Abstract
Background
Studies in depressed patients have demonstrated the presence of emotional bias toward negative stimuli, as well as dysregulated brain serotonin function. The present study compared the effects of acute tryptophan depletion (ATD) on both an emotional processing and a planning task in never-depressed healthy volunteers at high and low familial risk for depression.
Methods
Young adults with no personal psychiatric history were stratified into two groups based on family history (n = 25). Participants were enrolled in a randomized, double-blind, placebo-controlled crossover ATD study and completed the affective go/no-go and Tower of London tasks once during each condition.
Results
There was a significant treatment by valence by group interaction on the affective go/no-go, driven primarily by a greater frequency of inappropriate responses to sad than to happy distracters in the high-risk group during ATD. No group differences were observed on the Tower of London.
Conclusions
Asymptomatic individuals at high familial risk for depression showed abnormalities in emotional processing while undergoing experimentally induced tryptophan depletion. These findings support emotional processing disturbances as potential trait-level abnormalities associated with the risk of mood disorder.
Keywords: Affective go/no-go, emotional processing, family history, high risk, major depression, tryptophan depletion
Major depressive disorder (MDD) is characterized by an inability to disengage from distracting negative thoughts, memories, and events (1,2). Studies in depressed patients have reported evidence of emotional bias toward negative stimuli (3-5), as well as dysregulated brain serotonin (5-HT) function (6). Recent studies in healthy volunteers have linked 5-HT function specifically with performance on emotional processing or “hot” cognitive tasks (7-9).
To understand the role of 5-HT function in the pathophysiology of MDD, it is critical to study individuals at high familial risk (HR) for depression before first onset of the disorder. Acute tryptophan depletion (ATD) is a widely used experimental paradigm that manipulates availability of brain 5-HT by depletion of its precursor, tryptophan, allowing the study of differences in 5-HT function (6,10). Published ATD studies comparing affective processing in HR and low familial risk (LR) groups have yielded mixed results (11,12), with one study suggesting a differential effect of ATD when combined with stress (13).
The present study compared the effects of ATD on performance in an emotional processing task, the affective go/no-go (AGNG) (3), in a group of healthy volunteers with positive family history of MDD (HR) and a control group with negative family psychiatric history (LR). Both major depression and ATD have been shown to affect performance on this task. Studies with the AGNG have reported negative bias in currently depressed patients (3,4), positive bias in healthy volunteers with a negative family psychiatric history (4,8), and abolition of positive bias in healthy volunteers during ATD (7-9). The specific dependent measures revealing emotional bias (i.e., omission errors, distracter errors, reaction times) have varied across studies. The only published ATD study in remitted depressives reported positive bias both during ATD and placebo, an unexpected finding (14). To our knowledge, there are no published studies comparing performance on the affective go/no-go task in healthy volunteers at high and low risk for depression. In light of previous findings in ATD studies of healthy individuals (7-9) and the established link between MDD and 5-HT dysfunction (6), we predicted that ATD would differentially affect emotional processing in HR and LR participants. Specifically, we hypothesized that the HR group would show a negative emotional bias on the AGNG during ATD compared with the LR control group. By contrast, we hypothesized that ATD would not differentially impact performance in a comparison planning task not involving affective processing, the Tower of London (TOL) (15).
Methods and Materials
Participants
Procedures were approved by the Mount Sinai School of Medicine Institutional Review Board. Male and female volunteers (18–35 years) with no personal history of any Axis I disorders and free of medical illness, current medications, and lifetime 3,4-methylene-dioxymethamphetamine (ecstasy) use were stratified into two groups based on family history. The HR volunteers had at least one first-degree relative with recurrent or chronic MDD. The LR volunteers had no history of any Axis I disorders in first-degree relatives. Participants were assessed with the Structural Clinical Interview for DSM-IV and a clinical interview by a psychiatrist. Family history of psychiatric (Axis I) disorders was ascertained for all first-degree relatives by administering the Family Interview for Genetic Studies (16) to participants and whenever possible to a second family informant. For HR participants, the Family Interview for Genetic Studies was also used to identify at least one first-degree relative with recurrent or chronic MDD, formally diagnosed by a physician and prescribed antidepressant medication. Whenever possible (for 5 [38%] HR volunteers), the Structural Clinical Interview for DSM-IV was also administered to the affected relative to confirm the MDD history. The two groups were matched for age and gender.
