Abstract
Cluster analytic methods have examined the symptom presentation of chronic tic disorders (CTDs), with limited agreement across studies. The present study investigated patterns, clinical correlates, and treatment outcome of tic symptoms. 239 youth and adults with CTDs completed a battery of assessments at baseline to determine diagnoses, tic severity, and clinical characteristics. Participants were randomly assigned to receive either a comprehensive behavioral intervention for tics (CBIT) or psychoeducation and supportive therapy (PST). A cluster analysis was conducted on the baseline Yale Global Tic Severity Scale (YGTSS) symptom checklist to identify the constellations of tic symptoms. Four tic clusters were identified: Impulse Control and Complex Phonic Tics; Complex Motor Tics; Simple Head Motor/Vocal Tics; and Primarily Simple Motor Tics. Frequencies of tic symptoms showed few differences across youth and adults. Tic clusters had small associations with clinical characteristics and showed no associations to the presence of coexisting psychiatric conditions. Cluster membership scores did not predict treatment response to CBIT or tic severity reductions. Tic symptoms distinctly cluster with few difference across youth and adults, or coexisting conditions. This study, which is the first to examine tic clusters in relation to treatment, suggested that tic symptom profiles respond equally well to CBIT.
Keywords: Habit reversal training, Comprehensive Behavioral Intervention for Tics, Tic symptom profiles, Chronic Tic Disorders, Treatment outcome, Cluster analysis
1. Introduction
Tics are sudden motor movements or vocalizations that begin in childhood and may persist into adulthood (Leckman, 2002). Transient tics are common in school-age children affecting as many as 24% in this age group (Snider et al, 2002). Chronic tic disorders are delineated by tic type (motor, phonic or both) and duration. For example, Persistent (Chronic) Motor Tic Disorder is defined by the presence of a single or multiple motor tics that persist for more than a year. The diagnosis of Tourette Syndrome (TS) requires both multiple motor and one or more phonic tics (not necessarily concurrently) that last more than a year (American Psychiatric Association, 2013). The estimated prevalence of TS ranges from three to eight per 1,000 in children (Scahill et al., 2009). In community and clinical samples, chronic tic disorders are associated with a wide range of behavioral and emotional difficulties (Sukhodolsky et al, 2003; Storch et al., 2007; Scahill, et al, 2009; Specht et al, 2011; Kraft et al, 2012).
Tic disorders have a heterogeneous presentation, with tics varying across and within individuals according to type (motor or phonic), anatomical location, and complexity (number of muscle groups involved) (Leckman et al, 2006). Tics in individuals with TS often begin with eye blinking and movements of the face and head region. Motor tics usually precede phonic tics and simple tics precede more complex tics (Leckman, et al, 2006; Bloch and Leckman, 2009). Simple tics include brief, repetitive movements such as eye blinking, grimacing, head jerks, shrugging or vocalizations such as throat clearing, grunting. Complex motor tics involve larger muscle groups and appear more goal-directed in character (e.g., arm thrusts, gyrating, bending). Vocalizations such as words or short phrases (e.g., “oh boy”, “you bet”) can also occur. Although often believed to be prototypic of TS, coprolalia, or bouts of uncontrolled cursing, affects only an estimated 18.5% of patients (Freeman et al., 2009). Many individuals with TS experience premonitory urges associated with tics, with some difficulty in urge recognition among youth under 10 years of age (Woods et al., 2005). Although tic severity ranges from mild to severe across individuals, most cases exhibit a fluctuating course with peaks in symptom severity that stabilize over a period of weeks (Lin et al, 2002). Following the onset of tics in early school-age years, tics often increase in number, type and frequency into early adolescence and often subside in early adulthood (Bloch and Leckman, 2009). Nonetheless, tic symptoms and impairment may persist into adulthood, resulting in a diminished quality of life (Bloch and Leckman, 2009; Gorman et al, 2010). Although the cause of TS is unknown, available evidence suggests that dysregulation of cortical and subcortical motor circuits underlie tic symptoms (see Leckman et al., 2010 for a review).
Beyond the broad categories of simple and complex tics, there is little consensus regarding the existence and organization of symptom subtypes within tic disorders (Walkup et al, 2010). This lack of consensus may be due to differences in sample selection, assessment methods, and nomenclature used in prior studies. For instance, a tic could be classified as a ‘facial grimace’ on one measure, but as a ‘mouth/jaw movement’ or a ‘facial tic’ on another measure. In an attempt to circumvent measurement variability, several studies have relied upon factor and/or cluster analytic techniques (Alsobrook and Pauls, 2002; Mathews et al, 2007; Robertson and Cavanna, 2007; Robertson et al, 2008; Kircanski et al, 2010;). Collectively, these four studies have produced two consistent findings. First, tic symptoms cluster by complexity (simple versus complex) (Mathews et al., 2007; Robertson et al, 2008; Kircanski et al, 2010). Second, compulsive tic behaviors (e.g., touching, repetitive behaviors, echolalia) cluster separately from other tic symptoms (e.g., head movements, leg movements, coprolalia) (Alsobrook and Pauls, 2002; Robertson et al, 2008).
Kircanski and colleagues used agglomerative cluster analysis to examine clusters of tic symptoms from the Yale Global Tic Severity Scale (YGTSS; Leckman et al, 1989) and their clinical correlates in 99 children (Kircanski et al, 2010). The YGTSS is a reliable and valid clinician-rated instrument that is commonly used in clinical trials to measure tic severity (Leckman et al, 1989; Storch et al, 2005). Kircanski et al. (2010) identified four overlapping clusters; predominantly complex tics, simple head/face tics, simple body tics, and simple vocal/facial tics. Associations were reported between specific symptom clusters and symptom severity, age, and premonitory urge ratings (Kircanski et al, 2010). To date, no cluster analytic study has explored associations between tic clusters and treatment outcome.
