Neill J. P., Kawashima K., Hsie A. W. Correlation between changes in intracellular level of cyclic AMP, activation of cyclic AMP-dependent protein kinase, and the morphology of Chinese hamster ovary cells in culture. Arch Biochem Biophys. 1977 Jul;182(1):181–187. doi: 10.1016/0003-9861(77)90297-1. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Fishman W. H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J Biol Chem. 1972 May 25;247(10):3082–3087. [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Non-lytic release of acetylcholinesterase from erythrocytes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1977 Oct 1;82(1):143–146. doi: 10.1016/0014-5793(77)80905-8. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Release of alkaline phosphatase from membranes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1977 Oct 1;167(1):281–284. doi: 10.1042/bj1670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Specific release of plasma membrane enzymes by a phosphatidylinositol-specific phospholipase C. Biochim Biophys Acta. 1978 Apr 20;508(3):565–570. doi: 10.1016/0005-2736(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Low M. G., Zilversmit D. B. Role of phosphatidylinositol in attachment of alkaline phosphatase to membranes. Biochemistry. 1980 Aug 19;19(17):3913–3918. doi: 10.1021/bi00558a004. [DOI] [PubMed] [Google Scholar]
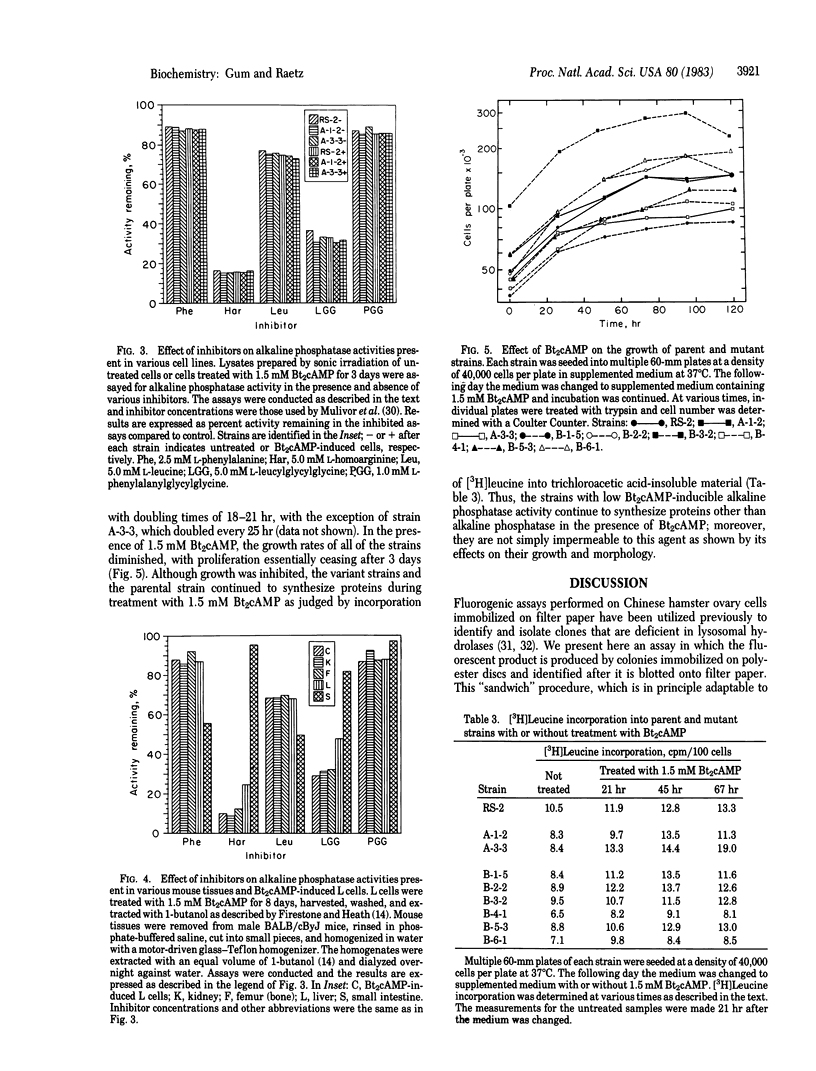
- MERCHANT D. J., HELLMAN K. B. Growth of L-M strain mouse cells in a chemically defined medium. Proc Soc Exp Biol Med. 1962 May;110:194–198. doi: 10.3181/00379727-110-27464. [DOI] [PubMed] [Google Scholar]
- MOSS D. W., KING E. J. Properties of alkaline-phosphatase fractions separated by starch-gel electrophoresis. Biochem J. 1962 Jul;84:192–195. doi: 10.1042/bj0840192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor R. A., Plotkin L. I., Harris H. Differential inhibition of the products of the human alkaline phosphatase loci. Ann Hum Genet. 1978 Jul;42(1):1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Wing D. C., Raetz C. R. Isolation of somatic cell mutants defective in the biosynthesis of phosphatidylethanolamine. J Biol Chem. 1981 Aug 10;256(15):7687–7690. [PubMed] [Google Scholar]
- Raetz C. R., Wermuth M. M., McIntyre T. M., Esko J. D., Wing D. C. Somatic cell cloning in polyester stacks. Proc Natl Acad Sci U S A. 1982 May;79(10):3223–3227. doi: 10.1073/pnas.79.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R. Isolation of lysosomal alpha-mannosidase mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1911–1915. doi: 10.1073/pnas.76.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Myerowitz R., Youle R. J., Murray G. J., Neville D. M., Jr The mannose 6-phosphate receptor of Chinese Hamster ovary cells. Isolation of mutants with altered receptors. J Biol Chem. 1981 Oct 25;256(20):10618–10622. [PubMed] [Google Scholar]
- Singh T. J., Roth C., Gottesman M. M., Pastan I. H. Characterization of cyclic AMP-resistant Chinese hamster ovary cell mutants lacking type I protein kinase. J Biol Chem. 1981 Jan 25;256(2):926–932. [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]