Abstract
Alzheimer’s disease (AD) is the most common form of dementia in the older people and 6th leading cause of death in the United States. Deposition of amyloid-beta (Aβ) plaques, hyperphosphorylation of microtubule associated protein tau (MAPT), neuroinflammation and cholinergic neuron loss are the major hallmarks of AD. Deposition of Aβ peptides, which takes place years before the clinical onset of the disease can trigger hyperphophorylation of tau proteins and neuroinflammation, and the latter is thought to be primarily involved in neuronal and synaptic damage seen in AD. To date, four cholinesterase inhibitors or ChEI (tacrine, rivastigmine, donepezil and galantamine) and a partial NMDA receptor antagonist (memantine) are the only approved treatment options for AD. However, these drugs fail to completely cure the disease, which warrants a search for newer class of targets that would eventually lead to effective drugs for the treatment of AD. In addition to selected pharmacological agents, botanical and medicinal plant extracts are also being investigated. Apart from its culinary use, garlic (Allium sativum) is being used to treat several ailments like cancer and diabetes. Herein we have discussed the effects of a specific ‘Aged Garlic Extract’ (AGE) and one of its active ingredients, S-allyl-L-cysteine (SAC) in restricting several pathological cascades related to the synaptic degeneration and neuroinflammatory pathways associated with AD. Thus, based on the reported positive preliminary results reviewed herein, further research is required to develop the full potential of AGE and/or SAC into an effective preventative strategy for AD.
Keywords: Aged garlic extract, aging, amyloid, Alzheimer’s disease, botanicals, brain, dietary, medicinals, neuroinflammation, nutraceuticals, nutrition, synapse, pleiotropic, resveratrol, tau, therapeutics
1. INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia affecting elderly populations all over the globe. Over 5.4 million people in the United States are diagnosed with AD and this number will triple by mid- century if definite curative or restrictive therapies are not developed[1]. Neuropathologically, deposition of amyloid β (Aβ) peptides in the brain parenchyma, hyperphosphorylation of microtubule associated protein tau (MAPT) and loss of cholinergic neurons comprise the major hallmarks of AD. It is believed that deposited Aβ peptides trigger a series of inflammatory reactions including activation of microglia, generation of reactive oxygen species (ROS) and cytochemokines result in widespread neuronal loss [2]. To date, four cholinesterase inhibitors (ChEI; tacrine, rivastigmine, donepezil and galantamine) and a partial NMDA receptor antagonist (memantine) are the only treatment options for AD. Although these drugs can provide some symptomatic relief, they fail to cure or restrict the disease progression. In this context, clinical trials with new drugs aiming to decrease Aβ production have been conducted. Unfortunately, some of these trials were halted because of serious adverse effects including cancer [3]. Moreover, these drugs primarily target a single pathological pathway such as Aβ precursor protein (APP) processing. It is indicated that deposition of Aβ peptides as diffuse plaques appear much earlier in the disease process and it is possible that people may remain cognitively intact for decades[4]. Neuroinflammation may be a key player responsible for the clinical onset of AD[5]. Since AD has multifactorial etiopathogenesis, such as neuritic plaques, neurofibirillary tangles, severe neuroinflammation, glutamate induced excitotoxicity, defective Aβ breakdown, we hypothesize that an agent effective in modulating multiple pathological pathways could be an appropriate therapeutic agent in preventing and restricting AD progression. Although sequence of these events is poorly understood, they result in widespread neuronal and synaptic loss.
In addition to pharmacological agents, botanical and medicinal plant extracts are also being investigated. Some of these studies are based on dietary and epidemiological studies, including incidence and prevalence of AD in different cultures and countries. For example, a large epidemiological study had depicted a significant (~4.4-fold) less incidence of AD in Indian elderly when compared with a reference American populace[6]. Another epidemiological study did not observe any association between AD and APOE ε4 in an elderly cohort in Nigeria. However, the latter was strongly associated with AD in African American populations[7]. These findings indicate a strong environmental contribution in the development of AD, which may include dietary factors. In this context, Mediterranean diets are believed to protect against the development of AD[8]. Resveratrol (a polyphenol present in fruits and red wine) increases cerebral blood flow in young adults [9]. Curcumin (a polyphenol present in turmeric and curry powder) was shown to have protective or curative roles in AD models [2]. However, curcumin failed to exert significant beneficial effects in clinical settings[10], which can be attributed to its poor bioavailability and rapid bio-transformation[11]. In this context, we have observed increased bioavailability and significant neuroprotective properties of a nanoformulation of curcumin (NanoCurc™) in vivo as well as in neuronal cells [11]. We studied further to evaluate potential beneficial roles of other naturally occurring compounds in the context of neurodegenerative disorders, such as AD, and observed significant neuroprotective and synaptopreservative properties of garlic derived compounds in animal and cell culture models [12]. In this review, we will discuss ‘pleiotropic’ properties of garlic components in modifying several pathological cascades involved in AD.
Apart from culinary purposes, garlic (Allium sativum) is being used to treat different ailments such as cardiovascular [13], gastrointestinal [14], hematological [15] and as cancer [16]. Recent studies have demonstrated beneficial effects of “Aged Garlic Extract” (AGE) and one of its active ingredients S-Allyl-L-Cysteine (SAC) in AD models [12, 17]. In these studies AGE and SAC treatments not only decreased Aβ loads in APP-transgenic (Tg) mice, but also ameliorated tau pathology and increased levels of synaptic protein markers versus vehicle treated mice. Previous studies also demonstrated potent antioxidant action of AGE [18].
2. ALZHEIMER’S DISEASE (AD) - BASIC PATHOLOGY AND NEUROCHEMISTRY
2.1. Aβ Deposition
Aβ peptide is a generated from APP by sequential enzymatic cleavage of β- and γ secretases. APP, which is encoded by a long gene localized in human chromosome 21, is a large (695–770 amino acids) glycosylated transmembrane protein. Apart from mammalian APP, two other non-mammalian homologous proteins were also identified which are appl (present in Drosophila melanogaster) and apl-1 (present in the worm Caenorhabditis elegans) [19–20]. APP is first cleaved by β secretase (also known as BACE-1) to produce sAPPβ (596 amino acids for APP 695 isoform) and a C-terminal 99 amino acid containing fragment (C99)[21]. In the final step of processing, C99 is cleaved by γ secretase to produce Aβ peptides, containing 39–43 amino acids residue [22]. The Aβ peptide with 40 amino acids (Aβ40) is abundantly produced in this process and considered as less pathogenic. However, the Aβ peptide with 42 amino acids residue (Aβ42), although produced in lower quantities, is fibrilar in nature and can potentially form aggregates [23–24]. In addition to aggregated Aβ, soluble oligomeric Aβ species are neurotoxic, and the brain load of the latter was found to be positively correlated with the clinical symptoms of AD [25–26]. It is believed that Aβ deposition can also trigger hyperphosphorylation of tau and neuroinflammation including generation of ROS.
