Abstract
Using behavioral and blood oxygen level dependent (BOLD) response indices through functional magnetic resonance imaging (fMRI), the current study investigated whether youths with disruptive behavior disorders (conduct disorder and oppositional defiant disorder) plus psychopathic traits (DBD + PT) show aberrant sensitivity to eye gaze information generally and/or whether they show particular insensitivity to eye gaze information in the context of fearful expressions. The participants were 36 children and adolescents (ages 10–17 years); 17 had DBD + PT and 19 were healthy comparison subjects. Participants performed a spatial attention paradigm where spatial attention was cued by eye gaze in faces displaying fearful, angry, or neutral affect. Eye gaze sensitivity was indexed both behaviorally and as BOLD response. There were no group differences in behavioral response: both groups showed significantly faster responses if the target was in the congruent spatial direction indicated by eye gaze. Neither group showed a Congruence × Emotion interaction; neither group showed an advantage from the displayer’s emotional expression behaviorally. However, the BOLD response revealed a significant Group × Congruence × Emotion interaction. The comparison youth showed increased activity within the dorsal endogenous orienting network (superior parietal lobule and inferior parietal sulcus) for fearful congruent relative to incongruent trials relative to the youth with DBD + PT. The results are discussed with reference to current models of DBD + PT and possible treatment innovations.
Youths with disruptive behavior disorders (DBD), including conduct disorder (CD) and oppositional defiant disorder, show increased aggression and antisocial behavior (Frick, Stickle, Dandreaux, Farrell, & Kimonis, 2005). Some of these youths also exhibit psychopathic traits (PTs), including callous–unemotional (CU; e.g., lack of guilt and empathy), narcissistic (e.g., brags excessively about abilities), and impulsive (acts without thinking) components (Barry et al., 2000). PTs are detectable early in childhood and persist into adulthood (Lynam, Caspi, Moffitt, Loeber, & Stouthamer-Loeber, 2007), and DBD + PT youths are at highest risk for recurrent behavioral problems (Dadds, Fraser, Frost, & Hawes, 2005; Frick & Dickens, 2006). However, functional magnetic resonance imaging (fMRI) work investigating their pathophysiology has only just begun (e.g., Finger et al., 2008, 2011; Jones, Laurens, Herba, Barker, & Viding, 2009; Marsh et al., 2008; Passamonti et al., 2010).
Two core impairments shown by youths with DBD + PT are (a) in reinforcement-based decision-making and (b) in responding to the distress cues of other individuals, that is, their displays of pain and sadness (Blair, 2007). With respect to distress cues, which is the focus of the current paper, these can be viewed as aversive reinforcers that reduce the probability that the behavior engendering these cues will be performed in the future (Blair, 2003). Impairment in reinforcement learning and the response to distress cues leads to a developmental condition where the individual is relatively indifferent to the distress of others. Studies have shown that antisocial individuals with PT show reduced autonomic responses to the pain and sadness of others (Aniskiewicz, 1979; Blair, 1999). Moreover, youths with DBD + PT show impaired recognition of fearful expressions (Blair, Colledge, Murray, & Mitchell, 2001; Dadds et al., 2006; Marsh & Blair, 2008; Stevens, Charman, & Blair, 2001) and reduced attention to this expression (Kimonis, Frick, Fazekas, & Loney, 2006).
At the neural level, the core neural systems that are implicated include the amygdala, striatum, and ventromedial prefrontal cortex (vmPFC; Blair, 2007; Finger et al., 2011). From a cognitive neuroscience perspective, these systems are highly interconnected but have some degree of functional specialization. The suggestion is that the role of the amygdala in stimulus-reinforcement learning, the role of the caudate and vmPFC in prediction error signaling (detecting that there is a difference between the reinforcement received and that expected), and the role of vmPFC in the representation of reinforcement outcome are all compromised (Blair, 2007; Finger et al., 2011). Moreover, the amygdala’s response to distress cues, particularly the process of learning the valence of objects from those distress cues (cf. Jeon et al., 2010), is thought to be compromised (Blair, 2007). In line with this hypothesis, youths with CD and PT show reduced amygdala responses to others’ fearful and sad expressions (Jones et al., 2009; Marsh et al., 2008; Passamonti et al., 2010).
In contrast to findings of reduced amygdala responses to fearful expression in youths with PT, there have been reports of increased responses, at least within the amygdala, to neutral expressions in youths with CD (Passamonti et al., 2010) and adult violent offenders (Pardini & Phillips, 2010). However, it should be noted that other studies have failed to observe this increased responding to neutral expressions (see Jones et al., 2009; Marsh et al., 2008). Moreover, the increased amygdala response to neutral expressions in CD was only found in the block of trials with angry expressions (and not in the blocks with other expressions; Passamonti et al., 2010). In contrast, the increased response to neutral expressions in the study with adult offenders was only found in the blocks of trials with happy expressions (and not in the blocks with other expressions, including angry; Pardini & Phillips, 2010).
The amygdala is not only responsive to fearful expressions (Murphy, Nimmo-Smith, & Lawrence, 2003), but it is also responsive to eye gaze information (Hoffman, Gothard, Schmid, & Logothetis, 2007; Sato, Kochiyama, Uono, & Yoshikawa, 2010) and appears to direct gaze/attention toward the eyes of others. Patients with amygdala damage show reduced gaze toward the eyes of individuals displaying fear and show an improvement in their fear recognition deficit when instructed to attend to the eyes (Adolphs et al., 2005). Similarly, youths with DBD + PT show reduced gaze toward the eyes (Dadds, El Masry, Wimalaweera, & Guastella, 2008) and an improvement in their fear recognition following instructions to attend to the eyes (Dadds et al., 2006). As such, an understanding of the neurobiology of eye gaze processing in DBD + PT is clearly of relevance to understanding the disorder.
Eye gaze information is important. Shifts in another person’s gaze tell the observer where they are attending and may direct his/her attention to a salient object in the environment. Combining information on an individual’s gaze toward an object with their emotional expression allows the rapid communication information regarding that object’s valence (Bayliss, Frischen, Fenske, & Tipper, 2007; Blair, 2003). This rapid transmission of valence information is seen in social referencing studies in humans (Klinnert, Emde, Butterfield, & Campos, 1987) and observational fear learning studies in monkeys (Mineka & Cook, 1993). Confirming the amygdala’s important role in this, recent animal work has shown that amygdala lesions block the acquisition and expression of observational fear (Jeon et al., 2010).