Experimental Procedure
Participants were enrolled in a randomized, double-blind, placebo-controlled crossover ATD study, using a modified methodology (17,18) (Supplement 1). The HR and LR groups were matched for treatment order. Blood samples for plasma tryptophan were obtained at baseline (T0) and 6 hours (T6) and 8 hours (T8) after capsule ingestion. Mood-lowering response was assessed by self-report with visual analogue scales at T0, 5 hours after capsule ingestion (T5), and T8. Participants completed the TOL task (15) at T6 and the AGNG task (3) at T8. Data from three LR participants who completed only one of the two sessions were excluded from analyses (Supplement 1).
Data Analysis
Biochemical, mood, and behavioral data were analyzed with repeated measures analysis of variance using SPSS 16 (SPSS, Inc., Chicago, Illinois), with group (HR vs. LR) as the between-subject factor. Analyses of plasma tryptophan levels and mood-lowering response to ATD included treatment (ATD vs. placebo) and time as within-subject factors. Analyses of performance on the AGNG task (omission and distracter errors, reaction times) included treatment, valence (happy vs. sad word blocks), and block type (shift vs. non-shift) as within-subject factors. For analyses with the TOL task, treatment and difficulty level were included as within-subject factors.
Results
The completer sample included 13 HR (10 female participants, mean age 26.5 years) and 12 LR (9 female participants, mean age 25.3 years) participants (Supplement 1). Group characteristics did not differ significantly between groups. For most HR participants (92%), age of onset of MDD in the affected relative was < 30 and MDD was recurrent.
Biochemical Effects
At T6, plasma free tryptophan levels were reduced from baseline by 72% in the HR group and by 71% in the LR group. Total tryptophan levels were reduced by 72% in the HR group and by 76% in the LR group. There were significant treatment by time interactions for both free [F(1,23) = 26.6, p < .0001] and total [F(1,22) = 97.5, p < .000001] tryptophan levels, with no significant group effect. Analyses of tryptophan levels at T8 confirmed persistence of a significant treatment by time interaction for free and total tryptophan levels (p < .001).
Mood Effects
There were no significant treatment by time by group interactions on the happy/euphoric [F(2,22) = .55, p = .59] or the sad [F(2,22) = 1.62, p = .22] visual analogue scales (Supplement 1).
Affective Go/No-Go
Analysis of distracter error rates showed a significant treatment by valence by group interaction [F(1,23) = 5.00, p = .035] (Figure 1, Table 1). Further analysis showed a significant valence by group interaction during ATD [F(1,23) = 4.43, p = .047] but not during placebo [F(1,23) = .67, p = .42]. During ATD, the HR group showed a higher number of inappropriate responses to sad than to happy distracters, which approached significance [t(12) = 1.93, p = .08]; in the LR group, responses to distracters during ATD did not differ significantly by valence [t(11) = 1.00, p = .34]. In within-group analyses, the treatment by valence interaction within the HR group fell just short of the trend level [F(1,12) = 3.17, p = .10]; there was no significant treatment by valence interaction in the LR group [F(1,11) = 1.95, p = .19].
Figure 1.
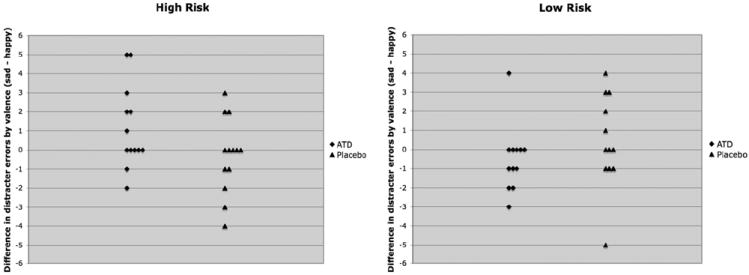
Performance on the affective go/no-go task. Figure 1 illustrates a significant treatment by valence by group interaction in distracter error rates on the affective go/no-go task [F(1,23) = 5.00, p = .035]. During acute tryptophan depletion, the high-risk group made more inappropriate responses to sad distracters during happy target blocks than to happy distracters during sad target blocks, a difference that approached significance [t(12) = 1.93, p = .08]. In the low-risk group, responses to distracters during acute tryptophan depletion did not differ significantly by valence [t(11) = 1.00, p = .34]. There were no significant findings during the placebo condition. Each diamond or triangle represents an individual participant. ATD, acute tryptophan depletion.
Table 1.