2.2. Hyperphosphorylation of Microtubule Associated Protein Tau (MAPT) (Tauopathies)- Evidence from Animal and Clinical Studies
MAPT or Tau is a microtubular protein found in neurons and coded by a gene located at chromosome 17 [27]. One of the primary functions of tau is to provide structural stability of microtubule by interacting with tubulin, another structural protein of the cell [28]. Tau is a phosphoprotein, and hyperphosphorylation can prevent binding of tau with tubulin, hence destabilize axonal structures. Phosphorylation at Ser and/or Thr residues at different positions (such as Ser 262, Thr 231, Ser 235) of tau protein is considered as the major forms of phosphorylation and observed in neurodegenerative disorders including AD [29]. Several protein kinases including glycogen synthase kinase-3 (GSK-3) and cyclin dependent kinase-5 (cdk-5) can phosphorylate tau protein [30]. Postmortem AD brain revealed 3-4-fold more hyperphosphorylation of tau protein versus post mortem non-AD brain tissue [31]. To date, it is not completely clear whether or not deposition of Aβ causes tau hyperphosphorylation. Intracerebral infusion of Aβ peptides in Tg mice models induced tauopathies [32]. Further, intracerebral infusion of deposited Aβ peptides collected from aged APP-Tg mice to young tau-Tg mice resulted in an increase in tau pathology in the brain of the injected mice [33]. In a triple transgenic mouse model (APP, presenilin-1 and tau), Aβ deposition was observed before the appearance of tangle pathology [34].
Beyond AD and in a clinical study, we also observed increased levels of CSF-phosphotau in normal pressure hydrocephalus (NPH) patients suffering for more than one year [35]. Cardinal pathology of NPH involves disruption of CSF hemodynamics, which may result in increased Aβ deposition [35]. However, tau pathology was not observed in the postmortem brain samples of patients suffered in a rare disease, hereditary cerebral hemorrhage with amyloidosis of Dutch type (HCHWA-Dutch), which also showed a considerable amount of Aβ burden [36]. Moreover, a growing body of evidence suggests a link between hyperphosphorylation of tau in diabetes. Brain tissues of streptozotocin induced diabetic rats’ revealed decreased levels of insulin in the brain due to altered transport of insulin across the blood brain barrier (BBB) [37]. Further, GSK-3β, a prime enzyme responsible for pathological phosphorylation of tau can be inactivated by insulin [38]. Thus apart from AD, other disorders such as NPH, diabetes can induce hyperphosphorylation of tau.
2.3. Neuroinflammation
As stated earlier, neuroinflammation is believed to play a central role in the clinical onset of AD[5]. This is consistent with the observations that deposited Aβ can activate microglia, which produces cytochemokines (such as TNFα, ILs) and result in neuroinflammation. Further, ROS, nuclear factor kappa beta (NFκB), peroxisome proliferators-activated receptor-γ (PPARγ), apolipoprotein E (APOE) are also thought to play important roles in neuroinflammation associated with AD (for review see [2]). Neuroinflammation can be an important cause for widespread neuronal damage observed in AD, especially cholinergic neurons in basal forebrain are more susceptible for degeneration [39].
3. CHEMICAL COMPOUNDS EXTRACTED FROM GARLIC
Garlic consists of several sulfur containing ingredients and alliin (S-allyl cysteine sulfoxide) is the major one. Alliin undergoes several chemical transformations to generate a series of other sulfur compounds such as allyl thiosulfinate, allicin, allyl methyl thiosulfinate, allyl propenyl thiosulfinate, diallyl disulfide, and allyl methyl sulfide [40]. SAC is an important component of garlic which is produced by hydrolysis from γ-glutamyl-S-allyl cysteine [40]. Some sulfur containing garlic compounds are sparely water soluble and upon ingestion can also cause adverse effects in humans[12]. AGE is prepared by soaking garlic in ethanol-water mixture for 20 months, which removes irritant compounds from garlic and solubilize some of the insoluble compounds [41]. SAC is one of the active ingredients of AGE. Previous report has proposed greater safety and efficacy of AGE than raw garlic as a therapeutic agent [42].
4. POTENTIAL ROLES OF AGE AND SAC IN AMELIORATING AD-LIKE PATHOLOGY
4.1. Aβ Related Pathology
AGE and SAC were observed to possess potential anti-amyloidogenic properties both in vitro and in vivo studies. Previous research has demonstrated protection of cellular structures by AGE from Aβ- mediated damage [43–44]. Mechanistically, SAC was shown to have Aβ disaggregation property in vitro [45]. In an AD animal model, four months of independent treatments of AGE and SAC resulted in a significant decrease of both Aβ load and numbers of Aβ plaques in the brain of APP-Tg mice (Tg-2576) versus non-treated Tg mice controls [17]. In addition to levels of Aβ peptides, brain lysate of the animals from the above study was analyzed in Western immunoblotting to measure intracellular APP. Notably, we observed a significant decrease in the levels of intracellular APP in AGE-treated APP-Tg mice versus untreated Tg mice (Ray and Lahiri unpublished). The exact mechanism of AGE and SAC in modulating APP levels or processing is still not properly elucidated and could be mediated through NFκB (discussed later).
Apart from that, it was also observed that SAC can prevent Aβ-mediated hippocampal neurodegeneration by attenuating endoplasmic reticulum (ER) stress [46]. Another important aspect of AGE and SAC’s mechanism is their ability in inhibiting the activation of caspase 3[47]. Deposited Aβ can increase the level of activated caspase 3 [48], which was also observed in APP-Tg mice brain versus wild type mice. It was also observed that activation of caspase 3 can lead to decreased synaptic function and postsynaptic density [49]. We have observed significant increase in the levels of pre-synaptic proteins SNAP25 and synaptophysin in brains of APP-Tg mice treated with either AGE or SAC versus non-treated APP-Tg mice [12]. Further, we have observed decreased levels of cleaved caspase 3, which indicates inhibition of the caspase 3 activity in AGE-treated APP-Tg mice brain samples versus untreated Tg mice (data not shown).