Numerous attentional cuing studies have demonstrated that gaze direction cues direct spatial attention to a gazed-at direction (Driver et al., 1999; for a review, see Frischen, Bayliss, & Tipper, 2007). This effect may be augmented if the gaze direction is shown by an individual displaying fear (Putman, Hermans, & van Honk, 2006; Tipples, 2006), although other studies have failed to show this effect (Hietanen & Leppanen, 2003) or shown it only in anxious participants (Mathews, Fox, Yiend, & Calder, 2003) or under threatening contexts (Friesen, Halvorson, & Graham, 2011; Kuhn & Tipples, 2011). The current study investigates the impact of gaze direction cues on spatial attention in youths with DBD + PT as well as the neural systems associated with this effect.
Our goal in this study was to determine whether any reduced behavioral or neural responsiveness to gaze information in individuals with DBD + PT occurred independently of emotional expression or whether it is more pronounced for fearful expressions. A generalized reduced sensitivity to eye gaze information would indicate this capacity to be dysfunctional in addition to the reinforcement-based impairments seen in DBD + PT. In contrast, if the reduced sensitivity was selective for fearful expressions, it might indicate that reduced attention to the eye region of fearful expressions (cf. Dadds et al., 2008) was a secondary consequence of reduced responsiveness to the expression. Under this account, the reduced responsiveness to the fearful expression would more weakly prime regions responsible for spatial attention orienting.
We examined this issue both behaviorally and with fMRI. With respect to the behavioral data, we predicted the following: first, there will be reduced priming of spatial location by eye gaze cues in youths with DBD + PT; second, youths with DBD + PT will fail to show any significant interaction of fearful expression with gaze cues (i.e., fail to show any enhanced priming of social location by the eye gaze of individuals displaying fearful expressions). With respect to the blood oxygen level dependent (BOLD) response data, we predicted atypical responding in the amygdala to eye gaze information in youths with DBD + PT. In addition, we were interested in examining the recruitment of attentional systems to eye gaze stimuli. Work has shown that the endogenous orienting of attention engages a dorsal network (comprising the superior parietal lobule [SPL], the inferior parietal sulcus [IPS], and the frontal eye fields; for a review, see Corbetta & Shulman, 2002). We predicted that DBD + PT youths might show reduced recruitment of this dorsal network to eye gaze stimuli (reflecting less cuing by eye gaze information). We also predicted that this might be particularly marked for fearful expressions. The current study tests these predictions.
Methods
Participants
Thirty-six youths participated: 17 youths with DBD + PT and 19 healthy comparison youths. Participants were recruited from the community through advertising (13 DBD youths, 5 healthy youths), fliers (1 DBD youth, 5 healthy youths), and referrals from other study participants (6 healthy youths) or mental health practitioners (2 DBD youths). The recruitment source was unknown for 1 DBD youth and 3 healthy youths (see Table 1). A statement of informed assent and consent was obtained from participating children and parents, respectively. This study was approved by the NIMH Institutional Review Board.
Table 1.
Characteristics of youth with DBD + PT and healthy youth
| Characteristic | Youth With DBD + PT (N =17)
|
Healthy Youth (N =19)
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 15.51 (2.33) | 15.22 (2.30) |
| IQa | 90.88*** (8.08) | 110.47*** (13.45) |
| APSD | 27.29*** (4.90) | 4.17*** (3.11) |
| N | % | N | % | |
|
| ||||
| Gender | 13 male, 4 female | 9 male, 10 female | ||
| Handedness | 3 left, 16 right | 4 left, 13 right | ||
|
| ||||
|
DSM-IV Diagnoses
| ||||
| CD | 16 | 94.1 | 0 | 0 |
| ODD | 1 | 5.9 | 0 | 0 |
| ADHD | 9 | 52.9 | 0 | 0 |
Note: DBD + PT, disruptive behavior disorder plus psychopathic traits; APSD, Antisocial Process Screening Device; CD, conduct disorder; ODD, oppositional defiant disorder; ADHD, attention-deficit/hyperactivity disorder.
Assessed with the Wechsler Abbreviated Scale of Intelligence (two-subtest form).
p < .001 significant difference.
All youths and parents completed Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997) assessments with an experienced clinician, who was trained and supervised by expert child psychiatrists, with good interrater reliability (κ > 0.75 for all diagnoses). The K-SADS assesses for substance abuse and substance dependence and, because of exclusion criteria, no children in either group met the criteria for these diagnoses. IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (two-subtest form). Exclusion criteria were pervasive developmental disorder; Tourette syndrome; lifetime history of psychosis; depression; bipolar disorder; generalized, social, or separation anxiety disorder; posttraumatic stress disorder; neurologic disorder; history of head trauma; history of substance abuse; and IQ < 75. In addition, parents completed the Antisocial Process Screening Device (APSD), a measure of PTs. Youths meeting K-SADS criteria for CD or oppositional defiant disorder who had APSD scores of 20 or greater were included in the PTs group, and those scoring <20 were excluded from the study. Comparison subjects did not meet the criteria for any K-SADS diagnosis and scored 11 or lower on the APSD. The groups did not differ significantly on age or gender breakdown, but healthy control youths did have significantly higher IQs, t (34) = 5.218, p < .001 (Table 1). Groups were also matched on handedness. In addition, two DBD + PT youths were taking medications that could not be withheld during scanning.
Study measures
The eye gaze task
In this task, participants were initially presented with face stimuli displaying neutral affect (Figure 1). After 300 ms, an “x” probe would appear to one side of the face. Simultaneously, the faces would direct the eyes either toward the probe (congruent trials) or away from the probe (incongruent trials). Concurrent with the eye gaze shift, the facial expression would either remain neutral (neutral trials) or change to an angry expression (angry trials) or a fearful facial expression (fearful trials). This image was presented for 1200 ms, during which participants responded via button press to indicate the location of the probe. This was followed by a 1000-ms fixation.
Figure 1.
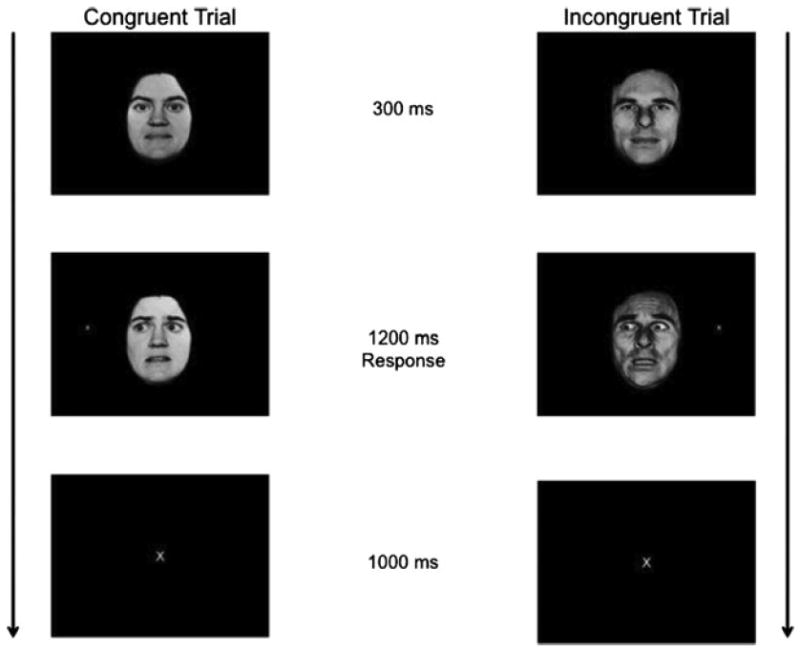
The eye gaze task.