In the present study, data were compiled from two multi-center, randomized clinical trials (RCTs) comparing the Comprehensive Behavioral Intervention for Tics (CBIT; Woods et al., 2008) to a structured psychoeducation and supportive therapy intervention (PST) in children (Piacentini et al, 2010) and adults (Wilhelm et al, 2012) with chronic tic disorders. The two RCTs employed identical designs and assessment methods, which included study participation lasting 10 weeks in duration, and blinded assessments of the YGTSS conducted at baseline, midpoint (Week 5) and endpoint (Week 10). The first RCT included 126 youth ages 9 to 17 years (Piacentini et al, 2010); the second RCT included 122 participants between 16 and 69 years of age (Wilhelm et al, 2012). Similar to Kircanski et al. (2010), we used an agglomerative cluster analysis to identify tic symptom clusters based on the YGTSS and examined associations of clinical characteristics and tic clusters. In addition, the present study also examined the association between specific tic clusters and response to CBIT.
2. Method
2.1 Participants
To be eligible, participants had to have a chronic tic disorder of at least moderate severity, and be fluent in English. Moderate severity was defined as having a CGI-Severity (CGI-S) rating of “moderately ill” (4) or greater (Guy, 1976). In the child CBIT trial, the YGTSS Total Tic score had to be greater than 13 (participants with only motor or vocal tics required a YGTSS score greater than 9). In the adult CBIT trial, the YGTSS Total Tic score had to be greater than 14 (participants with only motor or vocal tics requires a YGTSS score greater than 10). Cases with severe tics (greater than 30 on the YGTSS Total Tic score) were reviewed by a cross-site panel to confirm appropriateness for study participation.
A current or lifetime diagnoses of major depression, anxiety disorders (including obsessive compulsive disorder; OCD), and/or attention deficit hyperactivity disorder (ADHD) were acceptable for enrollment if the coexisting disorder was stable and did not necessitate immediate treatment. Participants on psychotropic medication (including tic medication) could enter the trial if the medication was stable for at least six weeks prior to the baseline assessment and there was no expected dose change during the 10-week trial. Exclusion criteria included: IQ less than 80; current diagnosis of substance abuse or substance dependence; lifetime diagnosis of pervasive developmental disorder, mania or psychotic disorder; or previously receiving four or more sessions of habit reversal training for tics.
Six youths and three adults were excluded from the collective sample (N = 248) due to missing or illegible YGTSS symptom checklist data. The final sample included 239 participants (171 males and 68 females) ranging in age from 9-69 years (Myears= 21.67, SDyears = 14.10). Most participants were diagnosed with Tourette Disorder (n = 212). Twenty-five participants met criteria for Chronic Motor Tic Disorder and two participants were diagnosed with Chronic Vocal Tic Disorder. Sample characteristics are presented in Table 1. Additional sample details can be found in Piacentini et al. (2010) and Wilhelm et al. (2012).
Table 1. Baseline Demographic and Clinical Characteristics for Youth, Adults, and Collective Sample.
| Youth (n = 142) | Adults(n = 97) | Total Sample (N = 239) | |
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 12.45 (2.83) | 35.17 (13.09) | 21.67 (14.10) |
| YGTSS Motor Total | 14.68 (3.78) | 15.23 (3.09) | 14.90 (3.52) |
| YGTSS Phonic Total | 9.68 (4.66) | 7.68 (5.29) | 8.87 (5.01) |
| YGTSS Total Tic Score | 24.35 (6.05) | 22.91 (6.83) | 23.77 (6.40) |
| YGTSS Impairment Total Score | 23.48 (8.29) | 24.45 (6.69) | 23.87 (7.68) |
| ADHD-RS Total Score | 14.58 (11.61) | 14.53 (10.88) | 14.56 (11.30) |
| PUTS Total Score | 17.82 (6.70) | 21.69 (5.98) | 22.08 (6.84) |
| CY-BOCS/Y-BOCS Total Score | 6.43 (8.00) | 4.62 (7.38) | 5.70 (7.79) |
|
| |||
| N (%) | N (%) | N (%) | |
|
| |||
| Male | 111 (78%) | 60 (62%) | 171 (72%) |
| Female | 31 (22%) | 37 (38%) | 68 (28%) |
| Race | |||
| White | 123 (87%) | 77 (80%) | 200 (83%) |
| Black | 3 (2%) | 1 (1%) | 4 (2%) |
| Hispanic | 10 (7%) | 13 (13%) | 23 (10%) |
| Asian/Pacific Islander | 4 (3%) | 5 (5%) | 9 (4%) |
| Other | 2 (1%) | 1 (1%) | 3 (1%) |
| CGI-Severity | |||
| Moderately Ill | 84 (59%) | 70 (72%) | 154 (65%) |
| Markedly Ill | 53 (37%) | 24 (25%) | 77 (32%) |
| Severely Ill | 5 (4%) | 3 (3%) | 8 (3%) |
| Medication Status | |||
| On Medication for Tics* | 54 (38%) | 19 (20%) | 73 (31%) |
| On S/SRI Medication | 29 (20%) | 22 (23%) | 51 (21%) |
| On Stimulant Medication | 17 (12%) | 5 (5%) | 22 (9%) |
| Concurrent Disorders | |||
| OCD | 27 (19%) | 17 (18%) | 44 (18%) |
| ADHD | 40 (28%) | 26 (27%) | 66 (28%) |
| Non-OCD Anxiety Disorder** | 44 (31%) | 11 (11%) | 55 (23%) |
| Generalized Anxiety Disorder | 26 (18%) | 10 (10%) | 36 (15%) |
| Social Phobia | 25 (18%) | 2 (2%) | 27 (11%) |
| Panic Disorder | 0 (0%) | 3 (3%) | 3 (1%) |
| Separation Anxiety Disorder | 11 (8%) | ---- | 11 (5%) |
Tic medications included antipsychotic medications and alpha-2 agonists.
may have more than one anxiety disorder.