4.2. AD, Diabetes Mellitus, Cholesterol and the Role of Insulin
Based on a variety of current research results, a strong positive correlation between diabetes mellitus and AD has been proposed. Insulin in the brain can display growth factor like functions [50]. Further, insulin increases the expression of choline acetyl transferase (ChAT) in cholinergic neurons of basal forebrain [51]. In addition, insulin degrading enzyme (IDE), which catalyzes insulin also takes part in breaking down of Aβ peptides in the brain [52]. Hence diabetes mellitus, characterized by reduced production or peripheral resistance of insulin may lead to the development of AD. Interestingly, garlic can increase insulin secretion from pancreatic β cells, i.e. considered as an insulin secretagogue [53]. Secretagogues, which are medicines that stimulate the beta cell to secrete insulin, include the sulfonylureas and glinides. In experimental settings one of the garlic derived compounds S-Allyl-L-cysteine sulfoxide (SACS) was shown to ameliorate symptoms of diabetic rats, and this action of SACS was comparable to glibenclamide, a known anti-diabetic agent [54]. Another important aspect of AGE’s and SAC’s beneficial roles in Aβ-related pathology is by augmenting cardiovascular health. Epidemiological and clinical studies have documented that cardiovascular lesions are common in AD and are also potential risk factors [55–56]. Hypercholesterolemia, the most important causative factor for cardiovascular pathology, was shown to enhance brain Aβ accumulation [57]. Refolo et al. demonstrated an alteration in APP processing towards amyloidogenic pathway by cholesterol in Tg mice model [58]. Further, radio imaging study has documented that higher serum cholesterol level is associated with increases frequency of APOE ε4 alleles, one of the well known risk factors for AD [59]. Interestingly, garlic and garlic derived compounds were shown to have potent antilipidemic properties in term of inhibiting cholesterol synthesis [60–62]. Several mechanisms of garlic and its components’ lipid lowering properties were proposed including inhibition of the rate limiting enzyme of cholesterol biosynthesis, HMGCoA-reductase [63].
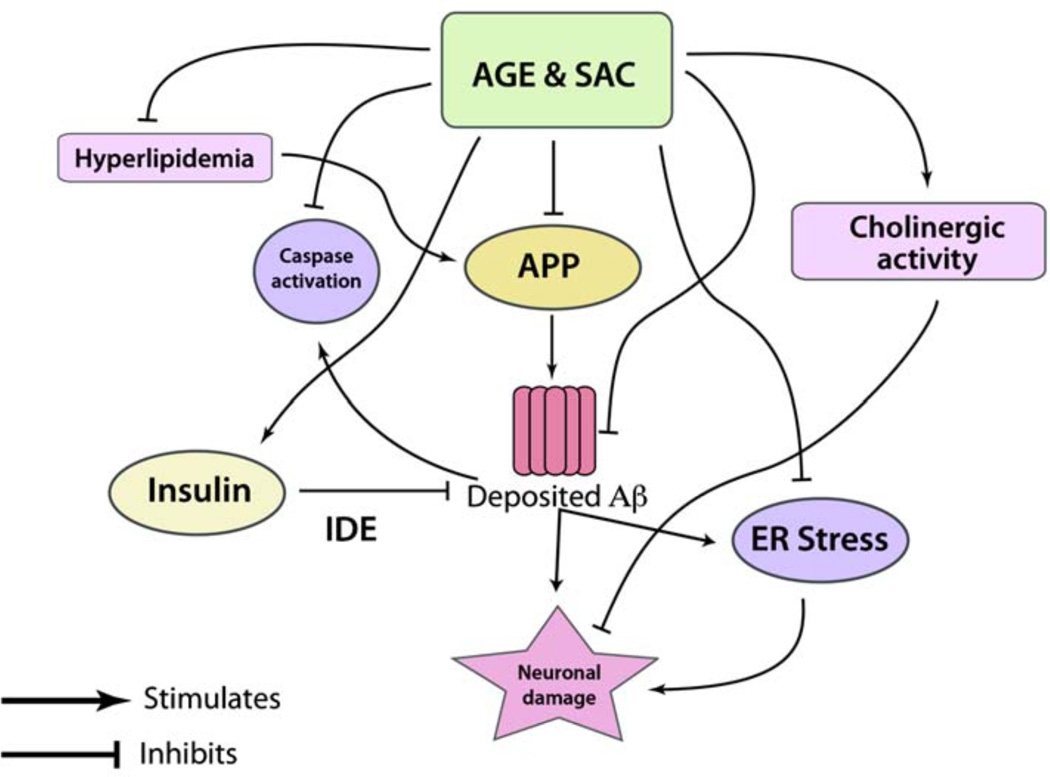
The summary of potential effects of AGE and SAC in ameliorating Aβ-related pathology is illustrated in Fig. (2).
Fig. (2).
The schematic shows potential roles of AGE and SAC in ameliorating APP and Aβ related pathologies in the context of AD. AGE treatment was shown to decrease the levels of APP in Alzheimer’s Tg mice (refer text), which was reflected in decreased Aβ levels. Further, SAC was shown to possess potential effects in disaggregating Aβ peptides. Deposited Aβ can activate caspases, and particularly caspase 3 activation can take a leading role in neuronal loss and disrupting synaptic structures. AGE was shown to inactivate caspase 3 in APP-Tg mice. Further, increased levels of insulin in the brain have neurotrophic as well as Aβ clearing function. The latter is thought to be carried out by up-regulation of insulin degrading enzyme (IDE). AGE is a natural secretagogue of insulin and indirectly capable of reducing brain Aβ load. It was observed that neuronal damage by deposited Aβ can be due to induction of endoplasmic reticulum (ER) stress, which is attenuated by garlic compounds, such as AGE and SAC. Further, hyperlipidemia can cause enhanced Aβ production (see the text), and AGE treatment can substantially reduce total cholesterol levels by inhibiting HMGCoA- reductase, the rate limiting enzyme of cholesterol biosynthesis.