Participants completed a brief practice run outside the scanner and then four task runs in the scanner. Each run contained identical trials, but the trial order was randomized within each run. Each run consisted of 108 trials: 24 neutral, 24 angry, and 24 fearful expression trials, and 36 fixation trials, which were of equal length to the task trials to provide a baseline. Two-thirds of the trials, within and across facial expressions, were congruent and one-third incongruent.
ASPD
This is a 20-item parent-completed rating of CU traits and conduct and impulsivity problems (Frick & Hare, 2001) that is designed to detect PTs in youth. Participants can score between 0 and 2 on each item. A three-factor structure has been characterized comprising CU, narcissism, and impulsivity (Frick & Hare, 2001). There is no established cutoff score for classification of a high level of PT (Frick & Hare, 2001). Following previous work (Finger et al., 2008; Marsh et al., 2008), we used a cutoff of 20 (all subjects >95th percentile). All of the healthy comparison subjects scored 11 or lower on this measure (all subjects <54th percentile). The ASPD was completed by the participants’ parents during screening prior to entry into the study.
MRI parameters
All participants received the following functional sequence: a total of 99 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time = 2900 ms, echo time = 27 ms, 64 × 64 matrix, 90-degree flip angle, 24-cm field of view). Whole-brain coverage was obtained with 46 axial slices (2.5-mm thickness with 0.5-mm spacing, 3.75 × 3.75 mm in-plane resolution). However, 15 participants (10 healthy controls, 5 DBD + PT) were scanned using a 1.5-T GE Signa scanner, and 17 participants (9 healthy controls, 12 DBD + PT) were scanned using a 3.0-T GE Signa Scanner. A high-resolution anatomical scan (three-dimensional spoiled gradient recalled acquisition in a steady state; 1.5 T: repetition time = 9 ms, echo time = 2.872 ms, 24-cm field of view, 20-degree flip angle, 128 axial slices, 1.5-mm thickness, 256 × 192 matrix; 3.0 T: repetition time = 7 ms, echo time = 2.984 ms, 24-cm field of view, 12-degree flip angle, 128 axial slices, 1.2-mm thickness, 256 × 192 matrix) in register with the EPI data set was obtained covering the whole brain.
Imaging data preprocessing
Imaging data were preprocessed and analyzed in AFNI (Cox, 1996). At the individual level, functional images from the first five repetitions, which were collected before equilibrium magnetization was reached, were discarded. Functional images from the four time series were motion corrected and spatially smoothed with a 6-mm full-width half-maximum Gaussian filter. The time series was normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100. The resultant regression coefficients represented a percentage of signal change from the mean.
In addition to six motion regressors, the following regressors were generated: neutral congruent, angry congruent, fearful congruent, neutral incongruent, angry incongruent, fearful incongruent, and incorrect response/no response trials. All regressors were created by convolving the train of stimulus events with a gamma variate hemodynamic response function to account for the slow hemodynamic response. Linear regression modeling was performed using the seven regressors described earlier plus regressors to model a first-order baseline drift function. This produced a β coefficient and associated t statistic for each voxel and regressor. In accordance with findings that normalization of brain volumes from age 7 to 8 years onward does not introduce major age-related distortions in localization or time course of the BOLD signal in event-related fMRI (Burgund et al., 2002; Kang, Burgund, Lugar, Petersen, & Schlaggar, 2003), the participants’ anatomical scans were individually registered to the Talairach–Tournoux atlas (Talairach & Tournoux, 1988). The individuals’ functional EPI data were then registered to their Talairach anatomical scan within AFNI.
fMRI data analysis
The group analysis of the BOLD data was then performed on regression coefficients from individual subject analyses using two 2 (Diagnosis: DBD + PT, Healthy Comparison) × 2 (Congruency: Congruent, Incongruent) × 2 (Facial Expression: Neutral or Fearful; Neutral or Angry) whole brain repeated measures analyses of variance (ANOVAs). Following previous work (Marsh et al., 2008), and in the interests of conserving power, two separate ANOVAs were conducted on the data. Initial threshholding was set at p < .005 with an extent threshold of 15 voxels, a slightly more stringent combination than that previously recommended (extent threshold of 10 voxels) to produce a desirable balance between Type I and Type II error rates (Lieberman & Cunningham, 2009). The average percentage of signal change was measured within each significant cluster of 15 voxels or greater. The post hoc analysis of significant main effects and interactions was assessed with t tests in SPSS 19.0 to further characterize the percentage of signal change. Because of the significant group difference in IQ scores, activity within the functional regions of interest identified by the ANOVA were further analyzed by 2 (Diagnosis: DBD + PT, Healthy Comparison) × 2 (Congruency: Congruent, Incongruent) × 2 (Facial Expression: Neutral, Fearful; Neutral, Angry) repeated measures analyses of covariance with IQ score as the covariate to determine whether group differences in this covariate could be determining the group effects. If IQ had a significant influence on response within a region, only the results that remained significant following the introduction of the IQ covariate are reported below.
Results
Behavioral results
Because the initial analyses of covariance revealed no significant main effects of, or interactions with, the covariate IQ, the response time and accuracy data were analyzed with two 2 (Diagnosis: DBD + PT, Healthy Comparison) × 3 (Facial Expression: Fear, Anger, Neutral) × 2 (Congruence) repeated measures ANOVAs. For response time, this revealed a main effect for congruence, F (1, 34) = 9.785, p = .004, where responses to congruent trials were faster than to incongruent trials, but no significant Group × Expression × Congruence, F (2, 33) = 0.518, p = .601, or Group × Congruence, F (2, 33) = 2.152, p = .152, interactions. The main effect of congruence found for response time was not observed for accuracy, F (1, 34) = 0.967, p = .332. There were also no significant Group × Expression × Congruence, F (2, 33) =1.830, p = .176, or Group × Congruence, F (2, 33) = 1.184, p = .284, interactions for the accuracy data.