Results of the Affective Go/No-Go Task
| Measure | Condition | High Risk
|
Low Risk
|
||
|---|---|---|---|---|---|
| Placebo | ATD | Placebo | ATD | ||
| Reaction Time, msec (mean, standard error) | Happy targets/sad distracters | 492.0 (14.3) | 484.6 (12.5) | 489.7 (14.9) | 478.0 (13.0) |
| Sad targets/happy distracters | 510.1 (13.6) | 498.6 (14.7) | 510.0 (14.1) | 493.2 (15.3) | |
| Total Distracter Errors (mean, standard error) | Happy targets/sad distracters | 2.62 (.7) | 3.46 (.7) | 2.92 (.7) | 2.75 (.7) |
| Sad targets/happy distracters | 2.92 (.5) | 2.31 (.5) | 2.50 (.5) | 3.25 (.5) | |
| Total Omission Errors (mean, standard error) | Happy targets/sad distracters | 1.62 (.7) | .85 (.4) | 1.33 (.8) | .50 (.4) |
| Sad targets/happy distracters | .69 (.3) | .69 (.3) | .75 (.3) | .58 (.3) | |
ATD, acute tryptophan depletion; msec, milliseconds.
There was also a main effect of block type, with a significantly higher number of distracter errors during shift than nonshift blocks [F(1,23) = 17.2, p < .001]. For reaction time (RT), there was a significant main effect of valence, with significantly longer RT for sad than happy words [F(1,23) = 13.8, p = .001]. There were no significant treatment by valence by group interactions in omission error rates or in RT.
Tower of London
On the TOL task, there was a significant main effect of difficulty [F(3,20) = 6.6, p = .003] but no significant treatment by difficulty by group interactions in performance [F(3,20) = 1.3, p = .30] (Table 2).
Table 2.
Results of the Tower of London Task
| Measure | Difficulty Level | High Risk
|
Low Risk
|
||
|---|---|---|---|---|---|
| Placebo | ATD | Placebo | ATD | ||
| Total Correct Responsesa (mean, standard error) | 1 | 4.9 (.06) | 4.9 (.1) | 5.0 (.06) | 4.8 (.1) |
| 2 | 4.8 (.1) | 4.9 (.08) | 4.8 (.1) | 4.9 (.08) | |
| 3 | 4.8 (.2) | 4.3 (.3) | 4.3 (.2) | 4.5 (.3) | |
| 4 | 4.5 (.3) | 4.4 (.3) | 4.3 (.3) | 4.4 (.3) | |
As participants were administered difficulty levels 5 through 6 only if they completed levels 1 through 4 without errors, totals for difficulty levels 4 through 6 were collapsed into one total (labeled 4 in the table) for data analysis.
ATD, acute tryptophan depletion.
n = 24 due to missing data for one high-risk participant.
Discussion
This is the first study to our knowledge to compare the effects of ATD on affective and nonaffective cognitive tasks in healthy individuals with a family history of highly familial forms of depression and low-risk control subjects. Acute tryptophan depletion unmasked a significant group by valence interaction in distracter error rates. In particular, the HR group made a higher number of inappropriate responses to sad than to happy distracters during ATD, a difference that approached significance. In contrast, emotionally neutral decision making was not affected by ATD.
These differential effects of ATD on task-based emotional processing across groups in the absence of overt impact on mood supports prior findings that 5-HT might be more directly linked to affective processing than to mood per se (8,19). Unlike prior studies in LR volunteers (7-9), we did not find a positive bias in the LR group during the placebo condition, possibly given our smaller sample size and the inclusion of both male and female participants. Of note, however, results from these prior studies were not always consistent, as they differed in the dependent measure showing positive bias—distracter errors versus reaction times (Supplement 1).
Findings in never-depressed HR individuals during ATD suggest a possible premorbid vulnerability in 5-HT regulation. Abnormal 5-HT function in HR individuals might induce bias toward sad stimuli, resulting in reduced engagement in potentially rewarding activities or interactions with others, and a complex iterative cycle ultimately leading to first onset of MDD (8,19,20). Of note, only a subset of HR participants showed a higher response to sad than to happy distracters during ATD (Figure 1). Longitudinal follow-up could help determine whether performance on the AGNG can identify HR individuals who will eventually develop MDD, representing over one third of first-degree relatives of probands with early-onset, recurrent MDD (21).