4.3. Potential Roles of AGE and SAC in Ameliorating Tauopathies
In the molecular sequel related to the pathogenesis of AD, hyperphosphorylation of tau proteins is believed to follow Aβ deposition and to form neurofibrillary tangles. Certain garlic compounds effects’ in ameliorating tau pathology in preclinical settings are encouraging. Working with APP-Tg mice, Chauhan observed a decreased level of tau phosphorylation in APP-Tg mice by independent treatments of AGE, SAC and diallyl disulfide (DADS), another compound present in garlic. Mechanistically, the decreased level of tau phosphorylation was most likely due to decreasing the activity of GSK-3β by garlic compounds [17]. No change in the levels of Cdk5 was observed in the treated animals versus controls. As discussed, deposition of Aβ peptides can induce hyperphosphorylation of tau protein, AGE or SAC’s anti-amyloid properties can also be responsible for preventing hyperphosphorylation of tau. Interestingly, insulin secretagogue action of garlic compounds can increase the brain levels of insulin and insulin like growth factor (IGF), which can decrease brain Aβ burden and inhibit the activation of GSK-3β and can potentially prevent tau phosphorylation. Further, studies revealed an increased level of tau phosphorylation both in cortex and hippocampus of rats that were fed with high cholesterol containing diet for a prolog period of time (6 months) [64]. In AD patients, increased in the levels of CSF-phosphotau is considered as one of the diagnostic criteria [65]. It was observed that 14-weeks treatment with an HMGCoA- reductase inhibitor (simvastatin) decreased the levels of CSF-phosphotau in hyperlipidemic patients with dementia [66], and this can be attributed as an indirect evidence that hyperlipidemia can potentially cause hyper-phosphorylation of tau proteins. Due to antilipidemic properties, garlic compounds can play an important role in ameliorating tauopathies. In addition, several studies have shown that inflammation can induce tau pathology. Induction of microglial activation by lipopolysaccharide (LPS) was shown to increase tauopathies in tau-transgenic mice [67]. Activated microglia produces interleukin-1 (IL-1), which was observed to participate in tau pathology [68]. Moreover, activation of microglia can occur by secreted APP as well [69]. Taken together, due to their anti-inflammatory (discussed later), ROS-scavenging and APP lowering properties, garlic compounds can ameliorate neuroinflammation, which in turn, can be effective in preventing excessive phosphorylation of tau.
4.4. Role of AGE and SAC in the Context of Neuroinflammation and Protection Against ROS
Sporadic AD is generally a disease of the old age. Mitochondrial DNA is damaged in aging, which is not only associated with low ATP production but also with higher production of ROS, including highly charged free radicals [70]. In AD, deposited Aβ can activate microglia and activated microglia generates ROS, which play an important role in damaging neurons [71]. Apart from ROS, activated microglia can give rise to a number of pro-inflammatory cytochemokines like interleukins (ILs) and TNFα, leading to further neuronal loss [72]. Because of the anti-amyloid properties, AGE and other garlic derived compounds like SAC can be helpful in ameliorating neuroinflammation. From a therapeutic point of view, since Aβ deposition precedes the onset of clinical dementia and the exact timing and sequence are unknown, lowering only Aβ loads may not be sufficient in halting or treating AD. In this context, ROS-scavenging property of AGE and SAC would be beneficial. Notably, we have observed a significant protection to neuronally differentiated rat PC12 cells by AGE and SAC from ROS (H2O2)-mediated insults [12].
Mechanistically, it is suggested that garlic compounds can modulate intracellular levels of glutathione (GSH), a key enzyme responsible for cellular protection against ROS. Upon interaction with ROS, GSH become converted to oxidized glutathione (GSSG) by the enzymatic action of glutathione peroxidase [73]. The ratio of GSH to GSSG is an important parameter to determine the redox state of the cells; an increased ratio of GSH:GSSG denotes a reduced intracellular environment and the ratio decreases in oxidative stressed condition, which was observed in some specific regions of postmortem AD brain [74]. AGE was observed to increase the levels of intracellular GSH [75]. Further, in a rat model, AGE treatment was detected to preserve the levels of glutathione peroxidase and glutathione reductase, and the latter is involved in conversion of GSSG to GSH [76]. Similar results were observed in SAC treatment in a cell culture-based study [77]. Further, independent treatments of AGE and SAC to APP-Tg mice revealed a significant decrease in IL-1β immunoreactive microglia numbers compared to untreated APP-Tg mice [16].
One of the important mediators of inflammation in AD is activated NFκB, which is responsible for the production of various proinflammatory cytochemokines [2]. In steady state, NFκB stays within cytosol of the cells and stays bound to an inhibitor protein IkB [78]. NFκB activations requires phosphorylation of IkB and once IkB is phosphorylated, NFκB translocates to the nucleus and binds with promoter regions of several pro-inflammatory cytokines genes [79]. One of the stimulatory factors for NFκB activation is Aβ peptide, which was observed to bind with a death receptor (75 kDa-neurotrophin receptor) resulting in NFκB activation [80]. In addition to Aβ peptides, generated ROS from Aβ-microglia interaction can also stimulate NFκB activation [81]. Promoter mapping of different genes shows the presence of NFκB binding sites within APP, presenilin-1 and BACE-1 genes, indicating up-regulation of these proteins following activation of NFκB [2]. In this context, prevention of NFκB activation is considered as a rational strategy to ameliorate inflammation related to AD. Curcumin, one of the potential therapeutic candidates in AD and cancer, was shown to suppress the activation of NFκB [82]. Suppression of NFκB activation was also observed by SAC in cultured cells when they were separately treated with ROS and TNF-α [83–84].
Peroxisome proliferators-activated receptors (PPAR) are a class of ligand-activated transcription factor and plays an important role in regulation of inflammation [85]. PPAR has three distinct subtypes α, β and γ; out of those PPAR-γ is involved in adipocyte differentiation and also a target for anti-diabetic drugs like pioglitazone [86]. It was shown that activation of PPAR-γ in microglia and macrophages is involved in alleviating inflammation by decreasing the production of pro-inflammatory cytochemokines [87–88]. Further, activation of PPAR-γ is involved in Aβ clearance [89]. Regarding garlic compounds, diallyl disulfide (DADS), a component of AGE was shown to increase the expression of PPAR-γ in cell culture, hence can act as an anti-inflammatory agent [90–91].
Neuronal destruction and synaptic loss are the endpoints of neuroinflammation seen in AD, and cholinergic neurons in the basal forebrain are most vulnerable by this process [92]. Hence, facilitating choline uptake by cholinergic neurons or preserving the rate limiting enzyme for choline synthesis, choline acetyl transferase (ChAT) can be considered as a possible intervention approach. Working with cholinergically differentiated human neuroblastoma (SK-N-SH) cells, we have observed significant ‘procholinergic’ properties of AGE in terms of increasing high affinity choline uptake (HACU) and ChAT activity when the cells were co-treated with AGE and ROS (H2O2)(Ray and Lahiri, unpublished data). Similar ‘procholinergic’ effects were observed in SAC and ROS cotreated cholinergic human SK-N-SH cells (data not shown).