YGTSS = Yale Global Tic Severity Scale; ADHD-RS = Attention Deficit Hyperactivity Disorder- Rating Scale; PUTS = Premonitory Urge for Tic Scale; CY-BOCS = Children's Yale-Brown Obsessive-Compulsive Scale; Y-BOCS = Yale-Brown Obsessive-Compulsive Scale; CGI = Clinical Global Impression; OCD = Obsessive Compulsive Disorder; ADHD = Attention Deficit Hyperactivity Disorder; S/SRI = Selective/Serotonin Reuptake Inhibitor.
2.2 Measures
Diagnostic Interviews
Age appropriate structured diagnostic interviews were used to assess current diagnoses. For participants in the child CBIT study, the Anxiety Disorders Interview Schedule for DSM-IV-TR: Child Version (ADIS; Silverman and Albano, 1996) with a supplementary tic module, were administered to parents and youth. The ADIS is a structured clinical interview that assesses current episodes of Axis I disorders, and differential diagnoses based on DSM-IV criteria. The ADIS has consistently demonstrated strong psychometric properties, including test-retest reliability, inter-rater reliability, and concurrent validity (Silverman et al, 2001; Wood et al, 2002). For participants in the adult CBIT trial, the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002) was administered to participants. The SCID-Patient version is a semi-structured interview that assess current episodes of Axis I disorders. Additionally for adult CBIT participants, a supplementary tic and ADHD interview were administered.
Tic Symptoms and Severity
The presence and severity of tics among participants was assessed using the Yale Global Tic Severity Scale (YGTSS; Leckman et al, 1989). The YGTSS is a clinician-rated scale with demonstrated reliability and validity designed to measure tic severity over the previous week (Leckman et al, 1989; Storch, et al, 2005). The initial section of the YGTSS consists of a checklist of 40 possible tics separately categorized as simple motor, complex motor, simple vocal and complex vocal. Different types of simple vocal tics (e.g., coughing, throat clearing, sniffing, grunting, animal noises) are subsumed under a single category titled “any simple phonic tic.” Inter-rater reliability of the symptom YGTSS symptom checklist has not been evaluated. Tics noted as present in the past week are then globally rated on a series of 5-point subscales (number, frequency, intensity, complexity, and inference) with motor and vocal tics rated separately. The YGTSS yields three tic severity scores: Total Motor (0 to 25); Total Phonic (0 to 25) and the combined Total Tic Score (0 to 50). Additionally, the YGTSS also includes an Impairment scale scored from 0 to 50.
Premonitory Urge Ratings
Across participants, ratings of premonitory urges were assessed using the Premonitory Urge for Tics Scale (PUTS; Woods et al, 2005). The PUTS is a 9-item, self-report questionnaire designed to establish the presence and current degree of premonitory sensations in patients with chronic tic disorders. The total score ranges from 9 to 36.
Obsessive Compulsive Symptom Severity
Obsessive compulsive symptom severity was assessed using either the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS; Scahill et al., 1997) or the Yale-Brown Obsessive Compulsive Scale (Y-BOCS; Goodman et al, 1989a, 1989b). The Y-BOCS and CY-BOCS are clinician-administered semi-structured interviews used to measure obsessive compulsive symptom severity, with total severity scores ranging betweeen 0-40. The CY-BOCS/Y-BOCS are identical in format and content, and have demonsrated reliability and validity.
Attention Deficit/Hyper activity Disorder Symptom Severity
Across participants, ADHD symptom severity was assessed using the Attention Deficit Hyperactivity Disorder Rating Scale (ADHD-RS; DuPaul et al, 1998). The ADHD-RS is a 20-item scale derived from the DSM-IV-TR ADHD criteria used to measure the current level of inattention, impulsiveness and hyperactivity. The scale uses an identical 4-point rating scale for each item, with a total score ranging from 0 to 60.
Global Severity and Improvement
Participants’ global severity was assessed using the Clinical Global Impression - Severity Scale (CGI-S; Guy, 1976). The CGI-S is a 7-point single-item clinician-rated scale commonly used in clinical trials to measure overall clinical severity. Scores on the CGI-S range from “normal presentation/no illness” (1) to “extreme illness” (7). Similarly, change in participants’ overall clinical presentation was assessed using the Clinical Global Impression - Improvement Scale (CGI-I; Guy, 1976). The CGI-I is a clinician rating of the overall change in clinical presentation from baseline. The CGI-I ranges from “very much improved” (1) to “very much worse” (7). Ratings of “very much improved” and “much improved” were used to classify positive treatment response to CBIT.
2.3 Procedures
The RCTs were approved by the Institutional Review Boards at each site and all participants provided consent (parental permission for minors). Participants were enrolled from six centers: Johns Hopkins University, University of Wisconsin at Milwaukee; University of California at Los Angeles (child sites); Massachusetts General Hospital/Harvard University; University of Texas Health Sciences Center at San Antonio; Yale University (adult sites). Screening and baseline assessments were completed to confirm eligibility and to establish pre-treatment symptom severity. All raters had a master's degree or higher in a mental health field and were trained to reliability on clinician-rated measures. Prior to the administration of the YGTSS and CGI-I scales, raters received training on the instruments and demonstrated reliability on three video-recorded assessments. Ongoing supervision of all raters was provided via monthly cross-site teleconference calls throughout the trials. Assessments by new raters were recorded on video and reviewed by an expert rater for quality assessment with feed back discussed on separate training calls. Eligible child and adult participants were randomly assigned in a 1:1 ratio to eight sessions of CBIT or PST over a 10-week period. The randomization was stratified on the presence of tic medication at baseline (see Piacentini et al, 2010 and Wilhelm et al, 2012 for details on methods and procedures).