BOLD response results
The goal of the current study was to assess whether youths with DBD + PT show aberrant recruitment of the amygdala with respect to eye gaze information, perhaps particularly for fearful expressions, and whether they show reduced recruitment of the dorsal attention network involved in endogenous orienting during task performance. The first 2 (Diagnosis: DBD + PT, Healthy Comparison) × 2 (Emotional Expression: Fear, Neutral) × 2 (Congruence) ANOVA focused on differential responsiveness to fearful relative to neutral expressions in youths with DBD + PT. The second 2 (Diagnosis: DBD + PT, Healthy Control) × 2 (Emotional Expression: Anger, Neutral) × 2 (Congruence) ANOVA focused on differential responsiveness to anger relative to neutral expressions in youths with DBD + PT. The key interactions (Diagnosis × Expression × Congruence, Diagnosis × Expression and Diagnosis × Congruence) are described in the following results to provide tests of our a priori hypotheses (see Table 2).
Table 2.
Brain regions demonstrating differential blood oxygen level dependent responses during task performance in youths with DBD + PT and healthy youths
| Regiona | Coordinates of Peak Activationb
|
F (1, 40) | Voxels | ||||
|---|---|---|---|---|---|---|---|
| Left/Right | BA | x | y | z | |||
| Fear Versus Neutral Contrast
| |||||||
| Diagnosis × Emotion × Congruence | |||||||
| Superior parietal lobule | Right | 7 | 22.5 | −61.5 | 44.5 | 18.31 | 80 |
| Superior parietal lobule | Left | 7 | −19.5 | −64.5 | 44.5 | 18.89 | 70 |
| Inferior parietal lobule | Right | 40 | 28.5 | −40.5 | 50.5 | 9.96 | 19 |
| Inferior parietal lobule | Left | 40 | −43.5 | −37.5 | 41.5 | 11.19 | 18 |
| Posterior cingulate cortexc | Right | 31 | 16.5 | −34.5 | 41.5 | 9.17 | 11 |
| Cuneus | Left | 18 | −4.5 | −82.5 | 17.5 | 11.63 | 29 |
|
| |||||||
| Anger Versus Neutral Contrast
| |||||||
| Diagnosis × Emotion | |||||||
| Superior frontal cortex | Left | 9 | −28.5 | 49.5 | 32.5 | 10.30 | 25 |
| Middle frontal cortex | Right | 6 | 31.5 | −7.5 | 56.5 | 9.05 | 15 |
| Diagnosis × Congruence | |||||||
| Middle temporal cortex | Right | 21 | 37.5 | −10.5 | −6.5 | 12.22 | 38 |
| Thalamus | Right | 13.5 | −16.5 | 2.5 | 9.24 | 18 | |
Note: DBD + PT, disruptive behavior disorder plus psychopathic traits; BA, Brodmann area.
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/).
Based on the standard brain template of the Montreal Neurological Institute.
Due to the potential theoretical importance of this region to the research question, an extent threshold of >10 voxels was adopted.
Eye gaze information modulated by fearful expressions
Regions showing a significant Diagnosis × Expression × Congruence interaction included regions in the dorsal endogenous attention-orienting network, including bilateral SPL and bilateral IP (Figure 2). There was also a region of the posterior cingulate cortex (PCC) that, although it did not survive our extent threshold (voxel N = 11), we mention because of suggestions that the amygdala primes this endogenous attention-orienting network through the PCC (McCoy & Platt, 2005). In line with suggestions that these regions are involved in endogenous attention orienting, healthy controls showed significantly greater activity within all of these regions (except left IPS) to fearful congruent relative to fearful incongruent trials (ts = 2.192–3.158, ps = .042–.005). It is interesting that this effect was selective for fearful expressions; in none of these regions was there a differential response to neutral congruent relative to neutral incongruent trials (ts = 0.436–2.079, ps = .668–.052). It is critical that in all of these regions the increase in activity seen in fear congruent relative to fear incongruent trials was significantly greater for the healthy controls relative to the youths with DBD + PT (ts = 4.339–8.771, ps = .033–.006). No activations survived corrections for the Diagnosis × Expression or Diagnosis × Congruence interactions.
Figure 2.
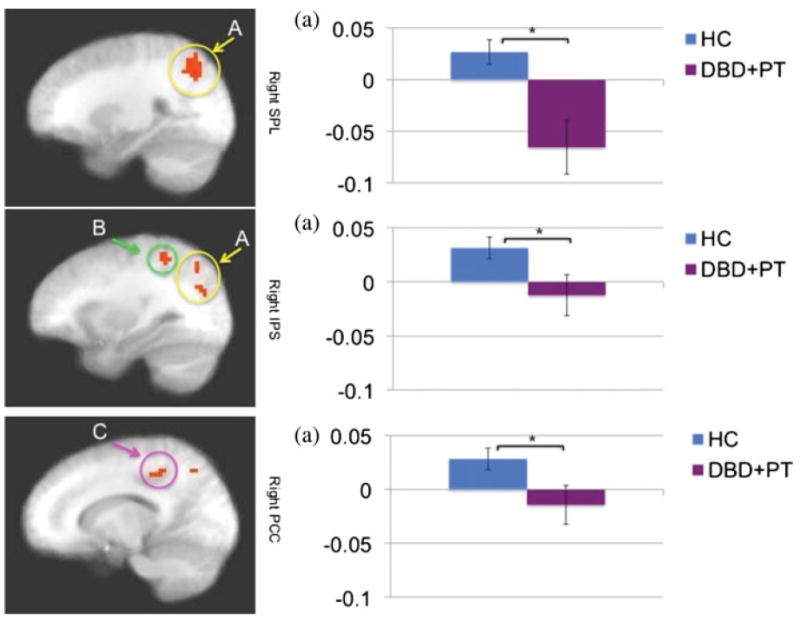
The difference between fear congruent trials and fear incongruent trials in Diagnosis × Expression × Congruence interaction in the right superior parietal lobule (SPL), right inferior parietal sulcus (IPS), and right posterior cingulate cortex (PCC) in 17 youths with disruptive behavior disorders (conduct disorder and oppositional defiant disorder) plus psychopathic traits (DBD + PT) and 19 healthy control (HC) youths. Healthy subjects showed significantly greater differences in activation between fear congruent trials and fear incongruent trials. The graphs show the average percentage of signal change across the identified region. *p < .05. [A color version of this figure can be viewed online at http://journals.cambridge.org/dpp]
Eye gaze information modulated by angry expressions
No activations survived corrections for the Diagnosis × Expression × Congruence interaction. However, there were significant Diagnosis × Expression interactions within the superior and middle frontal cortex. In both regions, there were no significant group differences in the response to angry stimuli (F = 0.824 and 0.082, p = .371 and .777, respectively). However, the youths with DBD + PT showed significantly greater responses to neutral stimuli relative to healthy controls (F = 5.606 and 11.943, p = .024 and .002, respectively). There were also significant Diagnosis × Congruence interactions within the right middle temporal cortex and right thalamus. In both regions, healthy controls showed a significantly greater increase in activity for incongruent relative to congruent trials relative to youths with DBD + PT (F = 12.212 and 7.711, p = .001 and .009, respectively).