5. SUMMARY
Development of novel drugs to treat AD is one of the priorities in current biomedical research as the number of AD patients and societal costs are steadily increasing. Current FDA-approved drugs for the treatment of AD fail to completely cure the disease. Several new drugs had been tried in clinical trials but most of them fail to demonstrate a definite curative or disease restrictive effects in those trials. In our view, the reasons for not achieving desirable effects with the new drugs can be due to the fact that these drugs primarily target a single molecular pathway such as APP processing. As we reviewed here, clinical manifestation of AD may not solely depend on deposited Aβ but hyper-phosphorylation of tau, and severe neuroinflammation can also play vital roles. Based on the aforementioned discussions, we support the proposal of the necessity of agents with ‘pleiotropic’ properties in the treatment of AD [93]. In fact, some pleiotropic properties have been observed in recent studies with ChEI (rivastigmine and phenserine) and memantine. Apart from their primary mode of actions, these drugs have been reported to display APP modulating and neuropreserving properties [94–96]. Rivastigmine, which increases acetylcholine levels by inhibiting acetylcholine esterase and butyrylcholine esterase was also observed to increase the levels of presynaptic proteins SNAP25 and synaptophysin in rat embryonic primary neurons [97].In this context, garlic compounds AGE and SAC can have significant effects in lowering brain amyloid load, reducing hyperphosphorylation of tau proteins and ameliorating neuroinflammation by modulating several interconnected pathways, however, how much these effects really translate into clinical trials remain to be seem.. In any case, the overall results of such intervention would have far reaching implications. Taken together, mechanistically, AGE and SAC can have great potential for an effective use in the prevention and treatment of AD. Particularly, as a pure synthesizable chemical compound, SAC can emerge as a potential therapeutic agent in preventing and treating neurodegenerative disorders, such as AD. In order to fully understand their efficacy, further research is warranted in larger clinical settings.
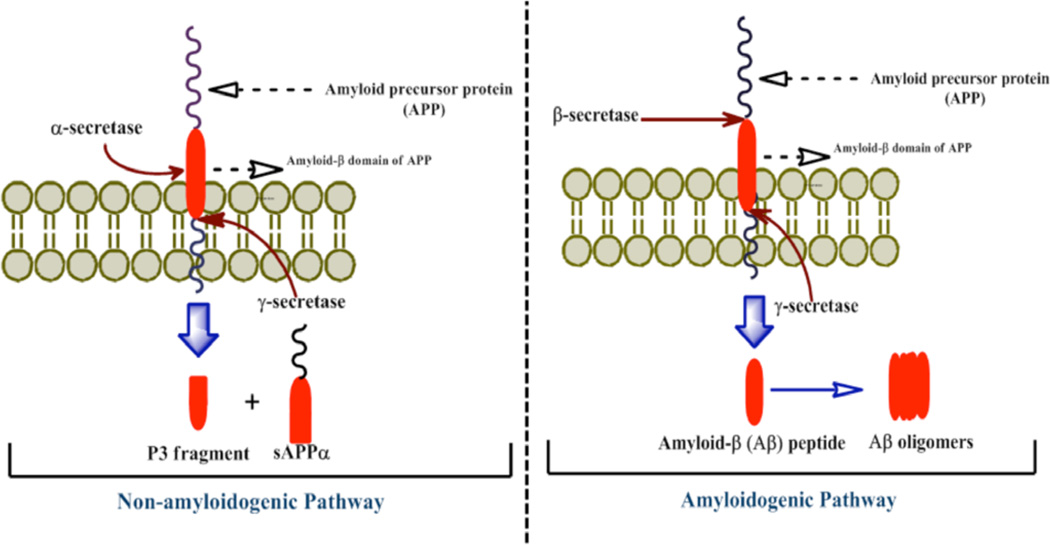
Fig. (1).
The schematic diagram shows APP processing by secretase enzymes. APP is a transmembrane protein consists of 695–771 amino acids. The major or primary cleavage pathway of APP is shown in the left, where it is initially cleaved by α-secretase enzyme. This enzyme cleaves APP within its Aβ domain; hence it precludes the generation of Aβ peptides and termed as ‘non-amyloidogenic pathway’. The second cleavage was carried out by γ-secretase. As shown in the figure, α-secretase pathway produces sAPPα and a small P3 fragment.
In contrast, the minor or β-secretase pathway is shown at the right side of the figure. Proteolytic cleavage of APP molecule by β-secretase and subsequent cleavage by γ-secretase produce sAPPβ and Aβ peptides and the latter is pathognomonic for AD. Generated Aβ can form aggregates and deposits as neuritic plaques within the brain parenchyma.
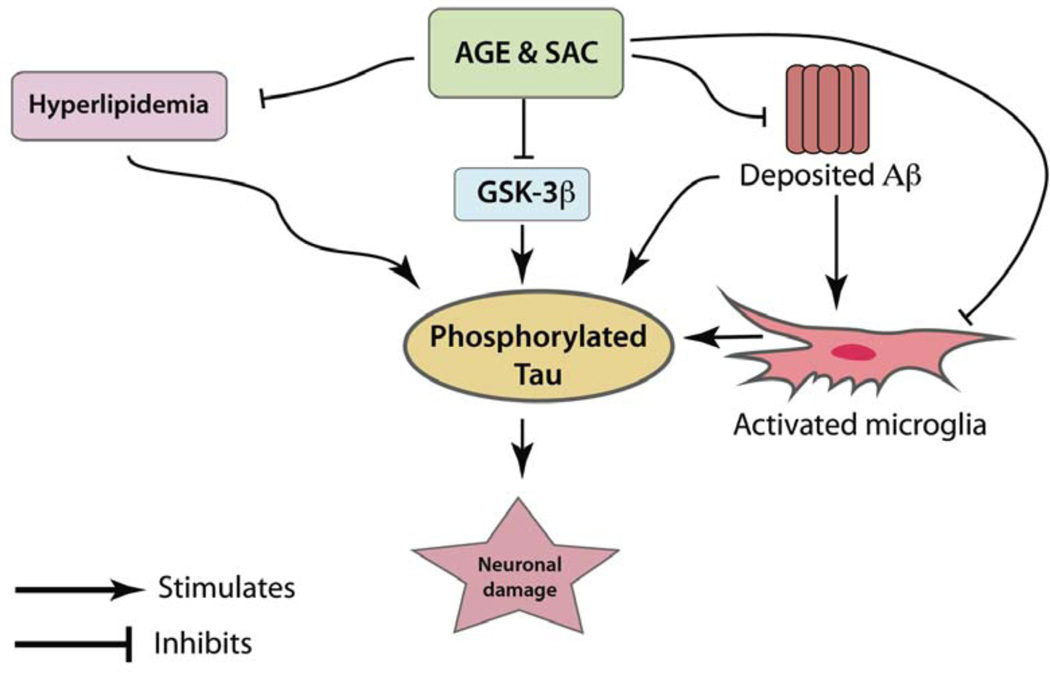
Fig. (3).