2.4 Treatment
The primary components of CBIT were Habit Reversal Training (HRT), which teaches individuals to manage premonitory tic urges without actually expressing their tics, and a functional intervention designed to identify and neutralize antecedent and consequent events associated with tic expression (Woods et al., 2008). Psychoeducation and supportive therapy (PST), which served as a comparison treatment for CBIT, was designed to mimic adjunctive psychosocial support for psychopharmacologic treatment (Scahill et al, 2006). Further information regarding specific treatments can be found in Piacentini et al, (2010) and Wilhelm et al, (2012). Given the small number of PST treatment responders across the two treatment trials (16/128), examination of tic clusters to treatment outcome were limited to the CBIT group only.
2.5 Analytic Plan
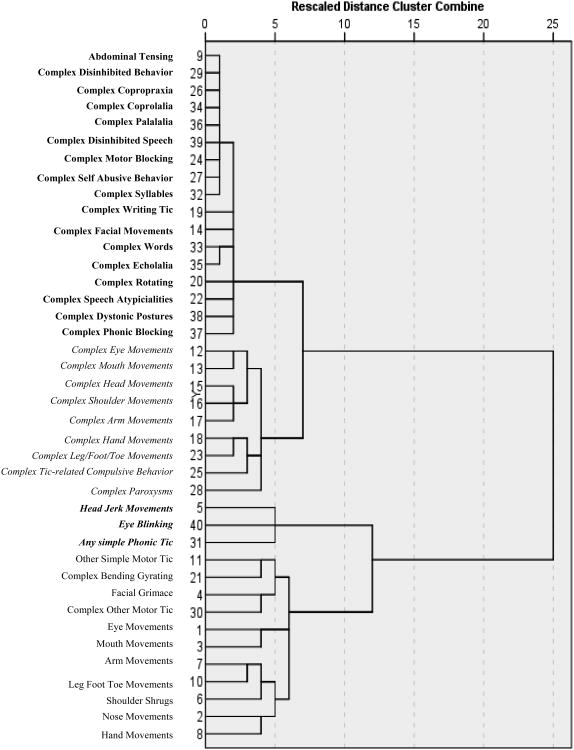
First, we examined age differences on tic severity, tic impairment, ADHD symptom severity, and obsessive compulsive symptom severity using independent sample t-tests. Based on the participant and the informant's response to the review of the YGTSS symptom checklist, tics were categorized as absent or present over the past week. The presence of individual tics was compared between children and adults using a chi-square test. Ward's hierarchical agglomerative cluster analysis was used to analyze the 40 tic types listed on the YGTSS checklist. This method progressively forms clusters of variables until all are subsumed into a single unifying cluster. The stages of agglomerations are displayed as a dendrogram with the formation of clusters plotted along a scaled, between-stage distance axis at each stage (Borgen and Barnett, 1987). The cluster models were reviewed by a panel of investigators (J.M., E.N., K.K., J.P., L.S.) based on the dendrograms and collective clinical experience. Consistent with Kircanski et al. (2010), tic symptoms were classified into a cluster when: (1) their dendrogram lines converged within a 10-unit window on the dendrogram cluster distance axis; and (2) convergence occurred before 50 (0 = individual symptoms, 100 = unitary cluster of all symptoms). Afterward, cluster models that met the above criteria were evaluated using investigator experience and clinical interpretability.
Cluster membership scores were calculated based on the number of symptoms endorsed in that particular cluster relative to other participants. For example, if a participant endorsed six out of the nine tics in a cluster, then that participant would receive a score of 0.66. The cluster score for each individual was subtracted by the mean cluster score of all participants for that cluster and divided by the standard deviation to yield the cluster membership score (Kircanski et al, 2010). An analysis of variance (ANOVA) was used to compare cluster membership scores across common coexisting presentations (i.e., TS, TS+OCD, TS+ADHD, TS+OCD+ADHD). Cluster membership scores were correlated with continuous clinical characteristics using Pearson's r for both youth and adult participants. A point bi-serial correlation was used to examine the association between medication use and cluster membership score. Significance levels for correlations was set at 0.05 and adjusted with Bonferroni correction for multiple comparisons. For participants receiving CBIT, logistic regression models examined the relationship between cluster membership scores and treatment response to CBIT on the CGI-I. Regression models were evaluated to determine whether baseline cluster membership scores predicted reductions in tic severity. All statistical analyses were completed using IBM SPSS version 20.
3. Results
3.1 Tic Symptom Clusters
Complete data were available on 239 participants. Independent sample t-tests showed no significant differences between youth and adults on measures of tic severity (p = 0.09), tic impairment (p = 0.34), ADHD symptom severity (p = 0.98), or obsessive compulsive symptom severity (p = 0.08). Therefore, youth and adult participants were combined for an examination of tic symptom clusters, clinical characteristics, and treatment outcome.