While we studied a carefully characterized sample of HR volunteers at particularly high familial risk for depression, our results are limited by sample size. Further, our findings might be specific to the particular tasks selected for study. Future studies should include a range of affective processing tasks and examine how differences in emotion processing might relate to neural responses and to behavioral responses in real-life situations.
Conclusions
In summary, ATD unmasked differences in emotional processing across HR and LR groups but did not affect planning ability. The possibility that individuals at high familial risk for depression exhibit disturbances in the processing of affective stimuli before and as a step toward the development of a mood disorder deserves further study.
Supplementary Material
Acknowledgments
This work was performed at the Mood and Anxiety Disorders Program, Department of Psychiatry, Mount Sinai School of Medicine, New York, New York, and was funded by Grant Number MO1-RR00071 from the National Center for Research Resources, a component of the National Institutes of Health.
We thank Marije aan het Rot, Ph.D., Douglas Brodman, M.A., Kathryn Keegan, M.A., James Murrough, M.D., Rebecca Price, Ph.D., Yasmina Rebani, B.A., Dana Sandor, M.D., Mara Steinbugler, M.A., William R. Taboas, M.A., Neelam Thapa, M.D., and the staff at the Mount Sinai Clinical Research Unit for excellent assistance. We thank Joseph Snow, Ph.D., for providing us with the affective go/no-go task.
Dr. Feder has received grant/research support from GlaxoSmithKline. Dr. Mathew has received grant/research support from Alexza Pharmaceuticals, GlaxoSmithKline, Novartis, National Alliance for Research on Schizophrenia and Depression, and Roche and has received consulting or lecture fees from AstraZeneca, Evotec Jazz Pharmaceuticals, Merck, and Pfizer. Dr. Neumeister has received grant/research support from Pfizer, Inc.; Eli Lilly; UCB Pharma, Inc.; and Ortho-McNeil Janssen Scientific Affairs, LLC. In addition, Drs. Charney and Mathew have been named as inventors on a use-patent of ketamine for the treatment of depression.
Footnotes
Supplementary material cited in this article is available online.
Dr. Charney reported no other biomedical financial interests or potential conflicts of interest. Ms. Skipper, Dr. Blair, Ms. Buchholz, Dr. Schwarz, Dr. Doucette, Ms. Alonso, and Ms. Collins reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Leppanen JM. Emotional information processing in mood disorders: A review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 2.Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: Implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- 3.Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 4.Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- 5.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Neumeister A. Tryptophan depletion, serotonin, and depression: Where do we stand? Psychopharmacol Bull. 2003;37:99–115. [PubMed] [Google Scholar]
- 7.Roiser JP, Levy J, Fromm SJ, Wang H, Hasler G, Sahakian BJ, Drevets WC. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33:1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson OJ, Sahakian BJ. A double dissociation in the roles of serotonin and mood in healthy subjects. Biol Psychiatry. 2009;65:89–92. doi: 10.1016/j.biopsych.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- 10.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: Review. Mol Psychiatry. 2003;8:951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 11.van der Veen FM, Evers EA, Deutz NE, Schmitt JA. Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology. 2007;32:216–224. doi: 10.1038/sj.npp.1301212. [DOI] [PubMed] [Google Scholar]
- 12.Evers EAT, van der Veen FM, Jolles J, Deutz NEP, Schmitt JAJ. The effect of acute tryptophan depletion on performance and the BOLD response during a Stroop task in healthy first-degree relatives of patients with unipolar depression. Psychiatry Res. 2009;173:52–58. doi: 10.1016/j.pscychresns.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Firk C, Markus CR. Effects of acute tryptophan depletion on affective processing in first-degree relatives of depressive patients and controls after exposure to uncontrollable stress. Psychopharmacology (Berl) 2008;199:151–160. doi: 10.1007/s00213-008-1125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry. 2009;66:441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell ME. Family Interview for Genetic Studies (FIGS): Manual for FIGS. Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- 17.Wolfe BE, Metzger ED, Jimerson DC. Comparison of the effects of amino acid mixture and placebo on plasma tryptophan to large neutral amino acid ratio. Life Sci. 1995;56:1395–1400. doi: 10.1016/0024-3205(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 18.Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 19.Harmer CJ. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 21.Zubenko GS, Zubenko WN, Spiker DG, Giles DE, Kaplan BB. Malignancy of recurrent, early-onset major depression: A family study. Am J Med Genet. 2001;105:690–699. doi: 10.1002/ajmg.1554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