Hyperphosphorylation of tau is one of the hallmarks of AD. Apart from reduction in Aβ-load, preventing excessive phosphorylation of tau is also considered to be a rational strategy for the treatment of AD. AGE and SAC treatments to the APP-Tg mice (as described in Fig.2) decreased the levels of hyperphosphorylated tau in the brain of treated animals versus untreated Tg mice [16]. Mechanistically, AGE and SAC were observed to inhibit the enzyme GSK-3β, a prime enzyme responsible for the hyperphosphorylation of tau. Deposited Aβ can also initiate hyperphosphorylation of tau protein. In this aspect, AGE and SAC’s Aβ lowering properties would be helpful in reducing hyperphosphorylation of tau. Further, activated microglia can release a series of cytochemokines, which also play important roles in hyperphosphorylation of tau. AGE and SAC treatment in APP-Tg mice were shown to decrease activated microglia load in treated Tg mice versus untreated Tg controls [16]. AGE and SAC treatment can also be beneficial in tauopathies by decreasing serum cholesterol levels.
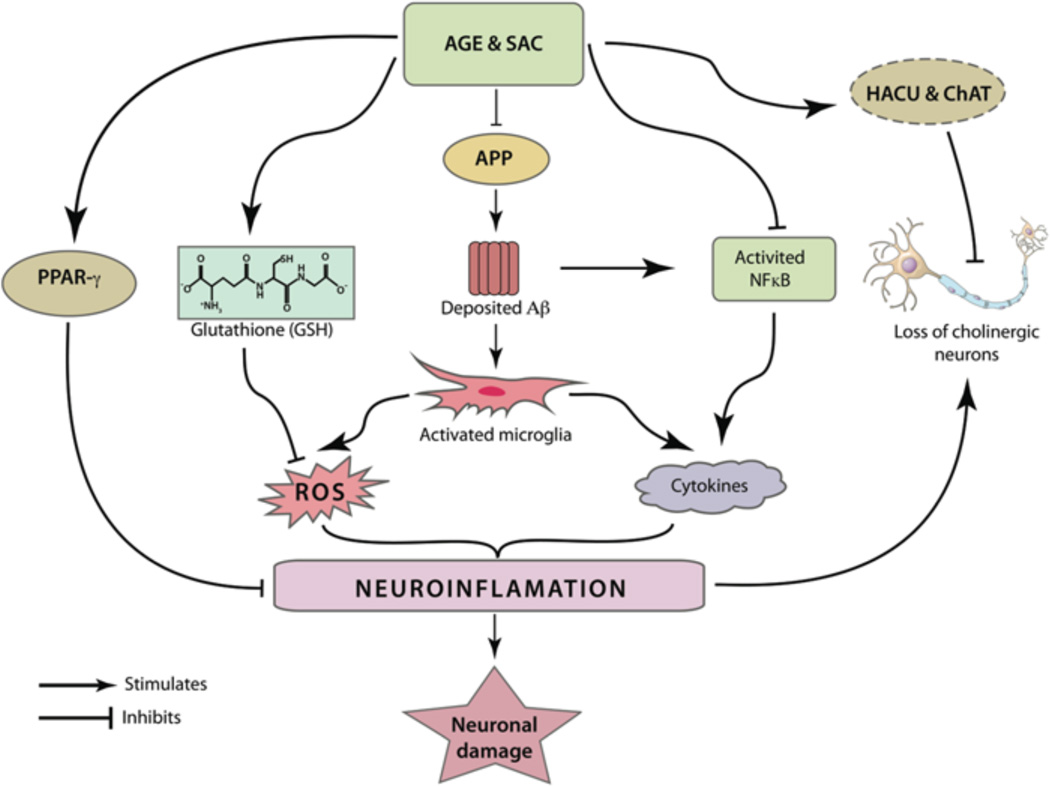
Fig. (4).
AGE and SAC can modulate different inflammatory pathways related to the pathogenesis of AD. Microglial activation by deposited Aβ peptides can be abridged by their potential roles in reducing the brain Aβ load. Further, SAC was found to inhibit the activation of the pro-inflammatory transcription factor NFκB. The latter plays an important role in the production of several cytochemokines. Activated NFκB is also responsible in generation of Aβ from APP. As mentioned in the text, apart from producing cytochemokines, activated microglia can produce ROS, leading to widespread neuronal damage. AGE and SAC treatments were independently shown to increase the production of glutathione (GSH), an important molecule to neutralize ROS. AGE treatment was also shown to have PPAR-γ agonistic property and the latter is believed to modulate expression of several pro-inflammatory genes. It was postulated that cholinergic neurons are most vulnerable by inflammation associated with AD. Notably, the increased HACU and ChAT activity, as observed by AGE and SAC treatment, is able to preserve cholinergic neurons from ROS or cytochemokines-mediated damage.
ACKNOWLEDGEMENT
We thank Jason Bailey and Justin Long, and grant supports from the National Institutes of Health (AG18379 and AG18884) to DKL.