The dendrogram displays the results from the agglomerative hierarchical cluster analysis of tic types (see Figure 1). Four tic symptom clusters emerged (Table 2). Cluster 1, operationally defined as “Impulse Control and Complex Phonic Tics” included 7 complex motor tics reflecting low impulse control (e.g., disinhibited behavior, copropraxia), 8 complex phonic tics, and one simple motor tic. Cluster 2 defined “Complex Motor Tics”, was composed of 9 complex motor tics. Cluster 3, which was comprised of 2 simple motor tics (eye blinking and head jerks) and the collapsed phonic tic category of ‘any simple phonic tic,’ was labeled “Simple Head Motor/Vocal Tics.” Cluster 4, labeled “Predominantly Simple Motor Tics”, included 9 simple motor tics, and 2 complex motor tics. Seventy (30%) participants endorsed at least one tic on all four clusters, 21 (9%) endorsed a least one tic on three of the four clusters, and 128 (54%) participants reported tics on two of the four clusters. Only 20 (8%) participants endorsed tics in only one cluster. Chi-square tests revealed few differences in the presence of specific tics between youth and adults. Simple phonic tics were more common in youth and some complex motor tics were more common in adults. Coprolalia was uncommon in both age groups.
Figure 1.
Hierarchical agglomerative cluster analysis on YGTSS Symptom Checklist Cluster 1 = Complex Phonic Tic&Impulse Control Tics; Cluster 2 = Complex Motor Tics; Cluster 3 = Simple Head Region Motor/Vocal Tics; Cluster 4 = Primarily Simple Motor Tics
Table 2. Comparison of baseline tics on the YGTSS Checklist in Youth(n = 142) and Adults (n = 97).
| Youth N (%) | Adults N (%) | Total Sample N (%) | |
|---|---|---|---|
| Cluster 1: Complex Phonic Tics and Impulse Control Tics | |||
|
| |||
| Abdominal Tensing | 0 (0%) | 0 (0%) | 0 (0%) |
| Complex Disinhibited Behaviors | 5 (4%) | 1 (1%) | 6 (3%) |
| Complex Copropraxia | 4 (3%) | 3 (3%) | 7 (3%) |
| Complex Coprolalia | 5 (4%) | 8 (8%) | 13 (5%) |
| Complex Palalalia | 8 (6%) | 2 (2%) | 10 (4%) |
| Complex Disinhibited Speech | 7 (5%) | 1 (1%) | 8 (3%) |
| Complex Motor Blocking | 5 (4%) | 6 (6%) | 11 (5%) |
| Complex Self-Abusive Behaviors | 4 (3%) | 7 (7%) | 11 (5%) |
| Complex Syllables | 9 (6%) | 3 (3%) | 12 (5%) |
| Complex Writing Tic | 11 (8%) | 5 (5%) | 16 (7%) |
| Complex Facial Movements | 6(4%) | 13 (13%)** | 19 (8%) |
| Complex Words | 10 (7%) | 6 (6%) | 16 (7%) |
| Complex Echolalia | 13 (9%) | 7 (7%) | 20 (8%) |
| Complex Phonic Blocking | 16 (11%) | 6 (6%) | 22 (9%) |
| Complex Dystonic Postures | 8 (6%) | 15 (16%)** | 23 (10%) |
| Complex Rotating Movements | 8 (6%) | 8 (8%) | 16 (7%) |
| Complex Speech Atypicalities | 12 (9%) | 6 (6%) | 18 (8%) |
|
| |||
| Cluster 2: Complex Motor Tics | |||
|
| |||
| Complex Eye Movements | 12 (9%) | 15 (16%) | 27 (11%) |
| Complex Mouth Movements | 9 (6%) | 22 (23%)** | 31 (13%) |
| Complex Head Movements | 11 (8%) | 26 (27%)** | 37 (16%) |
| Complex Shoulder Movements | 11 (8%) | 25 (26%)** | 36 (15%) |
| Complex Arm Movements | 12 (9%) | 17 (18%)* | 29 (12%) |
| Complex Hand Movements | 24 (17%) | 28 (29%)* | 52 (22%) |
| Complex Leg, Foot and Toe Movements | 13 (9%) | 21 (22%)** | 34 (14%) |
| Complex Tic Related Compulsive behaviors | 32 (23%) | 17 (18%) | 49 (21%) |
| Complex Paroxysms | 27 (19%) | 31 (32%)* | 58 (24%) |
|
| |||
| Cluster 3: Simple Head Motor/Vocal Tics | |||
|
| |||
| Eye Blinking | 82 (58%) | 65 (67%) | 147 (62%) |
| Head Jerk Movements | 78 (55%) | 59 (61%) | 137 (58%) |
| Any Simple Phonic Tic | 122 (86%)* | 72 (74%) | 194 (81%) |
|
| |||
| Cluster 4: Primarily Simple Motor Tics | |||
|
| |||
| Eye Movement | 53 (37%) | 33 (34%) | 86 (36%) |
| Mouth Movements | 63 (44%) | 37 (38%) | 100 (42%) |
| Nose Movements | 39 (28%) | 30 (31%) | 69 (29%) |
| Hand Movements | 47 (33%) | 24 (25%) | 71 (30%) |
| Shoulder Shrugs | 48 (39%) | 39 (40%) | 87 (37%) |
| Arm Movements | 41 (29%) | 31 (32%) | 78 (33%) |
| Leg, Foot and Toe Movements | 43 (30%) | 26 (27%) | 69 (29%) |
| Facial Grimace | 35 (25%) | 47 (49%)** | 82 (35%) |
| Complex Other Motor Tics | 41 (29%) | 18 (19%) | 59 (25%) |
| Other Simple Motor Tics | 32 (23%) | 15 (16%) | 47 (20%) |
| Complex Bending/Gyrating Movements | 31 (22%) | 35 (36%)* | 66 (28%) |
p < 0.05
p < 0.01
3.2 Clinical Correlates of Tic Clusters
Table 3 presents the correlations between cluster scores and clinical characteristics for youth and adults. Across participants, correlations ranged from −0.25 to 0.30. The magnitudes of these correlations were small with few reaching statistical significance, limiting clinical inferences. For youth, Cluster 1 exhibited a small association with the presence of a tic medication, whereas for adults, Cluster 1 had a small positive association with premonitory urge ratings. Additionally for adults, Cluster 4 had a small positive association with ratings of ADHD symptom severity. Collectively in youth and adults, a series of one-way ANOVAs indicated no significant differences in cluster membership scores across various TS/OCD/ADHD clinical profiles for any of the four clusters [Cluster 1: F(3, 235) = 136, p = 0.26; Cluster 2: F(3, 235) = 2.06, p = 0.11; Cluster 3: F(3, 235) = 0.70, p = 0.55;Cluster 4: F(3, 235) = 1.54, p = 0.21].
