Abstract
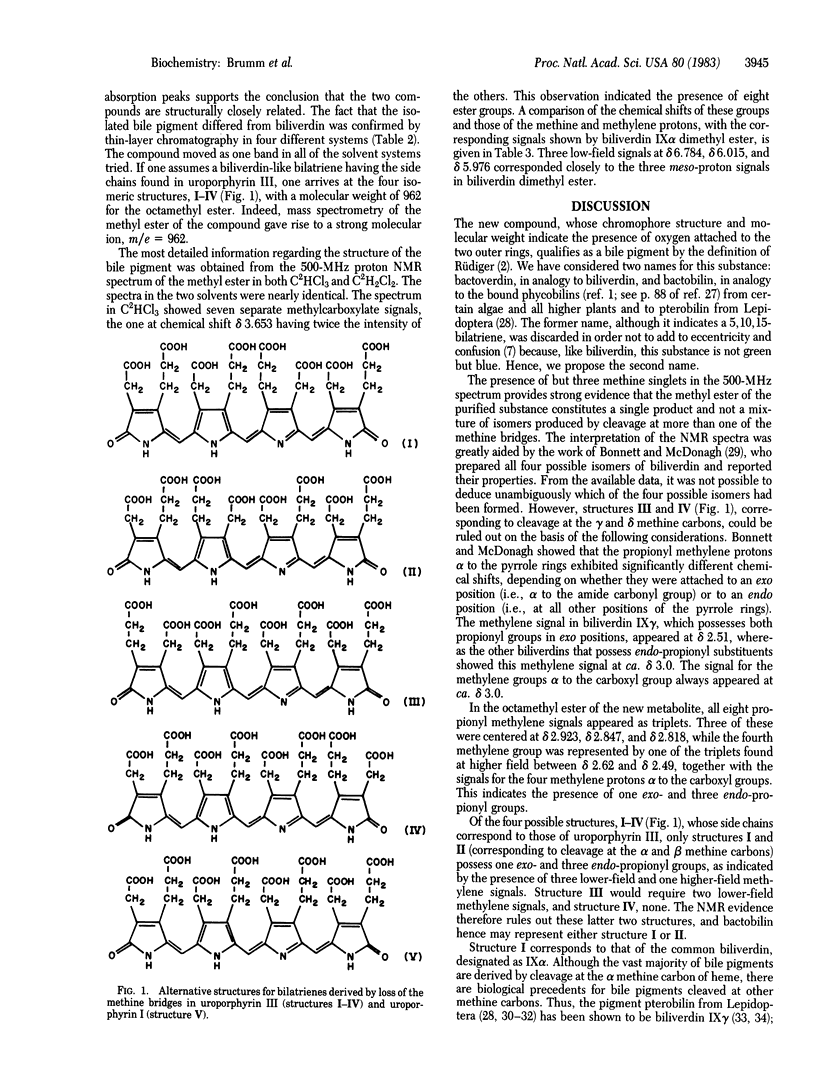
A blue bile pigment, possessing four acetic and four propionic acid side chains has been isolated from extracts of the anaerobic microorganism Clostridium tetanomorphum and in smaller amounts from Propionibacterium shermanii. The compound could be prepared in larger amounts by incubation of C. tetanomorphum enzyme extracts with added delta-aminolevulinic acid. The ultraviolet-visible, infrared, and proton magnetic resonance spectra of the pigment indicate a chromophore of the biliverdin type. Field-desorption mass spectrometry of the purified methyl ester showed a strong molecular ion at m/e = 962. This corresponds to the molecular weight expected for the octamethyl ester of a bilatriene type of bile pigment structurally derived from uroporphyrin III or I. Of the five possible structures, two could be eliminated by proton magnetic resonance spectroscopy. The name bactobilin is proposed for this previously unreported bile pigment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battersby A. R., Bushell M. J., Jones C., Lewis N. G., Pfenninger A. Biosynthesis of vitamin B12: identity of fragment extruded during ring contraction to the corrin macrocycle. Proc Natl Acad Sci U S A. 1981 Jan;78(1):13–15. doi: 10.1073/pnas.78.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann K. H., Deeg R., Gneuss K. D., Kriemler H. P., Müller G. Zur Cobyrinäure-Biosynthese. Gewinnung von Zwischenprodukten der Cobyrinsäure-Biosynthese mit Zellsuspensionen von Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1977 Oct;358(10):1315–1323. [PubMed] [Google Scholar]
- Bonnett R., McDonagh A. F. The meso-reactivity of porphyrins and related compounds. VI. Oxidative cleavage of the haem system. The four isomeric biliverdins of the IX series. J Chem Soc Perkin 1. 1973;9:881–888. doi: 10.1039/p19730000881. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Holroyd J. A., Troxler R. F., Offner G. D. Bile pigment synthesis in plants. Incorporation of haem into phycocyanobilin and phycobiliproteins in Cyanidium caldarium. Biochem J. 1981 Jan 15;194(1):137–147. doi: 10.1042/bj1940137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm P. J., Friedmann H. C. Succinylacetone pyrrole, a powerful inhibitor of vitamin B12 biosynthesis: effect of delta-aminolevulinic acid dehydratase. Biochem Biophys Res Commun. 1981 Oct 15;102(3):854–859. doi: 10.1016/0006-291x(81)91616-8. [DOI] [PubMed] [Google Scholar]
- Colleran E., Heirwegh K. P. Comparative aspects of bile pigment formation and excretion. Comp Biochem Physiol B. 1979;64(2):133–139. doi: 10.1016/0305-0491(79)90151-2. [DOI] [PubMed] [Google Scholar]
- Dauner H. O., Müller G. Bildung von Cobyrinsäure mittels eines zellfreien Systems aus Clostridium tetanomorphum. Hoppe Seylers Z Physiol Chem. 1975 Sep;356(9):1353–1358. doi: 10.1515/bchm2.1975.356.2.1353. [DOI] [PubMed] [Google Scholar]
- Deeg R., Kriemler H. P., Bergmann K. H., Müller G. Zur Cobyrinsäure-Biosynthese. Neuartige, methylierte Hydroporphyrine und deren Bedeutung bei der Cobyrinsäure-Bildung. Hoppe Seylers Z Physiol Chem. 1977 Mar;358(3):339–352. [PubMed] [Google Scholar]