Potential confounds
To account for possible the effects of medication use on the BOLD responses, the preceding analysis was repeated without the two youths in the DBD + PT group who were taking medication. The effects of interest in the Diagnosis × Emotion × Congruence interaction for fear versus neutral contrast were replicated with proximal activations in the same brain regions for each main effect and interaction. The effects of interest in the Diagnosis × Emotion interaction for the anger versus neutral contrast were also replicated; however, the effects seen in the Diagnosis × Congruence interaction were not.
To account for possible effects of comorbid attention-deficit/hyperactivity disorder (ADHD), the preceding analysis was also repeated without the nine youths in the DBD + PT group who met criteria for ADHD. Again, the effects of interest in the Diagnosis × Emotion × Congruence interaction for fear versus neutral contrast were replicated with proximal activations in the same brain regions for each main effect and interaction. However, the effects of interest in the Diagnosis × Emotion and the Diagnosis × Congruence interaction were not replicated after removing youths meeting the criteria for ADHD.
Symptom severity and the response modulated by gaze direction of fearful expressions
Considering the significant Diagnosis × Expression × Congruence interaction, we investigated whether there was a relationship between the differential response to fear congruent trials relative to fear incongruent trials and the severity of specific components of PT (i.e., CU, narcissistic, or impulsive). However, these analyses revealed no significant relationships between BOLD responses and symptom severity (rs = .078 to −.357, ps =.809−.159).
Discussion
The current study sought to determine whether youths with DBD + PT show aberrant sensitivity to eye gaze information, particularly in the context of fearful expressions. There were two main findings. First, at the behavioral level, there were no group differences in the spatial priming effect of eye gaze. Second, at the neural level, youths with DBD + PT showed reduced recruitment of the dorsal endogenous attention-orienting network (SPL and IPS [although not frontal eye fields]; Corbetta & Shulman, 2002) in response to fearful expressions.
A major reason for conducting this study was to determine whether youths with DBD + PT showed a generalized reduced sensitivity to eye gaze information or whether this reduced sensitivity was selective for fearful expressions. A generalized reduced sensitivity to eye gaze information would indicate this capacity to be dysfunctional in addition to the reinforcement-based impairments seen in DBD + PT. In contrast, if the reduced sensitivity was selective for fearful expressions, it might indicate that reduced attention to the eye region of fearful expressions (cf. Dadds et al., 2008) was a secondary consequence of reduced responsiveness to the expression; this reduced responsiveness might have a weaker effect on priming shifts in direction. The results broadly support the latter position. There were no group differences in the priming effect of eye gaze (although there was also no interaction with fearful expression, this was not seen in healthy controls, a typical finding in the literature; Friesen et al., 2011; Hietanen & Leppanen, 2003; Kuhn & Tipples, 2011; Mathews et al., 2003). Moreover, there were clear Diagnosis × Emotion × Congruence interactions within the bilateral SPL and bilateral IPS. These regions (with the frontal eye fields) make up the dorsal endogenous attention-orienting network (Corbetta & Shulman, 2002; Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). Within these regions, healthy controls showed increased responsiveness to fearful congruent relative to fearful incongruent trials (but not increased responsiveness to neutral congruent relative to neutral incongruent trials). Moreover, the increase in activity seen to fear congruent relative to fear incongruent trials was significantly greater for the healthy controls relative to the youths with DBD + PT. These data would suggest reduced spatial priming by youths with DBD + PT that was selectively greater for fearful relative to neutral expressions at least at the neural level. Given data by Dadds and colleagues (2008), a strong prediction would be that the neural data would be complemented by eye gaze data; that is, the youths with DBD + PT would show reduced shifts in eye gaze direction that was selective for fearful expressions. Because of technical difficulties, eye-tracking data was not available for this study. However, it will be important to collect these data in future work.
A caveat to the claim that spatial priming for gaze direction in youths with DBD + PT is selectively impaired for fearful expressions is that Group × Congruence interactions were seen within the right middle temporal cortex and right thalamus. Within these regions, healthy controls showed a significantly greater increase in activity for incongruent relative to congruent trials relative to youths with DBD + PT. Because activity was greater for incongruent relative to congruent trials, these data would suggest a greater requirement for the reorientation of attention in healthy controls relative to youths with DBD + PT. This in turn would suggest less attentional priming in the youths with DBD + PT to eye gaze information generally. However, it should be noted that neither the middle temporal cortex nor thalamus are considered involved in the reorientation of attention (Corbetta & Shulman, 2002). Moreover, no regions show significant Group × Congruence interactions following either the removal of the two youths with DBD + PT on psychotropic medication or the nine youths with DBD + PT with comorbid ADHD. As such, these results must be treated with some caution and appear to reflect the impact of psychotropic medication and/or comorbid ADHD rather than the pathophysiology of DBD + PT.
On the basis of previous work showing reduced amygdala responses to fearful expressions in youths with DBD + PT (Jones et al., 2009; Marsh et al., 2008), work showing that the amygdala responds to eye gaze information (Hoffman et al., 2007; Sato et al., 2010), and work showing that gaze direction to the eye region is disrupted in DBD + PT (putatively due to amygdala dysfunction; Dadds et al., 2008), we had predicted atypical responding in the amygdala to eye gaze information in youths with DBD + PT. However, this was not found. Nor was there any indication of reduced responsiveness in the amygdala to fearful expressions in the youths with DBT + PT. The absence of group differences in amygdala responsiveness in this study may reflect a poor signal within this region in this study. There were no findings of significant amygdala activity for any of the interactions and main effects for either the fear or anger ANOVAs. Alternatively, it may result from parameters inherent to spatial cuing paradigms. Considerable work shows that increased task demands results in reduced amygdala responses to emotional stimuli (Mitchell et al., 2007; Pessoa, McKenna, Gutierrez, & Ungerleider, 2002). The previous studies reporting reduced amygdala responsiveness to fearful expressions in youths with DBD + PT (Jones et al., 2009; Marsh et al., 2008) and sad expressions in youths with CD (Passamonti et al., 2010) required participants to provide gender judgments of individuals displaying emotional and neutral expressions. The task demands for gender judgments are low. In contrast, the current task involved participants responding to small target stimuli spatially distinct from the face as rapidly as possible; as such, the task demands are presumably greater. It is notable that recent work has found that reduced amygdala responses in youths with DBD + PT to fearful expressions is only seen under low attentional load conditions; under high load conditions neither group shows an amygdala response to fearful expressions (White et al., in press).