Table 3. Correlations of Cluster Scores with Diagnoses, Age, Medication Use and Scores on Clinical Ratings for Youth and Adults.
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Impulse Control & Complex Phonic Tics | Complex Motor Tics | Simple Head Moto/Vocal Tics | Primarily Simple Motor Tics | |||||
| Clinical | Adults | Adults | Adults | Adults | ||||
| Characteristics | Youth | Youth | Youth | Youth | ||||
| Age | 0.11 | −0.06 | −0.03 | −0.02 | 0.01 | −0.12 | 0.03 | 0.04 |
| On Medication For | ||||||||
| Tics | 0.23* | 0.00 | −0.04 | −0.25 | 0.07 | −0.11 | 0.16 | 0.12 |
| YGTSS Impairment | 0.17 | 0.19 | −0.18 | −0.05 | 0.07 | 0.03 | 0.19 | 0.07 |
| Premonitory Urge | 0.16 | 0.30* | −0.01 | 0.18 | 0.07 | 0.09 | 0.13 | 0.14 |
| Total Score | ||||||||
| Y-BOCS/CY- | 0.11 | 0.10 | 0.11 | 0.07 | 0.11 | 0.10 | 0.19 | 0.25 |
| BOCS Total Score | ||||||||
| ADHD-RS Total Score | 0.15 | 0.03 | 0.07 | −0.01 | 0.14 | 0.09 | 0.07 | 0.28* |
Corrected for Multiple Comparison: Significance set at p < 0.008
Note: Non-OCD Anxiety Disorder included Generalized Anxiety Disorder, Social Phobia, Panic Disorder and Separation Anxiety Disorder.
YGTSS = Yale Global Tic Severity Scale; Y-BOCS/CY-BOCS = (Children's) Yale-Brown Obsessive Compulsive Scale; ADHD-RS = Attention Deficit Hyperactivity Disorder- Rating Scale
3.3 Tic Clusters and Treatment Outcome
Combining data from two previous reports, (Piacentini et al., 2010; Wilhelm et al, 2012), 56 (47%) of participants with complete YGTSS data showed a positive response on the CGI-I (Much Improved or Very Much Improved) at Week 10. Logistic regression models indicated that cluster membership scores did not predict treatment response to CBIT (Cluster 1: OR = 0.95, 95% CI = 0.64 – 1.41; Cluster 2: OR = 1.18, 95% CI = 0.81- 1.72; Cluster 3: OR = 0.97, 95% CI = 0.65 – 1.46; Cluster 4: OR = 1.26, 95% CI = 0.85 – 1.86), nor did baseline cluster membership predict reductions in total tic severity on the YGTSS for these individuals [F(4, 115) = 0.47, p= 0.77, R2 = 0.02]. Further examination of individual motor and phonic subscales on the YGTSS revealed that cluster membership scores did not predict reductions for either motor tic severity [F (4, 115) = 0.60, p = 0.66, R2 = 0.02] or phonic tic severity [F (4, 115) = 1.64, p = 0.17, R2 = 0.05]
4. Discussion
This study examined empirically-derived tic clusters in a large sample of treatment-seeking youth and adults with chronic tic disorders. Facial grimace was the only simple motor tic that significantly differed between age groups occurring more commonly in adults than youth. As anticipated, complex motor tics were also more common in adults than youth, however complex phonic tics did not differ by age group. Although tics are believed to emerge in a developmental progression (motor before phonic, simple before complex), the similar distribution of complex phonic tics across the lifespan suggest that complex phonic tics may be indicative of greater tic severity regardless of age.
Consistent with prior research (Kircanski et al, 2010), a four-cluster model of tic symptoms was identified (C1: Complex Phonic Tics and Impulse Control Tics; C2: Complex Motor Tics; C3: Simple Head Motor/Vocal Tics; C4: Primarily Simple Motor Tics). Three of these four tic symptom clusters (C2, C3 and C4) were similar to those found by Kircanski and colleagues. The fourth cluster (C1) consisted primarily of complex phonic tics (e.g., disinhibited speech, complex syllables) and impulse control tics (e.g., copropraxia, self-abusive behavior, writing tics). By contrast, the fourth cluster reported by Kircanski and colleagues included simple phonic (e.g., sniffing, grunting, throat clearing) and facial tics (e.g., facial grimace, nose movements). Differences in findings may be due the inclusion of adults in the present sample, sample size, or our decision to collapse simple phonic tics into a single tic type. In contrast to the current four cluster model, other investigators have reported two clusters based on simple and complex tics (Mathews et al, 2007; Robertson et al, 2008). Although a two tic cluster model has some empirical support, this binary tic model did not meet the outlined criteria that dendrogram lines converge within a 10-unit window on the distance axis, and the convergence occurred before 50. Moreover, a binary tic cluster model would not fully explain tic symptom associations in the current sample as one cluster (C4) contained both simple and complex motor tics.