REFERENCES
- 1.Alzheimer's Association 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol. 2009;9(4):434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Sambamurti K, Greig NH, Utsuki T, Barnwell EL, Sharma E, Mazell C, Bhat NR, Kindy MS, Lahiri DK, Pappolla MA. Targets for AD treatment: conflicting messages from gamma-secretase inhibitors. J Neurochem. 2011;117(3):359–374. doi: 10.1111/j.1471-4159.2011.07213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in "normal" aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46(3):707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 5.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18(8):2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57(6):985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 7.Murrell JR, Price B, Lane KA, Baiyewu O, Gureje O, Ogunniyi A, Unverzagt FW, Smith-Gamble V, Gao S, Hendrie HC, Hall KS. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Arch Neurol. 2006;63(3):431–434. doi: 10.1001/archneur.63.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A, Solfrizzi V. Nutraceutical properties of Mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimers Dis. 2010;22(3):715–740. doi: 10.3233/JAD-2010-100942. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010;91(6):1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 10.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 11.Ray B, Bisht S, Maitra A, Lahiri DK. Neuroprotective and Neurorescue Effects of a Novel Polymeric Nanoparticle Formulation of Curcumin (NanoCurc) in the Neuronal Cell Culture and Animal Model: Implications for Alzheimer's disease. J Alzheimers Dis. 2011;23(1):61–77. doi: 10.3233/JAD-2010-101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by Aged Garlic Extract (AGE) and S-allyl-L-Cysteine (SAC) treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.07145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobenin IA, Pryanishnikov VV, Kunnova LM, Rabinovich YA, Martirosyan DM, Orekhov AN. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010;9:119. doi: 10.1186/1476-511X-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millen AE, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, Yokochi LA, Ziegler RG. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am J Clin Nutr. 2007;86(6):1754–1764. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 15.Takasu J, Uykimpang R, Sunga MA, Amagase H, Niihara Y. Aged garlic extract is a potential therapy for sickle-cell anemia. J Nutr. 2006;136(3 Suppl):803S–805S. doi: 10.1093/jn/136.3.803S. [DOI] [PubMed] [Google Scholar]
- 16.Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol. 2010;37(3):258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Chauhan NB. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer's transgenic model Tg2576. J Ethnopharmacol. 2006;108(3):385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131(3s):1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 19.Rosen DR, Martin-Morris L, Luo LQ, White K. A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci U S A. 1989;86(7):2478–2482. doi: 10.1073/pnas.86.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daigle I, Li C. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc Natl Acad Sci U S A. 1993;90(24):12045–12049. doi: 10.1073/pnas.90.24.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha S, Lieberburg I. Cellular mechanisms of beta-amyloid production and secretion. Proc Natl Acad Sci U S A. 1999;96(20):11049–11053. doi: 10.1073/pnas.96.20.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahiri DK, Farlow MR, Sambamurti K, Greig NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer's disease. Curr Drug Targets. 2003;4(2):97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- 23.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 25.Whalen BM, Selkoe DJ, Hartley DM. Small non-fibrillar assemblies of amyloid beta-protein bearing the Arctic mutation induce rapid neuritic degeneration. Neurobiol Dis. 2005;20(2):254–266. doi: 10.1016/j.nbd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155(3):853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118(1):53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Liang Z, Shi J, Yin D, El-Akkad E, Grundke-Iqbal I, Iqbal K, Gong CX. PKA modulates GSK-3beta- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett. 2006;580(26):6269–6274. doi: 10.1016/j.febslet.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B. Defective brain microtubule assembly in Alzheimer's disease. Lancet. 1986;2(8504):421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 32.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 33.Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171(6):2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Ray B, Reyes PF, Lahiri DK. Biochemical studies in Normal Pressure Hydrocephalus (NPH) patients: Change in CSF levels of amyloid precursor protein (APP), amyloid-beta (Abeta) peptide and phospho-tau. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 37.Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18(10):1577–1584. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 38.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56(7):1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse PJ, Struble RG, Hedreen JC, Clark AW, Price DL. Alzheimer's disease and related dementias: selective involvement of specific neuronal systems. CRC Crit Rev Clin Neurobiol. 1985;1(4):319–339. [PubMed] [Google Scholar]
- 40.Lawson LD, Gardner CD. Composition, stability, and bioavailability of garlic products used in a clinical trial. J Agric Food Chem. 2005;53(16):6254–6261. doi: 10.1021/jf050536+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borek C. Garlic reduces dementia and heart-disease risk. J Nutr. 2006;136(3 Suppl):810S–812S. doi: 10.1093/jn/136.3.810S. [DOI] [PubMed] [Google Scholar]
- 42.Park JH, Park YK, Park E. Antioxidative and antigenotoxic effects of garlic (Allium sativum L.) prepared by different processing methods. Plant Foods Hum Nutr. 2009;64(4):244–249. doi: 10.1007/s11130-009-0132-1. [DOI] [PubMed] [Google Scholar]
- 43.Selassie M, Griffin B, Gwebu N, Gwebu ET. Aged garlic extract attenuates the cytotoxicity of beta-amyloid on undifferentiated PC12 cells. In Vitro Cell Dev Biol Anim. 1999;35(7):369–370. doi: 10.1007/s11626-999-0109-2. [DOI] [PubMed] [Google Scholar]
- 44.Peng Q, Buz'Zard AR, Lau BH. Neuroprotective effect of garlic compounds in amyloid-beta peptide-induced apoptosis in vitro. Med Sci Monit. 2002;8(8):BR328–BR337. [PubMed] [Google Scholar]
- 45.Gupta VB, Rao KS. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer's beta-amyloid fibrils in vitro. Neurosci Lett. 2007;429(2–3):75–80. doi: 10.1016/j.neulet.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 46.Kosuge Y, Koen Y, Ishige K, Minami K, Urasawa H, Saito H, Ito Y. S-allyl-L-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunicamycin-induced neuronal death. Neuroscience. 2003;122(4):885–895. doi: 10.1016/j.neuroscience.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Jackson R, McNeil B, Taylor C, Holl G, Ruff D, Gwebu ET. Effect of aged garlic extract on caspase-3 activity in vitro. Nutr Neurosci. 2002;5(4):287–290. doi: 10.1080/10284150290032012. [DOI] [PubMed] [Google Scholar]
- 48.Marin N, Romero B, Bosch-Morell F, Llansola M, Felipo V, Roma J, Romero FJ. Beta-amyloid-induced activation of caspase-3 in primary cultures of rat neurons. Mech Ageing Dev. 2000;119(1–2):63–67. doi: 10.1016/s0047-6374(00)00172-x. [DOI] [PubMed] [Google Scholar]
- 49.D'Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci. 2011;14(1):69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 50.Kroner Z. The relationship between Alzheimer's disease and diabetes: Type 3 diabetes? Altern Med Rev. 2009;14(4):373–379. [PubMed] [Google Scholar]