There are at least three potential interpretations that can be offered for the current results, given the absence of significant amygdala dysfunction. First, the pathophysiology of youths with DBT + PT may additionally include SPL and IPS dysfunction. There have been previous reports of reduced activity in these regions in youths with DBD + PT in the context of passive avoidance learning (Finger et al., 2011), although not reversal learning (Finger et al., 2008). However, the reduced activity in these regions, when previously seen, has been considered a secondary consequence of reduced activity in regions known to be dysfunctional. Moreover, it is important to remember that in the current data, atypical responding was seen to fear expressions. This suggests a failure in recruitment of these regions to a specific type of stimulus rather than a failure in recruitment of a general functional capacity. This echoes the reinforcement learning data. Impaired stimulus-reinforcement learning was associated with reduced recruitment of these attentional regions (Finger et al., 2011). In contrast, intact recruitment of these regions was seen to explicit error information (Finger et al., 2008), information individuals with PT show intact use of behaviorally (Budhani, Richell, & Blair, 2006).
Second, the reduced activity in the SPL and IPS might be a secondary consequence of the observed PCC dysfunction. Certainly, there have been suggestions that the PCC is dysfunctional in PT (Anderson & Kiehl, 2012). Of course, this would imply that the responsiveness of the PCC to fearful expression information was selectively impaired in youths with DBD + PT; it is not clear that that level of representational specificity occurs within the PCC. Instead, PCC activity is thought to more generally relate to eye movements (Olson, Musil, & Goldberg, 1996) and spatial localization (Harker & Whishaw, 2002). Moreover, as it is related to both reinforcement related regions of the brain such as the amygdala, caudate, and orbital frontal cortex as well as oculomotor regions such as the parietal cortex, it has been argued that the PCC allows the functional linkage of motivational and oculomotor information (McCoy & Platt, 2005). A functional linkage-based account of PCC functioning would suggest that the primary source of the deficit occurred prior to the PCC; the region was not being differentially activated by the motivational information of the fearful expression.
Third, the findings are limited by the relatively poor temporal resolution of BOLD response data. Recent magnetoencephalography work has differentiated the early (<100 ms after stimulus onset) from the later (>150 ms after stimulus onset) amygdala response to fearful expressions (Luo et al., 2010). The suggestion is that, although top-down attention control systems can prime task demand representations and consequently reduce the representation of emotional information through representational competition (cf. Desimone & Duncan, 1995), this occurs relatively slowly (>150 ms after stimulus onset). As such, an early response to fearful relative to neutral expressions can be seen within the amygdala before the recruitment of systems involved in top-down attention control (Luo et al., 2010). On the bases of these data, we could speculate that comparison individuals, but not youths with DBD + PT, showed early recruitment of the amygdala, priming spatial attention within the SPL and IPS through the PCC. Of course, this third speculation requires testing with magnetoencephalography methodology.
The results of the Group × Emotion interaction for the anger ANOVA are worth mentioning. In both the superior and middle frontal cortex, youths with DBD + PT showed significantly greater responses to neutral expressions relative to healthy controls (there were no group differences in the response to anger expressions), although it should be noted that this Group × Emotion interaction was not present following the exclusion of those youths with DBD + PT with comorbid ADHD. This represents a partial replication of two earlier studies that have reported increased responses to neutral expressions in youths with CD (Passamonti et al., 2010) and adult violent offenders (Pardini & Phillips, 2010). Moreover, similar to the findings of Passamonti et al. (2010), the increased responses to neutral expressions was only seen in the anger ANOVA. However, the replication must be considered very partial. The regions showing increased responsiveness in the current study were the superior and middle frontal cortex rather than the amygdala seen in previous work. The previous results of increased amygdala responsiveness to neutral expressions cannot be considered robust. They were not seen in two further studies (Jones et al., 2009; Marsh et al., 2008). In addition, they did not replicate within the studies that reported them. Thus, in the Passamonti et al. (2010) study, the increased amygdala response to neutral expressions in CD was only found in the block of trials with angry expressions (it did not replicate in the blocks with other expressions; Passamonti et al., 2010). In contrast, the increased response to neutral expressions in the study with adult offenders was only found in the blocks of trials with happy expressions (it did not replicate in the blocks with other expressions, including angry; Pardini & Phillips, 2010). As such, although the response to neutral expressions deserves continuing attention, we do not believe that the current literature unequivocally supports the suggestion of enhanced neutral expression processing in DBD.
There are four caveats that should be considered with respect to the current data. First, although there were high rates of comorbid ADHD within the DBD + PT group, we did not include an ADHD comparison group in the current study. This was because previous work had indicated that youths with ADHD do not present with the pathophysiology found in DBD + PT youths (Finger et al., 2008; Marsh et al., 2008; Posner et al., 2011). Recent work by Posner and colleagues (2011) found that youths with ADHD showed increased amygdala activation to fearful faces. Moreover, and mitigating this limitation, it is important to note that our subsequent group analysis excluding DBD + PT youths with comorbid ADHD revealed extremely similar results regarding our main interaction of interest: Diagnosis × Emotion × Congruence. Second, the medications of two of our DBD + PT youths could not be withheld at the time of scanning. However, again mitigating this limitation, the results of our subsequent ANOVA excluding these participants again identified proximal regions showing significant Diagnosis × Emotion × Congruence interactions. Third, we did not have a group of DBD youths without PT. As such, we cannot conclude whether the current findings are specific to PTs or to DBD more generally. However, it should be noted that we found no relationship between the severity of specific components of PT and the differential response to fear congruent trials relative to fear incongruent trials. This contrasts with other work that we have conducted showing that the responsiveness of the amygdala to fearful expressions/functional connectivity between the amygdala and orbital frontal cortex in response to fearful expressions does relate to symptom severity (Marsh et al., 2008, 2011; White et al., in press). This could indicate that the functional impairments identified in the current study are more a feature of DBD rather than DBD + PT. However, we can speculate, given the previous findings (Marsh et al., 2008, 2011; White et al., in press), that the failure to identify any relationship represents the potential indirect nature of the atypical BOLD responses; that is, if we had been able to identify the reduced amygdala responses to fearful expressions in the youths with DBD + PT that we assume underpins these results, the level of reduced responsiveness would have related to PT severity. Fourth, the behavioral performance of children with DBD + PT did not significantly differ from the comparison group. Specifically, we failed to see any enhanced priming of spatial location by the gaze direction of fearful relative to neutral expressions in the comparison youth. Neither group showed such an effect. Although the absence of an interaction between gaze direction and expression in behavioral data (Friesen et al., 2011; Hietanen & Leppanen, 2003; Kuhn & Tipples, 2011; Mathews et al., 2003) with the presence of such an interaction in neurobiological data has been previously reported (Fichtenholtz, Hopfinger, Graham, Detwiler, & LaBar, 2007, 2009), it limits our interpretation of the data. We certainly cannot conclude from these data that youths with DBD + PT show less responsiveness to gaze information in the emotional expressions of others, although data from Dadds and colleagues would suggest that this is actually the case (Dadds, Allen, et al., 2012; Dadds et al., 2008; Dadds, Jambrak, Pasalich, Hawes, & Brennan, 2011).