Across the four clusters, cluster membership scores were similar across common combinations of coexisting conditions. Although these patterns of clinical presentation have been suggested as starting points for tic subtypes analyses (Robertson, 2008), purported differences are not likely accounted for by differing tic presentation. Exploring the clinical characteristics associated with each of the four tic clusters, few associations emerged as statistically significant and were small in magnitude. Cluster 1 (Impulse Control-Complex Phonic Tics) exhibited a positive association with tic medication in children. Given these findings for tic medication use in youth, Cluster 1 may be an indicator of more severe tics. The moderate association between Cluster 1 scores and premonitory urge severity in adults provides further evidence that more complex tics are related to premonitory urges. For Cluster 4 (Primarily Simple Motor Tics), there was a modest positive association between the cluster membership score and ratings of ADHD severity in adults. Given that this tic cluster included the two tics categories “other simple motor tic” and “other complex motor tic”, this association may be related to motor restlessness.
Study participants were drawn from two randomized behavior therapy trials, which permitted examination of tic cluster and treatment outcome. Cluster membership did not predict reductions in treatment response to Comprehensive Behavioral Intervention for Tics (CBIT; Woods, et al, 2008), a structured behavioral treatment protocol based on habit reversal training. To our knowledge, this is the first study to examine whether tic symptom clusters predicted treatment outcome. This implies that CBIT is equally effective across a range of tic types as no cluster emerged as predicting benefit or lack of efficacy. Conversely, however, it is also possible that the degree of heterogeneity within tic clusters may have obscured our ability to detect an association between tic cluster and treatment outcome.
There are several limitations of this study. First, agglomerative hierarchical cluster analysis does not use a goodness of model fit statistic. As such, the four tic cluster model was selected by an expert panel from several possible cluster models. Nonetheless, this approach allowed the current findings to be compared with previous tic cluster analyses. Future research using latent class analysis could examine alternative models concerning tic types and cluster of tics. Second, simple phonic tics were collapsed into a single category. It is possible that different simple phonic tics have stronger associations with other tics not associated with the head region area. It may be useful for the YGTSS symptom checklist to list simple phonic tics separately in a manner similar to the motor tic checklist. Third, the examination of tic cluster membership and treatment outcome was within the confines of a systematically-administered behavioral intervention for tics (CBIT). Future studies will need to examine if tic cluster membership predicts response to tic medications. Finally, the sample was predominantly Caucasian, which may limit the extent to which findings generalize to the larger population of individuals with tic disorders.
In summary, these findings suggest that tic symptom clusters have relatively discrete symptom groupings that do not significantly differ in presentation across common coexisting conditions. Tic cluster have few associations with specific clinical characteristics and were small in magnitude. The fact that tics across all of four clusters responded equally well to CBIT counters prior criticisms of behavioral approaches to tic management (Woods et al, 2007), and provides further evidence of the broad application of this intervention.
Acknowledgments
This research was supported by NIMH Grants R01MH070802 (Dr. Piacentini), 5R01MH069877 (Dr. Wilhelm), R01MH069874 (Dr. Scahill), and RO1MH069875 (Dr. Petersen) from the National Institute of Mental Health with subcontracts to Drs. Piacentini and Woods. Dr Walkup consulted on this grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsobrook JP, Pauls DL. A factor analysis of tic symptoms in Gilles de la Tourette's syndrome. The American Journal of Psychiatry. 2002;159(2):291–296. doi: 10.1176/appi.ajp.159.2.291. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bloch MH, Leckman JF. Clinical course of Tourette syndrome. Journal of Psychosomatic Research. 2009;67(6):497–501. doi: 10.1016/j.jpsychores.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgen FH, Barnett DC. Applying cluster analysis in counseling psychology research. Journal of Counseling Psychology. 1987;34:456–468. [Google Scholar]
- DuPaul GJ, Power TJ, McGoey KE, Ikeda MJ, Anastopoulos AD. Reliability and validity of parent and teacher ratings of attention-deficit/hyperactivity disorder symptoms. Journal of Psychoeducational Assessment. 1998;16(1):55–68. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Freeman RD, Zinner SH, Muller-Vahl KR, Fast DK, Burd LJ, Kano Y, Rothenberger A, Roessner V, Kerbeshian J, Stern JS, Jankovic J, Loughin T, Janik P, Shady G, Robertson MM, Lang AE, Budman C, Magor A, Bruun R, Berlin CM., Jr Coprophenomena in Tourette syndrome. Developmental Medicine and Child Neurology. 2009;51(3):218–227. doi: 10.1111/j.1469-8749.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Archives of General Psychiatry. 1989a;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C. The Yale-Brown Obsessive Compulsive Scale: II. Validity. Archives of General Psychiatry. 1989b;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, Peterson BS. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. British Journal of Psychiatry. 2010;197(1):36–44. doi: 10.1192/bjp.bp.109.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. Clinical Global Impressions ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute for Mental Health; 1976. pp. 218–222. [Google Scholar]