- 51.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71(3):365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Numata K, Kaplan DL. Mechanisms of enzymatic degradation of amyloid Beta microfibrils generating nanofilaments and nanospheres related to cytotoxicity. Biochemistry. 2010;49(15):3254–3260. doi: 10.1021/bi902134p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang ML, Johnson MA. Effect of garlic on carbohydrate metabolism and lipid synthesis in rats. J Nutr. 1980;110(5):931–936. doi: 10.1093/jn/110.5.931. [DOI] [PubMed] [Google Scholar]
- 54.Augusti KT, Sheela CG. Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experientia. 1996;52(2):115–120. doi: 10.1007/BF01923354. [DOI] [PubMed] [Google Scholar]
- 55.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 56.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 57.Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer's disease: a perspective on potential mechanisms and mediators. J Neurochem. 2010;115(3):551–562. doi: 10.1111/j.1471-4159.2010.06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7(4):321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 59.Reiman EM, Chen K, Langbaum JB, Lee W, Reschke C, Bandy D, Alexander GE, Caselli RJ. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging. Neuroimage. 2010;49(1):169–176. doi: 10.1016/j.neuroimage.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asdaq SM, Inamdar MN. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17(13):1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Singh DK, Porter TD. Inhibition of sterol 4alpha-methyl oxidase is the principal mechanism by which garlic decreases cholesterol synthesis. J Nutr. 2006;136(3 Suppl):759S–764S. doi: 10.1093/jn/136.3.759S. [DOI] [PubMed] [Google Scholar]
- 62.Sumioka I, Hayama M, Shimokawa Y, Shiraishi S, Tokunaga A. Lipid-lowering effect of monascus garlic fermented extract (MGFE) in hyperlipidemic subjects. Hiroshima J Med Sci. 2006;55(2):59–64. [PubMed] [Google Scholar]
- 63.Gebhardt R, Beck H. Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte cultures. Lipids. 1996;31(12):1269–1276. doi: 10.1007/BF02587912. [DOI] [PubMed] [Google Scholar]
- 64.Lu F, Li X, Suo AQ, Zhang JW. Inhibition of tau hyperphosphorylation and beta amyloid production in rat brain by oral administration of atorvastatin. Chin Med J (Engl) 2010;123(14):1864–1870. [PubMed] [Google Scholar]
- 65.Itoh N, Arai H, Urakami K, Ishiguro K, Ohno H, Hampel H, Buerger K, Wiltfang J, Otto M, Kretzschmar H, Moeller HJ, Imagawa M, Kohno H, Nakashima K, Kuzuhara S, Sasaki H, Imahori K. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer's disease. Ann Neurol. 2001;50(2):150–156. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- 66.Riekse RG, Li G, Petrie EC, Leverenz JB, Vavrek D, Vuletic S, Albers JJ, Montine TJ, Lee VM, Lee M, Seubert P, Galasko D, Schellenberg GD, Hazzard WR, Peskind ER. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis. 2006;10(4):399–406. doi: 10.3233/jad-2006-10408. [DOI] [PubMed] [Google Scholar]
- 67.Lee DC, Rizer J, Selenica ML, Reid P, Kraft C, Johnson A, Blair L, Gordon MN, Dickey CA, Morgan D. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation. 2010;7:56. doi: 10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23(5):1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76(3):846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 70.Ma YS, Wu SB, Lee WY, Cheng JS, Wei YH. Response to the Increase of Oxidative Stress and Mutation of Mitochondrial DNA in Aging. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 71.Schilling T, Eder C. Amyloid-beta-induced reactive oxygen species production and priming are differentially regulated by ion channels in microglia. J Cell Physiol. 2011 doi: 10.1002/jcp.22675. [DOI] [PubMed] [Google Scholar]
- 72.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer's disease? Neurobiol Aging. 1995;16(4):661–674. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- 74.Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, Marsden CD, Schapira AH. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci. 1998;158(1):24–29. doi: 10.1016/s0022-510x(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 75.Pinto JT, Krasnikov BF, Cooper AJ. Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J Nutr. 2006;136(3 Suppl):835S–841S. doi: 10.1093/jn/136.3.835S. [DOI] [PubMed] [Google Scholar]
- 76.Maldonado PD, Barrera D, Medina-Campos ON, Hernandez-Pando R, Ibarra-Rubio ME, Pedraza-Chaverri J. Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Life Sci. 2003;73(20):2543–2556. doi: 10.1016/s0024-3205(03)00609-x. [DOI] [PubMed] [Google Scholar]
- 77.Ide N, Lau BH. S-allylcysteine attenuates oxidative stress in endothelial cells. Drug Dev Ind Pharm. 1999;25(5):619–624. doi: 10.1081/ddc-100102217. [DOI] [PubMed] [Google Scholar]
- 78.Delgado M, Varela N, Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56(10):1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- 79.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 80.Kuner P, Schubenel R, Hertel C. Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J Neurosci Res. 1998;54(6):798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 81.Tobar N, Villar V, Santibanez JF. ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem. 2010;340(1–2):195–202. doi: 10.1007/s11010-010-0418-5. [DOI] [PubMed] [Google Scholar]
- 82.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 83.Geng Z, Rong Y, Lau BH. S-allyl cysteine inhibits activation of nuclear factor kappa B in human T cells. Free Radic Biol Med. 1997;23(2):345–350. doi: 10.1016/s0891-5849(97)00006-3. [DOI] [PubMed] [Google Scholar]
- 84.Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J Nutr. 2001;131(3s):1020S–1026S. doi: 10.1093/jn/131.3.1020S. [DOI] [PubMed] [Google Scholar]
- 85.Neri T, Armani C, Pegoli A, Cordazzo C, Carmazzi Y, Brunelleschi S, Bardelli C, Breschi MC, Paggiaro P, Celi A. Role of NF-{kappa}B and PPAR-{gamma} in lung inflammation induced by monocyte-derived microparticles. Eur Respir J. 2010 doi: 10.1183/09031936.00023310. [DOI] [PubMed] [Google Scholar]
- 86.Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 87.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14-prostaglandin J2. Proc Natl Acad Sci U S A. 1999;96(8):4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 89.Espuny-Camacho I, Dominguez D, Merchiers P, Van Rompaey L, Selkoe D, De Strooper B. Peroxisome proliferator-activated receptor gamma enhances the activity of an insulin degrading enzyme-like metalloprotease for amyloid-beta clearance. J Alzheimers Dis. 2010;20(4):1119–1132. doi: 10.3233/JAD-2010-091633. [DOI] [PubMed] [Google Scholar]
- 90.Lee JH, Kim KA, Kwon KB, Kim EK, Lee YR, Song MY, Koo JH, Ka SO, Park JW, Park BH. Diallyl disulfide accelerates adipogenesis in 3T3-L1 cells. Int J Mol Med. 2007;20(1):59–64. [PubMed] [Google Scholar]
- 91.Zhou Z, Tan HL, Xu BX, Ma ZC, Gao Y, Wang SQ. Microarray analysis of altered gene expression in diallyl trisulfide-treated HepG2 cells. Pharmacol Rep. 2005;57(6):818–823. [PubMed] [Google Scholar]
- 92.Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer's pathology. J Cell Mol Med. 2008;12(6A):2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frautschy SA, Cole GM. Why pleiotropic interventions are Needed for Alzheimer's disease. Mol Neurobiol. 2010;41(2–3):392–409. doi: 10.1007/s12035-010-8137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. The experimental Alzheimer's disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther. 2007;320(1):386–396. doi: 10.1124/jpet.106.112102. [DOI] [PubMed] [Google Scholar]
- 95.Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK. Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res. 2009;88(1):143–154. doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ray B, Banerjee PK, Greig NH, Lahiri DK. Memantine treatment decreases levels of secreted Alzheimer's amyloid precursor protein (APP) and amyloid beta (A beta) peptide in the human neuroblastoma cells. Neurosci Lett. 470(1):1–5. doi: 10.1016/j.neulet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bailey JA, Lahiri DK. A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer's disease. J Neurochem. 2010;112(4):843–853. doi: 10.1111/j.1471-4159.2009.06490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]