With respect to these caveats, it is worth considering how future work should consider them. First, with respect to ADHD, the ideal study would involve four groups: patients with CD only, patients with ADHD only, patients with CD and ADHD, and healthy comparison youths. The problem with this recommendation is its practicality. CD and ADHD are highly comorbid (Hinshaw, 1987), just over 50% in the current study. Recruitment would be lengthy. Moreover, the inclusion of four groups would require significantly greater total numbers in each group. As such, although ideal, it may difficult to achieve. Questions regarding the pathophysiology of CD can be addressed with the current approach. (If the DBD vs. healthy controls group differences remain significant after the removal of the comorbid patients, it is clear that they are relatively robust, given that they survive even with the smaller total numbers.) However, important questions cannot be addressed such as the degree to which CD differs from ADHD. (Although there would be no reason to believe that patients with ADHD would show a selective effect for fearful expressions, they might show significantly less modulation by gaze congruence given their attentional deficits.) Moreover, it would be interesting to note whether comorbid CD + ADHD represents a more severe form of CD. Second, with respect to medication, ideally only unmedicated patients would be studied. However, again, this recommendation may not always be practical. A considerable number of youths with CD receive atypical antipsychotics, for example, aripiprazole (abilify) and risperidone (risperdal). There have been estimates that over 70% of youths with DBDs in the United States in some institutional settings are given antipsychotics (Zito et al., 2008). Perhaps the recommendation must be that we should conduct studies to understand the impact of these medications on the pathophysiology of CD. Clearly, as in the case of two patients in the current study, it remains possible to reach the criterion for CD despite the medication. Third, with respect to youths with CD (or DBD) without PT, one recommendation would be for future studies to include this group of patients to determine whether findings are specific to PTs or to DBD more generally. An alternative, related approach is to follow the recommendations of the fifth edition of the Diagnostic and Statistic Manual of Mental Disorders (DSM-V) that has proposed modifications of the CD diagnosis to include a CU (reduced guilt and empathy) specifier. Under this approach, all participants meeting the diagnosis for CD would be included and the modulation by CU traits would be examined. It is this latter approach that our group has adopted in our current work given the DSM-V recommendations.
Finally, the current findings suggest the possibly utility of a novel treatment approach for DBD + PT youths, borrowing from new anxiety treatments. Like cognitive behavior therapy, attention bias modification treatment (ABMT) draws from the notion that cognitive biases underlie anxiety disorders (Hakamata et al., 2010). In contrast to cognitive behavior therapy, ABMT attempts to target attentional biases. Utilizing a dot-probe task, targets are always associated with neutral stimuli, as opposed to anxiety/fear provoking stimuli, biasing attention away from anxiety provoking environmental cues (Hakamata et al., 2010). ABMT has been successful in both adults and children (Bar-Haim, Morag, & Glickman, 2011; Hakamata et al., 2010). From the current results and previous work (Marsh & Blair, 2008; Marsh et al., 2008; White et al., in press), DBD + PT youths appear to lack an appropriate emotional response and consequent attentional bias to fearful faces. Therefore, a modified ABMT modality in which attention was biased toward fear/anxiety provoking stimuli might work to counteract this deficit. It is important to note, however, that successful treatment of DBD + PT will almost certainly require more than one approach, even for a single individual, because DBD + PT seems to require more significant treatment resources relative to DBD youths without PT (e.g., Caldwell, Skeem, Salekin, & Van Rybroek, 2006).
In summary, the current data extends previous findings of impaired processing of information from the eye region in youths with DBD + PT (Dadds, Allen, et al., 2012; Dadds et al., 2008; Dadds, Jambrak, et al., 2011). They suggest that this impairment may be selective for emotional expressions, perhaps particularly those emotional expressions such as fear that are more reliant on the amygdala for processing (Murphy et al., 2003) and have implications for attention-based accounts of the disorder (Newman, Brinkley, Lorenz, Hiatt, & MacCoon, 2007). They also suggest that eye-tracking information in youths with DBD + PT would be a useful additional level of data in future work to disentangle explanations of the expression-processing deficit in DBD + PT. Finally, the current data suggest the potential for success with new treatment modalities in DBD + PT.