- Kircanski K, Woods DW, Chang SW, Ricketts EJ, Piacentini JC. Cluster analysis of the Yale Global Tic Severity Scale (YGTSS): Symptom dimensions and clinical correlates in an outpatient youth sample. Journal of Abnormal Child Psychology. 2010;38(6):777–788. doi: 10.1007/s10802-010-9410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft JT, Dalsgaard S, Obel C, Thomsen PH, Henriksen TB, Scahill L. Prevalence and clinical correlates of tic disorders in a community sample of school-age children. European Child and Adolescent Psychiatry. 2012;21(1):5–13. doi: 10.1007/s00787-011-0223-z. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Tourette's syndrome. Lancet. 2002;360(9345):1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Scahill L, King RA. Tourette Syndrome: The Self Under Siege. Journal of Child Neurology. 2006;21(8):642–649. doi: 10.1177/08830738060210081001. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette's disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20(4):237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Lin H, Yeh CB, Peterson BS, Scahill L, Grantz H, Findley DB, Katsovich L, Otka J, Lombroso PJ, King RA, Leckman JF. Assessment of symptom exacerbations in a longitudinal study of children with Tourette's syndrome or obsessive-complusive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(9):1070–1077. doi: 10.1097/00004583-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Jang KL, Herrera LD, Lowe TL, Budman CL, Erenberg G, Naarden A, Bruun RD, Schork NJ, Freimer NB, Reus VI. Tic symptom profiles in subjects with Tourette Syndrome from two genetically isolated populations. Biological Psychiatry. 2007;61(3):292–300. doi: 10.1016/j.biopsych.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, Walkup JT. Behavior therapy for children with Tourette disorder: A randomized controlled trial. Journal of the American Medical Association. 2010;303(19):1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 2: tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. Journal of Psychosomatic Research. 2008;65(5):473–486. doi: 10.1016/j.jpsychores.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Althoff RR, Hafez A, Pauls DL. Principal components analysis of a large cohort with Tourette syndrome. British Journal of Psychiatry. 2008;193(1):31–36. doi: 10.1192/bjp.bp.107.039909. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Cavanna AE. The Gilles de la Tourette Syndrome: A principal component factor analytic study of a large pedigree. Psychiatric Genetics. 2007;17(3):143–152. doi: 10.1097/YPG.0b013e328015b937. [DOI] [PubMed] [Google Scholar]
- Scahill L, Bitsko RH, Visser SN, Blumberg SJ. Centers for Disease Control: Prevalence of diagnosed Tourette syndrome in persons aged 6-17 years - United States, 2007. Morbidity and Mortality Weekly Report. 2009;58(21):581–585. [PubMed] [Google Scholar]
- Scahill L, Erenberg G, Berlin CM, Jr, Budman C, Coffey BJ, Jankovic J, Kiessling L, King RA, Kurlan R, Lang A, Mink J, Murphy T, Zinner S, Walkup J. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV-Child and Parent Versions. San Antonio, TX: Graywinds Publications; 1996. [Google Scholar]
- Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with anxiety disorders interview schedule for DSM-IV : Child and parent versions. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(8):937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- Snider LA, Seligman LD, Ketchen BR, Levitt SJ, Bates LR, Garvey MA, Swedo SE. Tics and problem behaviors in school children: prevalence, characterization, and associations. Pediatrics. 2002;110(2):331–336. doi: 10.1542/peds.110.2.331. [DOI] [PubMed] [Google Scholar]
- Specht MW, Woods DW, Piacentini J, Scahill L, Wilhelm S, Peterson AL, Chang S, Kepley H, Deckersbach T, Flessner C, Buzzella BA, McGuire JF, Levi-Pearl S, Walkup JT. Clinical characteristics of children and adolescents with a primary tic disorder. Journal of Developmental and Physical Disabilities. 2011;23(1):15–31. doi: 10.1007/s10882-010-9223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Lack CW, Simons LE, Goodman WK, Murphy TK, Geffken GR. A measure of functional impairment in youth with Tourette's Syndrome. Journal of Pediatric Psychology. 2007;32(8):950–959. doi: 10.1093/jpepsy/jsm034. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Sajid M, Allen P, Roberti JW, Goodman WK. Reliability and validity of the Yale Global Tic Severity Scale. Psychological Assessment. 2005;17(7):486–491. doi: 10.1037/1040-3590.17.4.486. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Zhang H, Peterson BS, King RA, Lombroso PJ, Katsovich L, Findley D, Leckman JF. Disruptive behavior in children with Tourette's syndrome: Association with ADHD comorbidity, tic severity, and functional impairment. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(1):98–105. doi: 10.1097/00004583-200301000-00016. [DOI] [PubMed] [Google Scholar]
- Walkup JT, Ferrao Y, Leckman JF, Stein DJ, Singer H. Tic disorders: some key issues for DSM-V. Depression and Anxiety. 2010;27(6):600–610. doi: 10.1002/da.20711. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, Chang S, Liu H, Dziura J, Walkup JT, Scahill L. Randomized trial of behavior therapy for adults with tourette syndrome. Archives of General Psychiatry. 2012;69(8):795–803. doi: 10.1001/archgenpsychiatry.2011.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Piacentini JC, Bergman RL, McCracken J, Barrios V. Concurrent validity of the anxiety disorders section of the Anxiety Disorders Interview Schedule for DSM-IV: Child and Parent Versions. Journal of Clinical Child and Adolescent Psychology. 2002;31(3):335–342. doi: 10.1207/S15374424JCCP3103_05. [DOI] [PubMed] [Google Scholar]
- Woods DW, Conelea CA, Walther MR. Barriers to dissemination: Exploring the criticisms of behavior therapy for tics. Clinical Psychology: Science and Practice. 2007;14(3):279–282. [Google Scholar]
- Woods DW, Piacentini J, Chang SW, Deckersbach T, Ginsburg GS, Peterson AL, Scahill L, Walkup JT, Wilhelm S. Managing Tourette Syndrome: A Behavioral Intervention for Children and Adolescents. New York: Oxford University Press; 2008. [Google Scholar]
- Woods DW, Piacentini J, Himle MB, Chang S. Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. Journal of Developmental and Behavioral Pediatrics. 2005;26(6):397–403. doi: 10.1097/00004703-200512000-00001. [DOI] [PubMed] [Google Scholar]