- Doss M. Trennung, Isolierung und Bestimmung von Proto-, Kopro-, Pentacarboxy-, Heptacarboxy- und Uroporphyrin. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):499–502. doi: 10.1515/bchm2.1969.350.1.499. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Tomaro M. L., Buldain G., Awruch J., Díaz L., Frydman B. Specificity of heme oxygenase: a study with synthetic hemins. Biochemistry. 1981 Sep 1;20(18):5177–5182. doi: 10.1021/bi00521a012. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Blanckaert N., Compernolle F., Fevery J., Zaman Z. Detection and properties of the non-alpha-isomers of bilirubin-IX. Biochem Soc Trans. 1977;5(1):316–317. doi: 10.1042/bst0050316. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Ibrahim N. G., Kappas A. Solubilization and partial purification of heme oxygenase from rat liver. J Biol Chem. 1977 Aug 25;252(16):5900–5903. [PubMed] [Google Scholar]
- Mombelli L., Nussbaumer C., Weber H., Müller G., Arigoni D. Biosynthesis of vitamin B12: nature of the volatile fragment generated during formation of the corrin ring system. Proc Natl Acad Sci U S A. 1981 Jan;78(1):11–12. doi: 10.1073/pnas.78.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Dieterle W., Siebke G. Gewinnung von Porphyrinen mittels Propionibacterium shermanii. Z Naturforsch B. 1970 Mar;25(3):307–309. [PubMed] [Google Scholar]
- O'Carra P., Colleran E. Separation and identification of biliverdin isomers and isomer analysis of phycobilins and bilirubin. J Chromatogr. 1970 Aug 12;50(3):458–468. doi: 10.1016/s0021-9673(00)97973-1. [DOI] [PubMed] [Google Scholar]
- Petryka Z. J., Watson C. J. Separation of bile pigments by thin layer chromatography. J Chromatogr. 1968 Sep 24;37(1):76–82. doi: 10.1016/s0021-9673(01)99073-9. [DOI] [PubMed] [Google Scholar]
- Rüdiger W., Klose W., Vuillaume M., Barbier M. On the structure of pterobilin, the blue pigment of Pieris brassicae. Experientia. 1968 Oct 15;24(10):1000–1000. doi: 10.1007/BF02138705. [DOI] [PubMed] [Google Scholar]
- Schacter B. A., Nelson E. B., Marver H. S., Masters B. S. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J Biol Chem. 1972 Jun 10;247(11):3601–3607. [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Chapman D. J., Cole W. J. The bile pigments of plants. Biochem Soc Symp. 1968;28:107–120. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., Lund G. Isolation of uroporphyrin III from Clostridium thermoaceticum. Biochem Biophys Res Commun. 1979 Sep 27;90(2):575–581. doi: 10.1016/0006-291x(79)91274-9. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Purification and properties of heme oxygenase from pig spleen microsomes. J Biol Chem. 1978 Jun 25;253(12):4224–4229. [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Sequence of the reaction of heme catabolism catalyzed by the microsomal heme oxygenase system. FEBS Lett. 1974 Nov 15;48(2):256–261. doi: 10.1016/0014-5793(74)80481-3. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982 Jul 10;257(13):7778–7785. [PubMed] [Google Scholar]