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute of Mental Health.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Anderson NE, Kiehl KA. The psychopath magnetized: Insights from brain imaging. Trends in Cognitive Science. 2012;16:52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniskiewicz AS. Autonomic components of vicarious conditioning and psychopathy. Journal of Clinical Psychology. 1979;35:60–67. doi: 10.1002/1097-4679(197901)35:1<60::aid-jclp2270350106>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Morag I, Glickman S. Training anxious children to disengage attention from threat: A randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52:861–869. doi: 10.1111/j.1469-7610.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- Barry CT, Frick PJ, DeShazo TM, McCoy MG, Ellis M, Loney BR. The importance of callous–unemotional traits for extending the concept of psychopathy to children. Journal of Abnormal Psychology. 2000;109:335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, Frischen A, Fenske MJ, Tipper SP. Affective evaluations of objects are influenced by observed gaze direction and emotional expression. Cognition. 2007;104:644–653. doi: 10.1016/j.cognition.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences. 1999;27:135–145. [Google Scholar]
- Blair RJR. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philosophical Transaction of the Royal Society London B: Biological Sciences. 2003;358(1431):561–572. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Science. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: Probabilistic response reversal deficits in adult individuals with psychopathy. Journal of Abnormal Psychology. 2006;115:552–558. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Caldwell M, Skeem J, Salekin R, Van Rybroek G. Treatment response of adolescent offenders with psychopathy features: A 2-year follow-up. Criminal Justice and Behavior. 2006;33:571–596. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Allen JL, Oliver BR, Faulkner N, Legge K, Moul C, et al. Love, eye contact and the developmental origins of empathy v. psychopathy. British Journal of Psychiatry. 2012;200:191–196. doi: 10.1192/bjp.bp.110.085720. [DOI] [PubMed] [Google Scholar]
- Dadds MR, El Masry Y, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:455–463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Fraser J, Frost A, Hawes DJ. Disentangling the underlying dimensions of psychopathy and conduct problems in childhood: A community study. Journal of Consulting and Clinical Psychology. 2005;73:400–410. doi: 10.1037/0022-006X.73.3.400. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Jambrak J, Pasalich D, Hawes DJ, Brennan J. Impaired attention to the eyes of attachment figures and the developmental origins of psychopathy. Journal of Child Psychology and Psychiatry. 2011;52:238–245. doi: 10.1111/j.1469-7610.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, et al. Attention to the eyes and fear-recognition deficits in child psychopathy. British Journal of Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers visuospatial orienting by adults in a reflexive manner. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fichtenholtz HM, Hopfinger JB, Graham R, Detwiler JM, LaBar KS. Happy and fearful emotion in cues and targets modulate event-related potential indices of gaze-directed attentional orienting. Social Cognitive and Affective Neuroscience. 2007;2:323–333. doi: 10.1093/scan/nsm026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenholtz HM, Hopfinger JB, Graham R, Detwiler JM, LaBar KS. Event-related potentials reveal temporal staging of dynamic facial expression and gaze shift effects on attentional orienting. Social Neuroscience. 2009;4:317–331. doi: 10.1080/17470910902809487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Americal Journal of Psychiatry. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Dickens C. Current perspectives on conduct disorder. Current Psychiatry Reports. 2006;8:59–72. doi: 10.1007/s11920-006-0082-3. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Hare RD. The Antisocial Process Screening Device. Toronto: Multi-Health Systems; 2001. [Google Scholar]
- Frick PJ, Stickle TR, Dandreaux DM, Farrell JM, Kimonis ER. Callous–unemotional traits in predicting the severity and stability of conduct problems and delinquency. Journal of Abnormal Child Psychology. 2005;33:471–487. doi: 10.1007/s10648-005-5728-9. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Halvorson KM, Graham R. Emotionally meaningful targets enhance orienting triggered by a fearful gazing face. Cognition and Emotion. 2011;25:73–88. doi: 10.1080/02699931003672381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Impaired spatial performance in rats with retrosplenial lesions: Importance of the spatial problem and the rat strain in identifying lesions in a swimming pool. Journal of Neuroscience. 2002;22:1155–1164. doi: 10.1523/JNEUROSCI.22-03-01155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen JK, Leppanen JM. Does facial expression affect attention orienting by gaze direction cues? Journal of Experimental Psychology: Human Perception and Performance. 2003;29:1228–1243. doi: 10.1037/0096-1523.29.6.1228. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. On the distinction between attentional deficits/hyperactivity and conduct problems/aggression in child psychopathology. Psychological Bulletin. 1987;101:443–463. [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Current Biology. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neuroscience. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous–unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression, and the processing of emotional stimuli in non-referred girls and boys. Behavioral Sciences & the Law. 2006;24:21–37. doi: 10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Emde RN, Butterfield P, Campos JJ. Social referencing: The infant’s use of emotional signals from a friendly adult with mother present. Annual Progress in Child Psychiatry and Child Development. 1987;22:427–432. [Google Scholar]
- Kuhn G, Tipples J. Increased gaze following for fearful faces. It depends on what you’re looking for! Psychonomic Bulletin Review. 2011;18:89–95. doi: 10.3758/s13423-010-0033-1. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Rebalancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Majestic C, Cheng X, Schechter JC, Blair RJR. Emotional automaticity is a matter of timing. Journal of Neuroscience. 2010;30:5825–5829. doi: 10.1523/JNEUROSCI.BC-5668-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M. Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. Journal of Abnormal Psychology. 2007;116:155–165. doi: 10.1037/0021-843X.116.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience and Biobehavioral Reviews. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz ITN, Schechter JC, Yu HH, et al. Reduced amygdala/orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research: Neuroimaging. 2011;194:279–286. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous–unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Mathews AM, Fox E, Yiend J, Calder AJ. Effects of eye gaze and emotion on visual attention. Visual Cognition. 2003;10:823–835. doi: 10.1080/13506280344000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AN, Platt ML. Expectations and outcomes: Decision-making in the primate brain. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2005;191:201–211. doi: 10.1007/s00359-004-0565-9. [DOI] [PubMed] [Google Scholar]
- Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. Journal of Experimental Psychology: General. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. NeuroImage. 2007;34:1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Newman JP, Brinkley CA, Lorenz AR, Hiatt KD, MacCoon DG. Psychopathy as psychopathology: Hare’s essential contributions. In: Herve H, Yuille JC, editors. The psychopathy: Theory, research, and practise. Mahwah, NJ: Erlbaum; 2007. pp. 173–206. [Google Scholar]
- Olson CR, Musil SY, Goldberg ME. Single neurons in posterior cingulate cortex of behaving macaque: Eye movement signals. Journal of Neurophysiology. 1996;76:3285–3300. doi: 10.1152/jn.1996.76.5.3285. [DOI] [PubMed] [Google Scholar]
- Pardini DA, Phillips M. Neural responses to emotional and neutral facial expressions in chronically violent men. Journal of Psychiatry and Neuroscience. 2010;35:390–398. doi: 10.1503/jpn.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, et al. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:828–837. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman P, Hermans E, van Honk J. Anxiety meets fear in perception of dynamic expressive gaze. Emotion. 2006;6:94–102. doi: 10.1037/1528-3542.6.1.94. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. Amygdala integrates emotional expression and gaze direction in response to dynamic facial expressions. NeuroImage. 2010;50:1658–1665. doi: 10.1016/j.neuroimage.2010.01.049. [DOI] [PubMed] [Google Scholar]
- Stevens D, Charman T, Blair RJR. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. Journal of Genetic Psychology. 2001;162:201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tipples J. Fear and fearfulness potentiate automatic orienting to eye gaze. Cognition and Emotion. 2006;20:309–320. [Google Scholar]
- White S, Marsh A, Fowler K, Schechter J, Adalio C, Pope K, et al. Reduced amygdala responding in youth with disruptive behavior disorder and psychopathic traits reflects a reduced emotional response not increased top-down attention to non-emotional features. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2012.11081270. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Sai D, Gardner JF, Thomas D, Coombes P, et al. Psychotropic medication patterns among youth in foster care. Pediatrics. 2008;121:e157–e163. doi: 10.1542/peds.2007-0212. [DOI] [PubMed] [Google Scholar]


